Introduction
Gastric cancer (GC) is one of the most common human
malignant diseases and the second leading cause of
cancer-associated mortality worldwide. GC has a particularly high
incidence and mortality rate in China (1,2),
with 404,565 new cases of GC annually, and 312,432 patients
succumbing to disease each year (3). During GC progression, GC cells become
increasingly resistant to apoptosis, and therefore refractory to
therapy (4,5). Despite improvements in systemic
therapy, the prognosis of GC patients remains poor. Therefore,
investigating the molecular mechanisms underlying GC progression
may be beneficial for the development of novel targeted
therapies.
MicroRNAs (miRNAs) are small (19–24 nucleotides in
length), noncoding RNAs, that incompletely bind to the 3′
untranslated region of target genes and subsequently negatively
regulate expression of multiple genes by inducing translational
silencing or degrading the mRNA (4). Accumulating evidence suggests that
miRNAs act as oncogenes or tumor suppressors, and that the
dysregulation of specific miRNAs is associated with the cancerous
transformation of cells (6–8).
miRNA (miR)-146a is involved in cell proliferation, differentiation
and apoptosis (9). miR-146a
dysregulation and dysfunction correlates with tumorigenesis and the
development of various types of cancer (10,11).
However, miR-146a exerts opposite effects in different cancers. It
serves as an oncogene in anaplastic thyroid carcinoma and cervical
cancer, and as a tumor suppressor in breast and prostate cancers
(12–15). Although evidence has suggested that
the expression of miR-146a is downregulated in GC, and that
downregulation of miR-146a is associated with tumor size and poor
prognosis (16), the underlying
molecular mechanism of miR-146a and its effects remain largely
unclear.
Cancer cells typically acquire a constitutively
active nuclear factor (NF)-κB pathway to promote survival,
proliferation and metastatic potential (17). In the majority of cell types, NF-κB
complexes are retained in the cytoplasm in an inactive form by a
family of inhibitory proteins known as inhibitors of κB (IκBs),
which includes IκBα, IκBβ and IκBε (18,19).
Degradation of IκB facilitates the release and nuclear
translocation of NF-κB (18).
Transforming growth factor β-activated kinase 1 (TAK1), a member of
the mitogen-activated protein kinase kinase kinase family, has a
prosurvival role in the activation of the NF-κB signaling pathway
(20). Loss of TAK1 sensitizes
cells for apoptosis or death in the majority of tissue types
(21). These results indicate that
TAK1 is an important upstream effector of the NF-κB signaling
pathway and that altering the expression of TAK1 may affect
apoptosis. In addition, previous studies have demonstrated that
miR-146a serves as a negative regulator of constitutive NF-κB
activity in breast cancer (12,17).
However, whether miR-146a regulates GC cell apoptosis by negatively
regulating TAK1 expression remains to be fully elucidated. The
present study analyzed the function of miR-146a in GC and
demonstrated that it inhibits GC cell apoptosis by targeting TAK1,
with subsequent inhibition of the NF-κB signaling pathway.
Materials and methods
Cell culture and transfection
SGC-7901 human GC cells (Shanghai Institute of Cell
Biology, Shanghai, China) were cultured in RPMI 1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified 5% CO2 incubator at 37°C. The miR-146a mimic
and inhibitor were obtained from Biotend Biological Technology
(Shanghai, China). Scrambled negative control mimic or inhibitor
(Biotend Biological Technology) were transfected to serve as
matched controls. TAK1-Flag plasmid was obtained from Addgene, Inc.
(Cambridge, MA, USA). TAK1 siRNA and the scrambled negative
control were purchased from Shanghai GenePharma, Co., Ltd.
(Shanghai, China). Oligomers used are listed in Table I. Cells (5×105/well)
were seeded into 6-well plates the day prior to transfection to
ensure a suitable cell confluence (70%) on the day of transfection.
Cells were subsequently transfected using Lipofectamine®
RNAiMax (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h
according to the manufacturer's protocol. The miR-146a mimic,
inhibitor and respective controls were used at a final
concentration of 10 nM. si TAK1 and control were used at a
final concentration of 20 nM. A total of 3 µg TAK1-Flag plasmid was
used for overexpression of TAK1.
 | Table I.Oligomers and primers sequences and
used in the present study. |
Table I.
Oligomers and primers sequences and
used in the present study.
| Gene (Homo
sapiens) | Sequence
(5′-3′) |
|---|
| miR-146a mimic |
UGAGAACUGAAUUCCAUGGGUU |
| Mimic NC |
UUCUCCGAACGUGUCACGUTT |
| miR-146a
inhibitor |
AACCCAUGGAAUUCAGUUCUCA |
| Inhibitor NC |
ACGUGACACGUUCGGAGAATT |
| si TAK1 |
AAAGCGTTTATTGTAGAGCTT |
| si TAK1
NC |
ACGUGACACGUUCGGAGAATT |
|
miR-146a-forward |
CAGTGCGTGTCGTGGAGT |
|
miR-146a-reverse |
GGGTGAGAACTGAATTCCA |
|
U6-forward |
CTCGCTTCGGCAGCACA |
|
U6-reverse |
AACGCTTCACGAATTTGCGT |
|
TAK1-forward |
ATCAGCAGAGTAGCTGCGGT |
|
TAK1-reverse |
GAGGAGCTTGCTGCAGAGT |
|
GAPDH-forward |
AGGGCTGCTTTTAACTCTGGT |
|
GAPDH-reverse |
CCCCACTTGATTTTGGAGGGA |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Single-stranded
cDNA was synthesized from total RNA using Moloney Murine Leukemia
Virus Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific,
Inc.) and oligo (dT) 18. qPCR was performed in a 96-well plate on a
Bio-Rad CFX Real-Time system using iQ™
SYBR®-Green Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). For miR-146a detection, the following
thermocycling conditions were used: An initial predenaturation step
at 50°C for 2 min, followed by 40 cycles of denaturation at 95°C
for 10 min and annealing at 60°C for 1 min. For TAK1, the
thermocycling conditions were as follows: An initial
predenaturation step at 94°C for 5 min, followed by 35 cycles of
denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 20 sec. Each run was performed in triplicate.
The primer sequences used are listed in Table I. The data were expressed as fold
change. The comparative quantitation cycle method was used to
quantify the expression levels of target genes relative to
endogenous controls (22). To
normalize the relative abundance of miR-146a and TAK1, U6 and GAPDH
served as endogenous controls, respectively (23). For each individual analysis, the
control group was used as the calibrator and given a relative value
of 1.0. All quantities were expressed as n-fold relative to the
calibrator.
Flow cytometry
An Annexin V-Fluorescein Isothiocyanate
(FITC)/Propidium Iodide (PI) Staining kit (BD Biosciences, Franklin
Lakes, NJ, USA) was used to detect apoptotic cells. Cells were
harvested 48 h following transfection and resuspended in 1X binding
buffer at a concentration of 1×106 cells/ml.
Subsequently, cells were stained with 5 µl Annexin V-FITC and 5 µl
PI for 15 min at room temperature in the dark. Cells were acquired
using a BD Accurri™ C6 flow cytometer (BD Biosciences)
and data was analyzed using FlowJo software version 7.6.2 (Tree
Star, Inc., Ashland, OR USA).
Caspase-3 activity assay
Following transfection with miR-146a mimic or
inhibitor, SGC-7901 cells were seeded into 96-well plates at a
density of 1×105 cells/well, incubated overnight and
processed using a Caspase-3 Activity assay kit (Cell Signaling
Technology, Inc., Danvers, MA, USA). Cells were lysed in 30 µl 1X
PathScan® Sandwich ELISA Lysis Buffer. The cell lysate
was mixed with the substrate solution and incubated at 37°C in the
dark for 2 h, following which the relative fluorescent was measured
using a fluorescence plate reader (BioTek Instruments, Inc.,
Winooski, VT, USA) with an excitation wavelength of 380 nm and an
emission wavelength of 420 nm.
Cell proliferation assay
SGC-7901 cell proliferation was detected using Cell
Counting kit-8 (CCK8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Following transfection, SGC-7901 cells were
seeded into 96-well plates at a density of 4×103
cells/well. CCK8 solution (10 µl) was added to each well and cells
were incubated for 2 h at 37°C. Optical density (OD) values were
obtained at a wavelength of 450 nm using a microplate reader
(BioTek Instruments, Inc.). The growth curve was constructed using
the mean OD value every 24 h for 3 days.
Western blotting
After 48 h transfection, cultured SGC-7901 cells
were washed with cold PBS once and lysed with lysis buffer (0.5%
NP40). Equal quantities of protein (30 µg) were separated by 10%
SDS-PAGE and transferred to nitrocellulose membranes. Membranes
were blocked with 5% skimmed milk and incubated with the following
primary antibodies, diluted 1:1,000, overnight at 4°C: Rabbit
anti-TAK1 (catalog no. 4505), rabbit anti-IκBα (catalog no. 4812),
anti-rabbit B-cell lymphoma 2 (Bcl-2; catalog no. 4223), rabbit
anti-β-actin (catalog no. 4970) and rabbit anti-Flag (catalog no.
14793), all purchased from Cell Signaling Technology, Inc. Blots
were washed with TBS containing Tween 20 (TBST) three times for 10
min each time. Subsequently, membranes were incubated with a
horseradish peroxidase-conjugated goat anti-rabbit antibody
(1:3,000; catalog no. A00098; GenScript, Piscataway, NJ, USA) for 2
h at room temperature and washed with TBST three times for 10 min
each time. Protein bands were visualized using Enhanced
Chemiluminescence Plus (Thermo Fisher Scientific, Inc.) and scanned
by Typhoon™ FLA 9500 (GE Healthcare Life Sciences,
Chalfont, UK). Densitometry was quantified using Image J software
(National Institutes of Health, Bethesda, MD, USA) and normalized
to β-actin.
Statistical analysis
Statistical analysis was performed using SPSS
software version 19 (IBM SPSS, Armonk, NY, USA). Data are expressed
as the mean ± standard deviation. Paired Student's t-tests or
one-way analyses of variance followed by the Bonferroni post hoc
test were used to determine statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-146a serves as an apoptotic
regulator
Abnormal apoptosis of tumor cells is critical for
cancer progression (5). Previous
studies have suggested that miR-146a provides negative feedback
inhibition of tumor progression (8,24,25).
Therefore, the present study evaluated the effect of altering
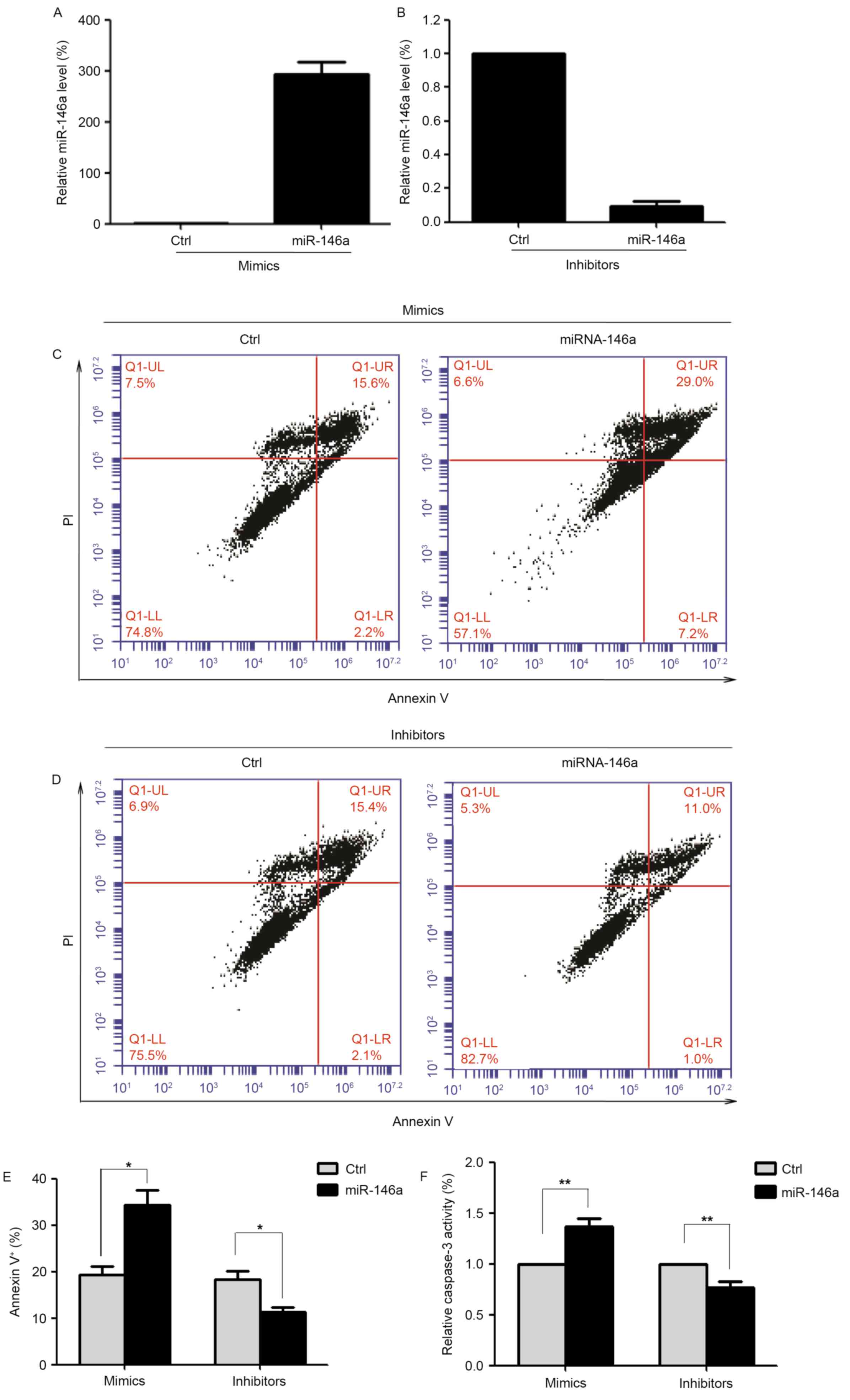
miR-146a expression levels on SGC-7901 cell apoptosis. SGC-7901
cells were transfected with an miR-146a mimic or scrambled control
for 48 h. Cells transfected with an miR-146a mimic demonstrated a
~300-fold increase in expression levels of miR-146a compared with
control cells, as assessed by RT-qPCR (Fig. 1A). In addition, SGC-7901 cells were
transfected with an miR-146a inhibitor or scrambled control for 48
h. Cells transfected with an miR-146a inhibitor exhibited a
decrease in miR-146a expression levels to ~10% of the levels of
control cells (Fig. 1B). Apoptosis
was assessed in transfected cells using Annexin V/PI staining
(Fig. 1C and D). Compared with the
scrambled control-transfected cells, overexpression of miR-146a
significantly increased the proportion of apoptotic cells (34.4±7.9
vs. 19.4±4.3%; n=6; P<0.05; Fig.
1E). The proportion of apoptotic cells following transfection
with an miR-146a inhibitor was significantly decreased compared
with scrambled control-transfected cells (11.3±2.6 vs. 18.4±4.1%;
n=6; P<0.05; Fig. 1E).
In addition, the effect of miR-146a on apoptosis of
SGC-7901 cells was assessed by measuring caspase-3 activity, a
feature of apoptosis. Overexpression of miR-146a significantly
increased caspase-3 activity, whereas inhibition of miR-146a
decreased caspase-3 activity (Fig.
1F). These results demonstrated that miR-146a may serve a
pro-apoptotic role in GC cells.
miR-146a modulates GC cell
proliferation
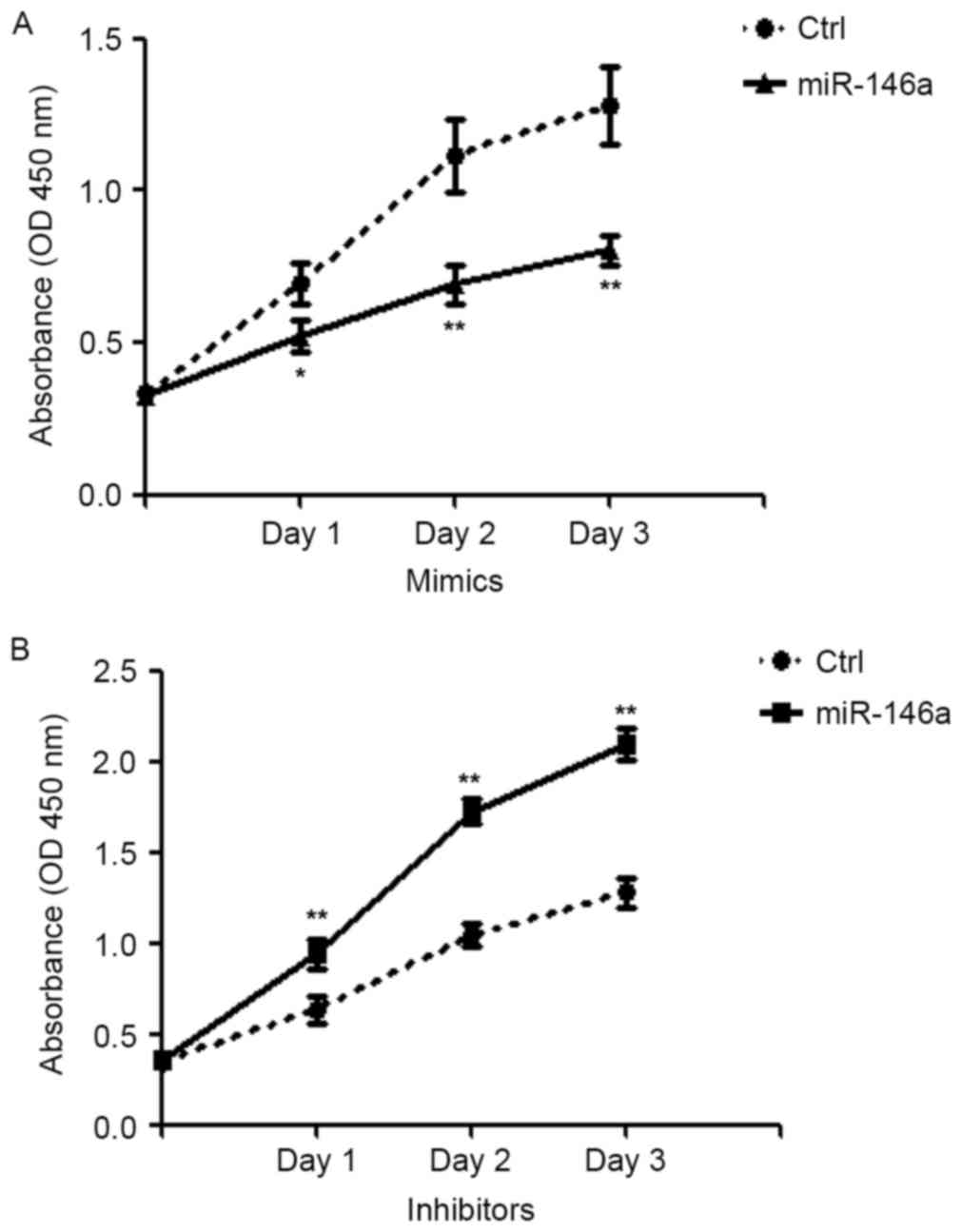
As the rapid proliferation of tumor cells serves a
pivotal role in tumor growth and development (5), the present study assessed the effects
of altering miR-146a expression levels on the proliferation of
SGC-7901 cells using a CCK8 assay. Compared with scrambled
control-transfected cells, miR-146a overexpression caused a
significant inhibition of cell proliferation (day 1, P<0.05;
days 2 and 3, P<0.01; Fig. 2A).
By contrast, knockdown of miR-146a led to a significant increase in
cell proliferation compared with the scrambled control (P<0.01;
Fig. 2B). These data suggested
that miR-146a expression is inversely correlated with cell
proliferation.
TAK1 expression is inversely
correlated with miR-146a expression
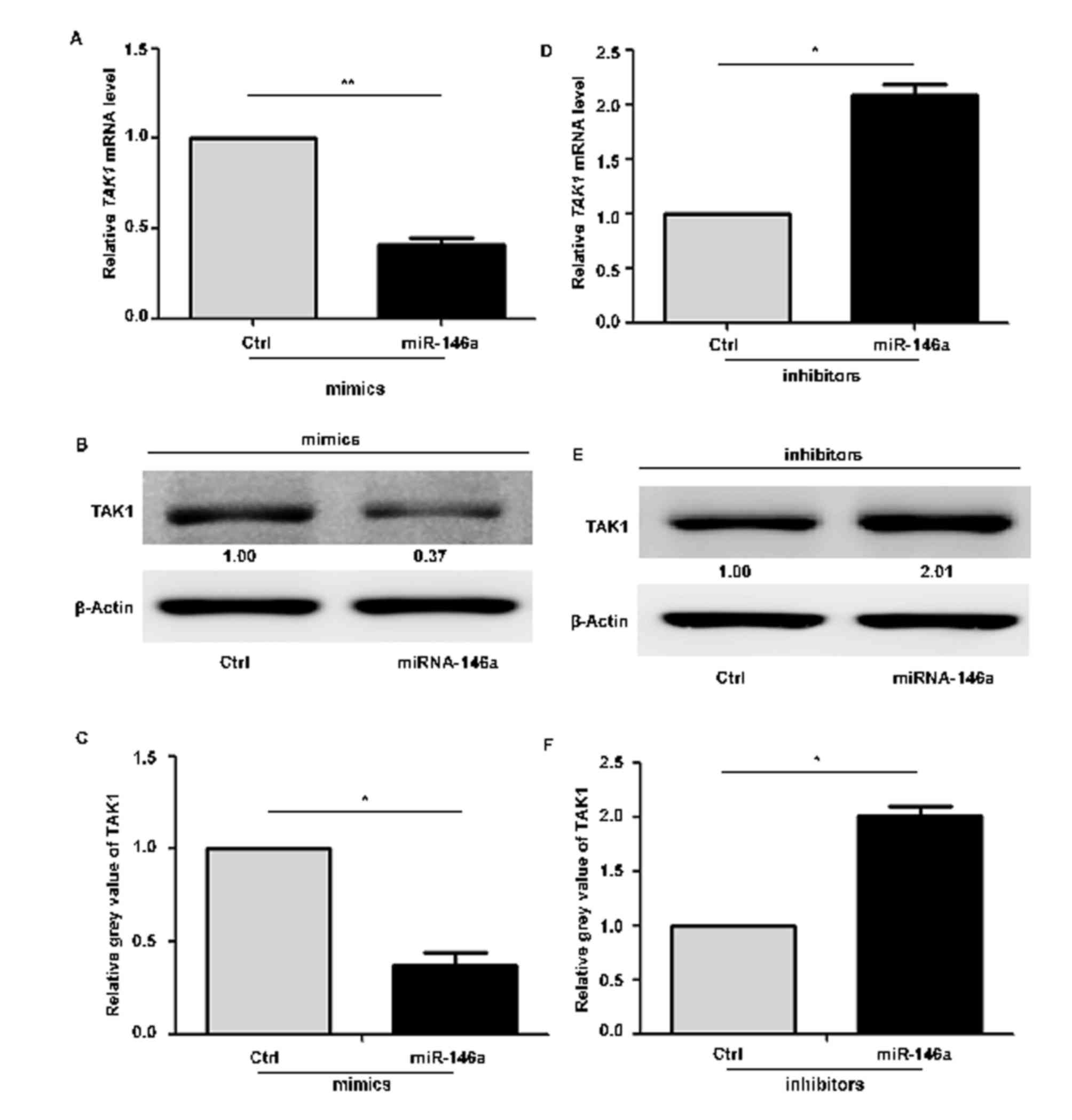
To determine whether miR-146a regulates TAK1 in GC,
the present study assessed the expression of TAK1 following
inhibition or overexpression of miR-146a in SGC-7901 cells, using
RT-qPCR and western blotting. Compared with scrambled
mimic-transfected cells, TAK1 expression at the mRNA (Fig. 3A) and protein (Fig. 3B and C) levels was decreased
following transfection with an miR-146a mimic. By contrast,
inhibition of miR-146a resulted in significant increases in TAK1
mRNA (Fig. 3D) and protein
(Fig. 3E and F) expression levels
compared with scrambled controls. These data indicated that
miR-146a expression is inversely correlated with TAK1, which may be
a potential target molecule of miR-146a in GC cell apoptosis.
Knockdown of TAK1 induces GC cell
apoptosis
To investigate whether TAK1 is involved in
regulating miR-146a-mediated SGC-7901 apoptosis, the present study
knocked down TAK1 using siRNA (si TAK1) to determine
the effect on GC cell apoptosis. TAK1 mRNA expression levels
were significantly decreased following siRNA transfection in
SGC-7901 cells compared with scrambled controls (Fig. 4A). Overexpression of TAK1 using a
TAK1-Flag plasmid increased protein expression levels of TAK1
compared with control cells (Fig. 4B
and C). Apoptosis was assessed in transfected cells using
Annexin V/PI staining (Fig. 4D and
E). Compared with scrambled control siRNA, the percentage of
apoptotic SGC-7901 cells following transfection with si TAK1
was significantly increased (37.33±8.87 vs. 19.18±6.30%; n=6;
P<0.05; Fig. 4F), in accordance
with the results obtained with miR-146a overexpression. By
contrast, overexpression of TAK1 led to increased SGC-7901 cell
survival compared with control cells (Fig. 4G). These data indicated that
miRNA-146a mediates apoptosis in human GC via targeting
TAK1.
miR-146a induces apoptosis via the
NF-κB pathway in GC
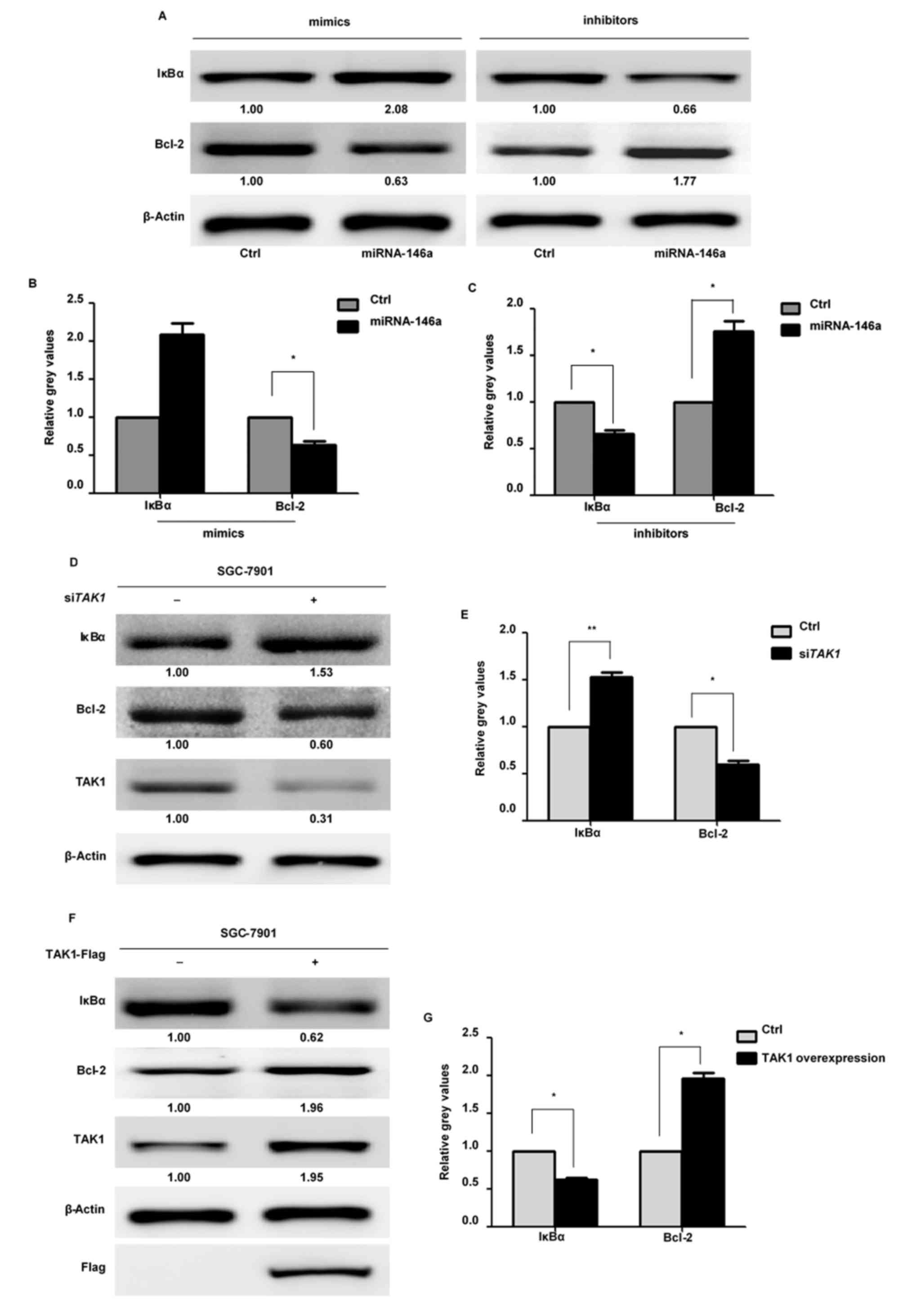
To confirm that miR-146a-induced GC cell apoptosis
involves inhibition of the NF-κB signaling pathway, at least
partially via downregulation of the NF-κB pathway mediator TAK1,
the present study detected IκBα protein expression levels by
western blotting. Following transfection with an miR-146a mimic,
IκBα protein expression levels were significantly upregulated,
whereas these levels were downregulated following transfection of
an miR-146a inhibitor (Fig. 5A-C).
Knockdown of TAK1 by siRNA additionally led to increased
IκBα protein expression levels (Fig.
5D and E). By contrast, overexpression of TAK1 resulted in
decreased expression levels of IκBα (Fig. 5F and G). These results indicated
that miR-146a inhibits the NF-κB signaling pathway. The protein
expression levels of Bcl-2, a known anti-apoptotic downstream
effecter of NF-κB, were also assessed. Transfection with an
miR-146a mimic or si TAK1 induced a decrease in Bcl-2
expression, whereas transfection of an miR-146a inhibitor led to an
increase in Bcl-2 expression in SGC-7901 cells. Increased
expression levels of Bcl-2 were additionally observed following
overexpression of TAK1. Taken together, these data suggested that
miR-146a modulates SGC-7901 cell apoptosis through suppression of
the NF-κB signaling pathway via targeting TAK1.
Discussion
The present study revealed three important findings
concerning the function of miR-146a in human GC progression.
Firstly, miR-146a may be a critical regulator of GC cell
proliferation and apoptosis. Overexpression of miR-146a
significantly enhanced apoptosis and inhibited proliferation of
SGC-7901 cells, whereas inhibition of miR-146a resulted in reduced
apoptosis and increased survival. Secondly, miR-146a inversely
affected the expression of TAK1 in GC cells. Finally, miR-146a
targeted TAK1, leading to inhibition of NF-κB and reduced
expression of Bcl-2. Therefore, miR-146a may regulate GC cell
apoptosis and proliferation by inhibiting the NF-κB signaling
pathway via targeting TAK1, suggesting a novel negative feedback
mechanism for miR-146a in the regulation of GC development.
The capacity of tumor cell populations to expand in
number is attributed to the rate of cell growth and apoptosis.
Rapid proliferation and evasion of apoptosis, two of the primary
features of cancer cells, are the leading causes of GC progression
(5,26). Hou et al (25) revealed that miR-146a enhances
apoptosis and inhibits survival in MKN-45 cells, a poorly
differentiated GC cell line. Consistent with these results, the
present study demonstrated that overexpression of miR-146a promoted
apoptosis and inhibited proliferation in SGC-7901 cells, a
moderately differentiated GC cell line, whereas inhibition of
miR-146a decreased apoptosis and increased proliferation. These
data suggested that miR-146a is an important regulator of GC cell
survival. Previously, up- and down-regulated expression of miR-146a
has been reported in GC tissues (16,25,27).
miR-146a expression was revealed to be high in noncancerous
prostatic epithelium and gradually decreased with cancer
progression (28). Reduced levels
of miR-146a have been associated with lymph node metastasis and
venous invasion (16).
Furthermore, the loss of miR-146a may be a late event in the
progression of GC (10).
Therefore, the conflicting data regarding the expression of
miR-146a may be attributed to different tumor progression status
and clinical stages. of GC. Although the expression of miR-146a in
GC remains controversial, there is a consensus that miR-146a acts
as a tumor suppressor in GC progression by inhibiting proliferation
and promoting apoptosis.
In GC, the NF-κB signaling pathway serves a pivotal
role in modulating cell survival, apoptosis, immunity and
inflammation, and NF-κB activation is associated with poor
prognosis (27,29). Various regulatory proteins,
including IκBα and Bcl-2, are direct transcriptional targets of
NF-κB, thus forming a negative feedback loop (30). Recently, evidence has demonstrated
that phosphorylation of IκBα at Ser-32 is necessary for its
degradation, and that this phosphorylation is decreased by
overexpression of miR-146a in breast cancer cells (12). In addition, upregulation of IκBα by
overexpression of miR-146a has been revealed in non-small cell lung
cancer cells (31). Consistent
with this, the present study demonstrated that overexpression of
miR-146a increased IκBα expression and decreased Bcl-2 expression
in SGC-7901 cells. By contrast, inhibition of miR-146a
downregulated expression levels of IκBα and upregulated those of
Bcl-2. Therefore, the results of the present study supported
published data suggesting that miR-146a modulates SGC-7901
apoptosis by inhibition of the NF-κB signaling pathway.
TAK1, a key molecular component in the determination
of cell fate, is an upstream kinase of NF-κB (20,21).
Accumulating evidence suggests the existence of a TAK1-NF-κB loop
in various diseases (21,30). The present study identified the
mechanism underlying miR-146a regulation of GC cell apoptosis
through the miR-146a/TAK1/NF-κB axis. Expression of TAK1 was
inversely modulated by miR-146a. Furthermore, overexpression of
miR-146a or silencing of TAK1 led to increased expression levels of
IκBα in SGC-7901 cells. By contrast, inhibition of miR-146a or
overexpression of TAK1 decreased the protein expression levels of
IκBα. Previous studies have suggested that TAK1 may phosphorylate
the IκB kinase complex for degradation and release NF-κB (20,32),
which may subsequently translocate to the nucleus and activate a
range of genes involved in inhibition of apoptosis and promotion of
proliferation (20,21). Therefore, miR-146a may act as an
NF-κB signaling pathway negative regulator via repression of TAK1
in GC cells.
In conclusion, the results of the present study
suggested that miR-146a, serving as a tumor suppressor, may
significantly promote GC cell apoptosis by inhibition of the NF-κB
signaling pathway via targeting TAK1. The newly identified
miR-146a/TAK1/NF-κB axis provides a novel insight into GC
progression. These findings suggested that miR-146a may be a
potential therapeutic target for the treatment of GC.
Acknowledgements
The present study was supported by the Social
Programs of Wenzhou Technology Bureau (grant no. Y20130231).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
4
|
Schickel R, Park SM, Murmann AE and Peter
ME: miR-200c regulates induction of apoptosis through CD95 by
targeting FAP-1. Mol Cell. 38:908–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanaka Y, Muramatsu T, Uetake H, Kozaki K
and Inazawa J: miR-544a induces epithelial-mesenchymal transition
through the activation of WNT signaling pathway in gastric cancer.
Carcinogenesis. 36:1363–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Ge X, Zhang Z, Zhang X, Chang J, Wu
Z, Tang W, Gan L, Sun M and Li J: MicroRNA-940 promotes tumor cell
invasion and metastasis by downregulating ZNF24 in gastric cancer.
Oncotarget. 6:25418–25428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao Q, Cao Z, Tu C, Zhao Y, Liu H and
Zhang S: MicroRNA-146a acts as a metastasis suppressor in gastric
cancer by targeting WASF2. Cancer Lett. 335:219–224. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park H, Huang X, Lu C, Cairo MS and Zhou
X: MicroRNA-146a and microRNA-146b regulate human dendritic cell
apoptosis and cytokine production by targeting TRAF6 and IRAK1
proteins. J Biol Chem. 290:2831–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hou Z, Yin H, Chen C, Dai X, Li X, Liu B
and Fang X: microRNA-146a targets the L1 cell adhesion molecule and
suppresses the metastatic potential of gastric cancer. Mol Med Rep.
6:501–506. 2012.PubMed/NCBI
|
|
11
|
Garcia AI, Buisson M, Bertrand P, Rimokh
R, Rouleau E, Lopez BS, Lidereau R, Mikaélian I and Mazoyer S:
Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in
triple negative sporadic breast cancers. EMBO Mol Med. 3:279–290.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y and Wang L: FOXP3 controls an
miR-146/NF-κB negative feedback loop that inhibits apoptosis in
breast cancer cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stuckrath I, Rack B, Janni W, Jäger B,
Pantel K and Schwarzenbach H: Aberrant plasma levels of circulating
miR-16, miR-107, miR-130a and miR-146a are associated with lymph
node metastasis and receptor status of breast cancer patients.
Oncotarget. 6:13387–13401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pacifico F, Crescenzi E, Mellone S,
Iannetti A, Porrino N, Liguoro D, Moscato F, Grieco M, Formisano S
and Leonardi A: Nuclear factor-{kappa}B contributes to anaplastic
thyroid carcinomas through up-regulation of miR-146a. J Clin
Endocrinol Metab. 95:1421–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kogo R, Mimori K, Tanaka F, Komune S and
Mori M: Clinical significance of miR-146a in gastric cancer cases.
Clin Cancer Res. 17:4277–4284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Espinosa L, Margalef P and Bigas A:
Non-conventional functions for NF-kappaB members: The dark side of
NF-κB. Oncogene. 34:2279–2287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh A, Sweeney MF, Yu M, Burger A,
Greninger P, Benes C, Haber DA and Settleman J: TAK1 inhibition
promotes apoptosis in KRAS-dependent colon cancers. Cell.
148:639–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mihaly SR, Ninomiya-Tsuji J and Morioka S:
TAK1 control of cell death. Cell Death Differ. 21:1667–1676. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y, Liu
Y, Zheng D and Shi J: Functions of miR-146a and miR-222 in
Tumor-associated macrophages in breast cancer. Sci Rep.
5:186482015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Q, Zhao X, Liu X, Wang Y, Huang J,
Jiang B, Chen Q and Yu J: miR-146a functions as a tumor suppressor
in prostate cancer by targeting Rac1. Prostate. 74:1613–1621. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou Z, Xie L, Yu L, Qian X and Liu B:
MicroRNA-146a is down-regulated in gastric cancer and regulates
cell proliferation and apoptosis. Med Oncol. 29:886–892. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crone SG, Jacobsen A, Federspiel B,
Bardram L, Krogh A, Lund AH and Friis-Hansen L: microRNA-146a
inhibits G protein-coupled receptor-mediated activation of NF-κB by
targeting CARD10 and COPS8 in gastric cancer. Mol Cancer.
11:712012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avci CB, Harman E, Dodurga Y, Susluer SY
and Gunduz C: Therapeutic potential of an anti-diabetic drug,
metformin: Alteration of miRNA expression in prostate cancer cells.
Asian Pac J Cancer Prev. 14:765–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma H, Gao L, Li S, Qin J, Chen L, Liu X,
Xu P, Wang F, Xiao H, Zhou S, et al: CCR7 enhances TGF-β1-induced
epithelial-mesenchymal transition and is associated with lymph node
metastasis and poor overall survival in gastric cancer. Oncotarget.
6:24348–24360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Greve J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou DX, Luo D, Tanigawa S, Hashimoto F,
Uto T, Masuzaki S, Fujii M and Sakata Y: Prodelphinidin B-4
3′-O-gallate, a tea polyphenol, is involved in the inhibition of
COX-2 and iNOS via the downregulation of TAK1-NF-kappaB pathway.
Biochem Pharmacol. 74:742–751. 2007. View Article : Google Scholar : PubMed/NCBI
|