Introduction
Inflammation is a pathological response to protect
against tissue injury following microbial invasion and is a key
factor in various chronic and metabolic diseases (1,2).
Inflammation is a complex process that is regulated by an array of
inflammatory factors released by activated immune cells (3). Activation of macrophages, which are
key immune cells, by inflammatory stimuli is an important part of
initiating defensive reactions. Activated macrophages release
inflammatory mediators, including nitric oxide (NO), prostaglandin
E2 (PGE2) and pro-inflammatory cytokines that
enhance defense capacity and induce a cascade of immune processes,
including the activation of nitric oxide synthases (NOSs) and
cyclooxygenase-2 (COX-2), which are main targets of
anti-inflammatory agents (4,5).
Nuclear factor-κB (NF-κB) is largely associated with
anti-inflammatory mediators, including NO, PGE2,
interleukin (IL)-6 and tumor necrosis factor (TNF)-α (6,7).
Activated NF-κB is translocated into immune cell nuclei and
mediates the expression of various pro-inflammatory and
immune-regulatory cytokines (8).
Toll-like receptors (TLRs) are pathogen-recognition receptors
present on cell membranes and are major components of the innate
immune system. TLRs are major initiators of the immune responses
against various pathogens. In particular, TLR-4 is associated with
lipopolysaccharide (LPS) at the beginning of the inflammatory
response that occurs in various chronic diseases and complications,
including aging, diabetes and cancer (9). Studies have demonstrated a close
interaction between inflammatory factors in inflammation pathways
and the oxidative stress that underlies chronic diseases (10,11).
Therefore, functional treatments should be developed to treat the
underlying causes of clinical diseases.
Acanthopanax henryi (Oliv.) Harms is used in
northeast Asian countries as a traditional therapeutic agent for
the treatment of rheumatism, inflammation, sinew, bone pains,
lameness and liver disease (12).
A study (13) has suggested that
the Acanthopanax leaves possess potential antioxidant
activities and another (14) that
Acanthopanax root bark extracts exhibit anti-inflammatory
activities. Acanthopanax henryi (Oliv.) contains various
bioactive compounds; however, few studies have been conducted on
them. Therefore, the present study focused on araliasaponin II (AS
II), one of the bioactive compounds from the leaves of
Acanthopanax henryi, as a potential therapeutic agent for
inflammation. As part of our continuing screening program to
evaluate the anti-inflammatory potential of natural compounds, the
anti-inflammatory effects of AS II were investigated and the
potential mechanisms involved in its action on the LPS-stimulated
immune response in murine macrophages was established.
Materials and methods
Plant collection
The leaves of Acanthopanax henryi (Oliv.)
Harms were collected in October 2012 in Xinhua (Hunan, China), 230
km west from Hunan (latitude N 27 56′ 16′, longitude E 111° 21′
22′). The plant is not endangered or protected, and its identify
was confirmed by Professor Liu Xiang-Qian (Hunan Key Laboratory of
Traditional Chinese Medicine Modernization, Hunan University of
Chinese Medicine, Changsha, China) and a voucher specimen (no.
20121125) was deposited within the School of Pharmacy, Hunan
University of Chinese Medicine. No specific permission was required
for collecting the plant.
Extraction and isolation
The dried leaves of Acanthopanax henryi
(Oliv.) Harms (10 kg) were cut into small pieces and extracted
three times with methanol (3×100 l) by soaking at room temperature,
and then concentrated to give a dark-green residue (0.8 kg), which
was suspended in H2O and separated with petroleum ether.
The water fraction was fractionated by column chromatography (CC)
on macroporous resin eluted with a gradient ethanol/H2O
(0, 30, 50, 75 and 95%) into five fractions, 1–5. Fraction 3 (75%
ethanol, 14.0 g) was subjected to silica gel CC eluted with
CHCl3/methanol/H2O (25:1:0/1:1:0.2) to give
15 fractions, A-O.
Fraction M (75 mg) was subjected to silica gel CC
eluted with CHCl3/methanol/H2O
(4:1:0.1/1:1:0.2) to give four sub-fractions, M1-M4 and M2-M4 were
further separated using Sephadex LH-20 (methanol) to produce AS II
(51 mg) (15).
The structures of the compounds were identified by
mass spectrometry (MS), one-dimensional (1D)-nuclear magnetic
resonance spectroscopy (NMR) and 2D-NMR, with a comparison of the
spectral data with those reported previously in the literature
(16).
High performance liquid chromatography
(HPLC)
The purity and content of AS II in the dried leaves
of Acanthopanax henryi (Oliv.) Harms were determined by HPLC
as previously described (17).
Briefly, 4.88 mg AS II was dissolved in 50 ml 100% methanol to a
final concentration of 0.0976 mg/ml for HPLC analysis. The dried
leaves of Acanthopanax henryi (Oliv.) Harms (100 g) were cut
into small pieces and extraction was performed three times using
methanol (3X 1 l) and reflux extraction at 65°C for 2 h each time.
The extract was then concentrated to generate a dark-green residue,
which was suspended in 100 ml H2O and separated with
petroleum ether. The water fraction was fractionated by column
chromatography (80×100 mm) on D101 macroporous resin (600 g;
Tianjin Guangfu Fine Chemical Research Institute, Tianjin, China)
and eluted with a gradient of ethanol/H2O (0, 30 and
75%) into three fractions: Fractions 1 to 3. Fraction 3 was
concentrated, transferred into volumetric flasks and diluted with
100% menthol to 50 ml to produce the sample for HPLC content
analysis with a Kinetex XB-C18 analytical column (100×4.6×2.6 -mm;
Phenomenex, Inc., Torrance, CA, USA) at 30°C. Elution was conducted
using mobile phase A (water) and mobile phase B (acetonitrile) with
a gradient as follows: 0–2 min, 29–31% B; 2–13 min, 31–35% B; 13–15
min, 35–40% B; 15–23 min, 40–44% B; 23–25 min, 44–46% B; 25–31 min,
46–49% B; 31–38 min, 49–55% B. The flow rate was kept constantly at
1.0 ml/min, and the effluents were monitored at 210 nm using an
Agilent 1200 HPLC system with a variable wavelength detector
(Agilent Technologies, Inc., Santa Clara, CA, USA). The purity
value of AS II, as evaluated by HPLC, was observed to be >98% by
the peak area normalization method. The value of purity was
obtained by calculating the percentage of its peak area to that of
the total peaks in the HPLC chromatogram. The content of AS II in
the leaves of Acanthopanax henryi (Oliv.) Harms was 3.28
mg/100 g, which was determined using the external standard method
with the isolated AS II as standard.
General experimental procedures
Melting points (uncorrected) were measured using a
Boetius micromelting point apparatus. Hydrogen-1-NMR (600 MHz),
Carbon-13-NMR (150 MHz) and 2D-NMR were recorded at room
temperature in methanol or pyridine-d5 using a Bruker ACF-500 NMR
spectrometer (Bruker Corporation, Billerica, MA, USA) and chemical
shifts were presented in δ (ppm) values relative to
tetramethylsilane as an internal standard. Mass spectra were
obtained on an MS Agilent 1200 Series LC/MSD Trap Mass spectrometer
(ESI-MS; Agilent Technologies, Inc.). Column chromatography was
carried out on silica gel (200–300 mesh and 100–200 mesh; Qingdao
Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (Merck KGaA,
Darmstadt, Germany) and D101 macroporous resin (Tianjin Guangfu
Chemical Co., Ltd., Tianjin, China). Reversed-phase thin-layer
chromatography was performed on a precoated RP-18F254s plates
(Merck KGaA). Thin-layer chromatography was conducted on self-made
silica gel G (Qingdao Marine Chemical, Inc.) plates and spots were
visualized by spraying with 10% H2SO4 in
ethanol (v/v) followed by heating at 105°C.
Reagents
RPMI-1640, penicillin and streptomycin were obtained
from Hyclone (GE Healthcare Life Sciences, Logan, UT, USA). Bovine
serum albumin, LPS,
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from
Sigma-Aldrich (Merck KGaA). Inducible NOS (iNOS; cat. no. sc-651),
COX-2 (cat. no. sc-1745), TLR-4 (cat. no. sc-16240), NF-κB (cat.
no. sc-8008), β-actin (cat. no. sc-47778) and peroxidase-conjugated
secondary antibodies (anti-mouse, cat. no. sc-2005; anti-rabbit,
cat. no. sc-2004; anti-goat, cat. no. sc-2020) were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Mouse IL-6
ELISA kit (cat. no. 555240) and mouse TNF-α (mono/mono) ELISA kit
(cat. no. 555268) were purchased from BD Biosciences (San Jose, CA,
USA). PRO-PREP™ Protein Extraction Solution was purchased from
Intron Biotechnology, Inc. (Seongnam, Korea). An RNeasy Mini kit
and a QuantiTect Reverse Transcription kit were purchased from
Qiagen GmbH (Hilden, Germany). Finally, fluorochrome-conjugated
secondary antibodies (anti-mouse, cat. no. A-11029 and anti-goat,
cat. no. A-21432) and fluorochrome-conjugated LPS (cat. no.
L-23351) were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA).
Cell culture
The RAW 264.7 cells were obtained from the Korea
Research Institute of Bioscience and Biotechnology (Seoul, Korea)
and cultured in RPMI 1640 medium supplemented with 10% fetal bovine
serum and 100 U/ml of penicillin/streptomycin sulfate. The cells
were cultured in a humidified incubator with 5% CO2
atmosphere at 37°C. To stimulate the cells, the medium was replaced
with fresh RPMI 1640 medium followed by addition of LPS in the
presence or absence of AS II for the indicated periods.
MTS assay
An MTS assay was used to determine the viability of
RAW 264.7 cells. Cells (5×104 cells per well) were
plated in 96-well plates (SPL Life Sciences, Pocheon, Korea). Cells
were treated without or with AS II (10, 20 and 40 µM) and the
plates were incubated for 24 h at 37°C followed by a further 2 h
with MTS solution (5 mg/ml). Optical density was measured at 490
nm. Cell viability was calculated using the formula (mean
absorbance value of treated cells/mean absorbance value of
untreated cells)x100.
Nitrite production
The cells were seeded at 5×104 per well
in 96-well culture plates. The RAW 264.7 cells were stimulated with
LPS (200 ng/ml) without or with AS II (10, 20 and 40 µM) for 24 h.
Nitrite in the cultured RAW 264.7 cell supernatant was determined
using Griess reagent (1% sulfanilamide, 0.1%
naphthylethylenediamine dihydrochloride and 2.5% phosphoric acid).
An equal volume of Griess reagent was mixed with the supernatant
and incubated at room temperature for 5 min. Nitrite concentrations
were measured at 570 nm using an Epoch microplate spectrophotometer
(Biotek Instruments, Inc., Winooski, VT, USA). A sodium nitrite
(NaNO2) standard curve was used to calculate nitrite
concentration.
Prostaglandin E2
production
The amount of PGE2 was determined using a
PGE2 Enzyme Immuno-Assay kit (GE Healthcare Life
Sciences, Chalfont, UK) according to the manufacturer's protocols.
Briefly, 2.5×105 RAW 264.7 cells per well were cultured
in 24-well culture plates. AS II (10, 20 and 40 µM) and LPS (200
ng/ml) was added to each well and incubated at 37°C for 24 h. The
supernatant was collected and used to measure PGE2
production.
Enzyme-linked immunosorbent assay
(ELISA)
RAW 264.7 macrophages (2.5×105 per well)
were cultured in 24-well plates and treated with LPS (200 ng/ml) in
the presence or absence of AS II (10, 20 and 40 µM) for 24 h.
Levels of TNF-α and IL-6 in the culture media were quantified using
ELISA kits, according to the manufacturer's protocols (BD
Biosciences). Briefly, ELISA plates (Nalge Nunc International;
Thermo Fisher Scientific, Inc.) were coated overnight with coating
buffer including anti-mouse IL-6 and TNF-α antibodies. The wells
were washed and the samples and standards added. Following a 2 h
incubation, biotinylated anti-mouse IL-6 monoclonal antibody and
biotinylated anti-mouse TNF-α with streptavidin-horseradish
peroxidase reagent were added each well and incubated for 1 h. The
wells were washed and the tetramethylbenzidine substrate solution
added to the wells and incubated for 30 min in the dark. A stop
solution (2N H3PO4) was added and absorbance
was read at 450 nm.
Western blot analysis
RAW 264.7 cells were plated at 6×106
cells per well in 6-well culture plates and treated with LPS (200
ng/ml) in the presence or absence of AS II (10, 20 and 40 µM) for
24 h at 37°C. Following incubation, the cell pellets were lysed in
PRO-PREP™ Protein Extraction Solution on ice for 20 min. Protein
levels in collected supernatants were determined using the Bio-Rad
protein assay reagent (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) according to the manufacturer's protocols. Sodium dodecyl
sulfate-polyacrylamide gel (10% for iNOS and COX-2; 12% for TLR-4)
electrophoresis were performed with equal amounts of protein (240
ng/lane) and transferred onto polyvinylidene membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with TBS-T [20
mM/l Tris-HCl (pH 7.6), 137 mM/l NaCl, 0.05% Tween 20] containing
5% skimmed milk, the membranes were incubated overnight at 4°C with
primary antibodies (1:1,000). The membranes were washed with TBS-T
and incubated for 1 h at room temperature with anti-mouse,
anti-goat or anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies (1:2,000). The bands
were evaluated by using ECL Prime Western Blotting Detection
Reagent and an ImageQuant LAS 4000 Mini BioMolecular Imager (GE
Healthcare).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RAW 264.7 cells were plated at 6×106
cells per well in 6-well culture plates and treated with LPS (200
ng/ml) in the presence or absence of AS II (10, 20 and 40 µM) for
24 h at 37°C. Following incubation, an RNeasy Mini kit was used to
isolate total cell RNA. Then, 1 µg total RNA was
reverse-transcribed into cDNA using the QuantiTect Reverse
Transcription kit (Qiagen GmbH) according to the manufacturer's
protocols. RT-qPCR was performed using power SYBR® Green
PCR master mix according to the manufacturer's protocols. PCR
thermocycling conditions were as follows: Holding stage, 1 cycle of
95°C for 10 min; 40 cycles of 95°C for 15 sec and 60°C for 1 min; 1
cycle of 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec). The
PCR products were measured with a StepOnePlus Real-Time RT-PCR
System. Relative gene expression was calculated based on the
2−ΔΔCq method (18)
using StepOne software version 2.3 (Applied Biosystems, Foster
City, CA, USA). β-actin mRNA expression was used as an endogenous
control. Primer sets for RT-qPCR are presented in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| iNOS |
GCAGAGATTGGAGGCCTTGTG |
GGGTTGTTGCTGAACTTCCAGTC |
| COX-2 |
GCCAGGCTGAACTTCGAAACA |
CAGATGACCTAGTAACGGACT |
| IL-6 |
TCTATACCACTTCACAAGTCGGA |
GAATTGCCATTGCACAACTCTTT |
| TNF-α |
ATGAGCACTGAAAGCATGATC- |
CATCCGTAAAGACCTCTATGCCAAC |
| β-actin |
CATCCGTAAAGACCTCTATGCCAAC |
ATGGAGCCACCGATCCACA |
Immunofluorescence staining
RAW 264.7 cells were cultured in chambered cover
glasses (Nalge Nunc International; Thermo Fisher Scientific, Inc.)
for 24 h and stimulated with LPS in the presence or absence of AS
II. The cells were fixed in 4% formaldehyde in PBS for 15 min at
room temperature and permeabilized with 100% methanol for 10 min at
−20°C. Specimens were blocked with blocking buffer (PBS with 5%
serum and 0.3% Triton X-100) for 1 h and incubated overnight with
polyclonal antibodies (1:200) at 4°C. Fluorochrome-conjugated
secondary antibodies (1:500) were applied for 1 h at room
temperature in the dark. Following washing with PBS, the nuclei
were counterstained with DAPI and fluorescence was visualized using
a fluorescence microscope (Ziess AG, Oberkochen, Germany).
AlexaFluor 488-conjugated LPS was used to evaluate the effects of
AS II on LPS and TLR-4 binding.
Statistical analyses
The statistical analysis was performed using one-way
analysis of variance followed by Scheffe's test for multiple
comparisons. Data are presented as mean ± standard deviation (n=5).
All calculations were performed using SPSS statistics version 22
software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
AS II inhibits LPS-induced NO and
PGE2 production and downregulated iNOS and COX-2
expression
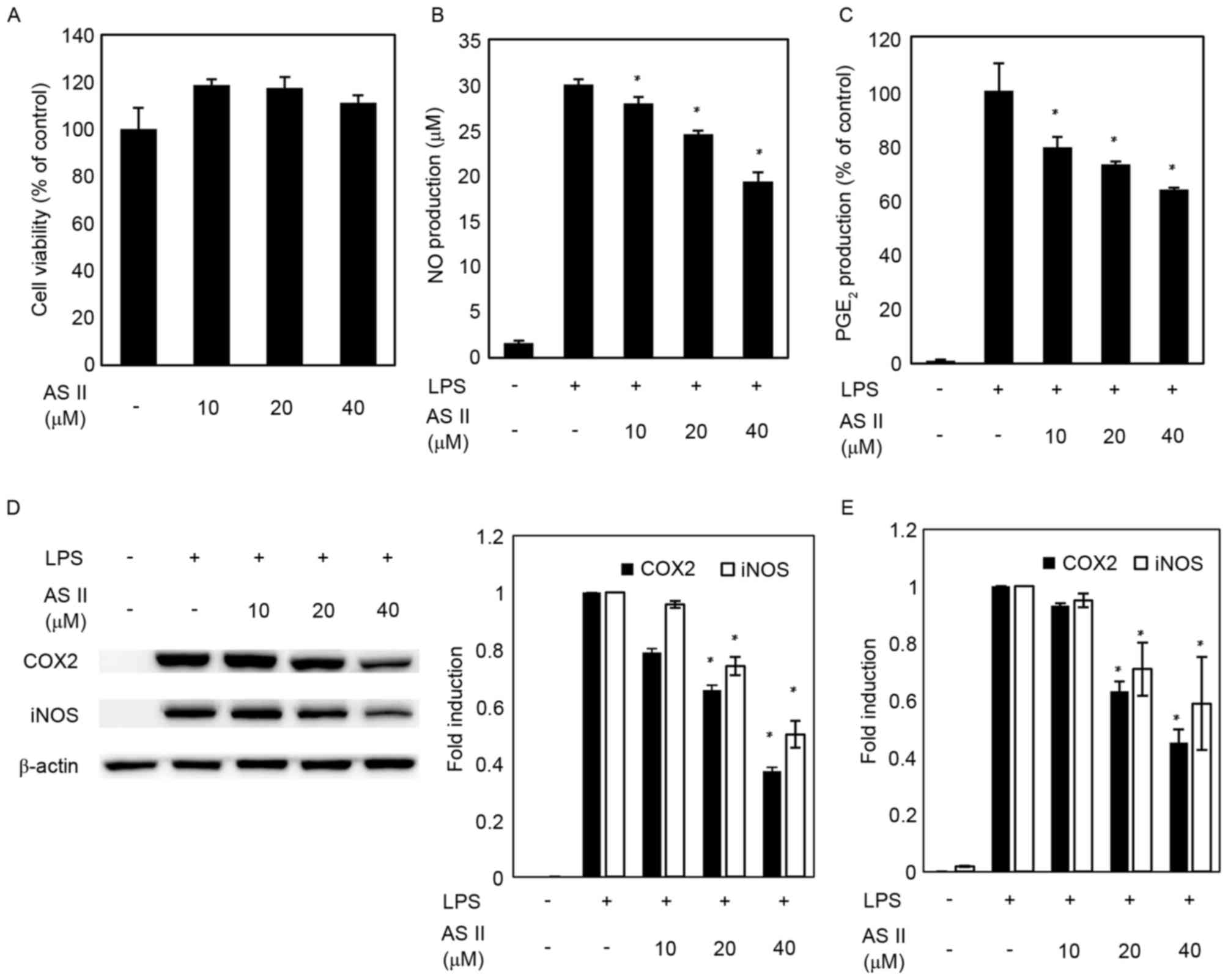
MTS assays were performed to determine the effects
of AS II on murine macrophage viability (Fig. 1A). The following experiments were
accomplished using 40 µM AS II to evaluate its anti-inflammatory
effects. As demonstrated in Fig. 1B
and C, LPS increased production of NO and PGE2
compared with the untreated group. However, groups pretreated with
AS II had significantly decreased levels of NO and PGE2
production in LPS-stimulated RAW 264.7 cells in a dose-dependent
manner up to 40 µM. The effects of AS II on iNOS and COX-2 protein
and mRNA expression were examined. iNOS and COX-2 mRNA and protein
expression levels were undetectable in the unstimulated group;
however, iNOS and COX-2 expression increased significantly in
response to LPS. AS II reduced COX-2 protein and mRNA expression by
37 and 45%, respectively, at the highest concentration compared
with LPS-treated cells. AS II (40 µM) decreased iNOS protein and
mRNA expression by 50 and 59%, respectively, relative to the
LPS=treated group (Fig. 1D and
E).
AS II suppresses LPS-induced IL-6 and
TNF-α production and mRNA expression
The effects of AS II on synthesis of the
pro-inflammatory cytokines IL-6 and TNF-α in LPS-stimulated murine
macrophages were investigated. Significantly increased levels of
IL-6 and TNF-α following treatment with LPS were reduced by
pretreatment with AS II (Fig. 2A).
The RT-qPCR data demonstrated that AS II reduced the mRNA of these
cytokines. IL-6 and TNF-α mRNA expression levels were decreased by
up to 49 and 56.8% respectively, compared with the LPS only treated
group (Fig. 2B).
AS II blocks NF-κB nuclear
translocation in murine macrophages
Previous studies have demonstrated that NF-κB is a
key transcriptional factor involved in regulating inflammatory
mediators, including iNOS, COX-2 and cytokines. As demonstrated in
Fig 3, NF-κB nuclear translocation
was increased in the LPS-stimulated group compared with the
untreated group. NF-κB nuclear translocation decreased
significantly when AS II was added with LPS (Fig. 3).
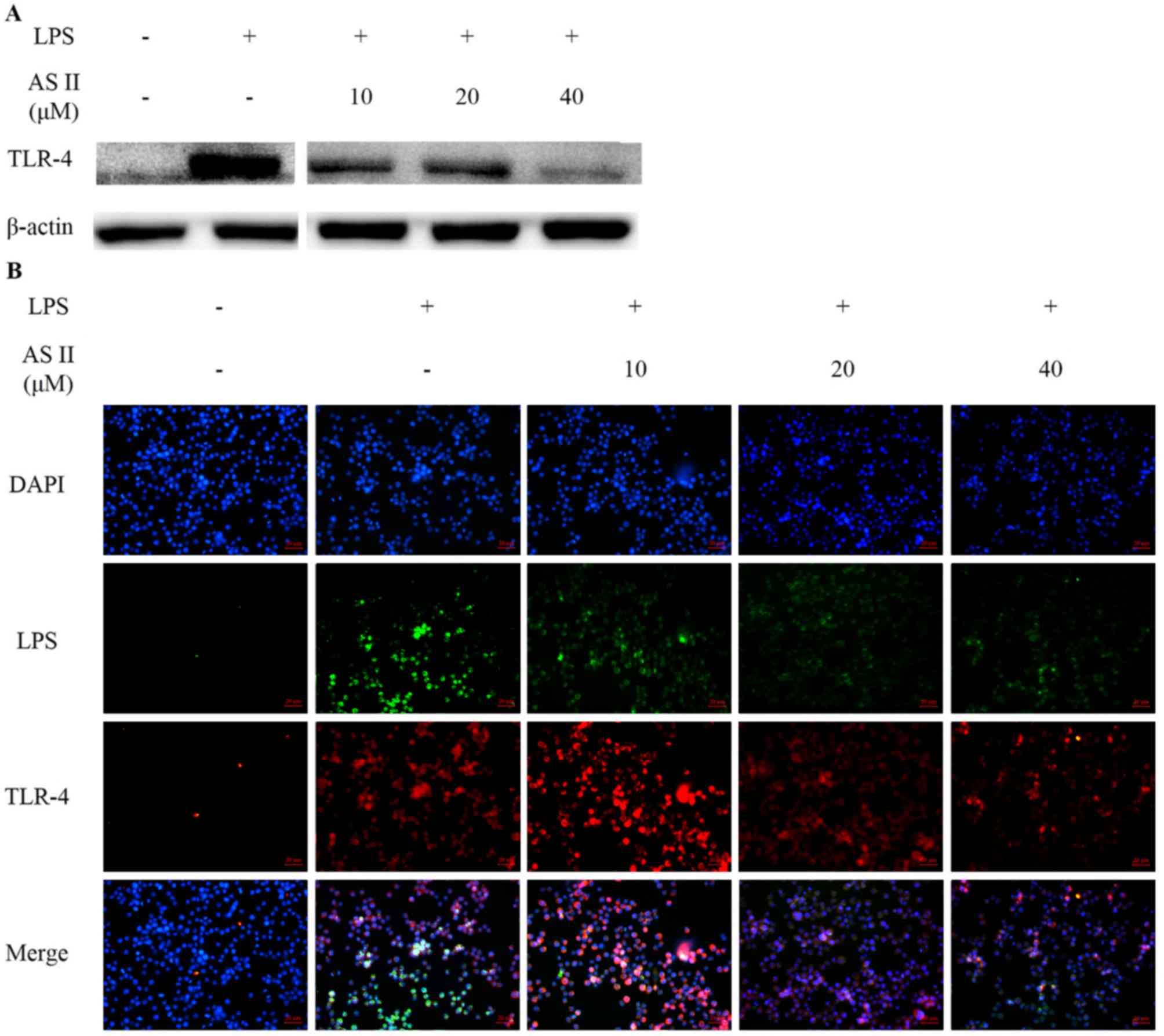
AS II decreases recognition of LPS and
downregulates TLR-4
The LPS-activated TLR-4 signaling pathway was
analyzed by western blot analysis and immunofluorescence staining.
Receptor expression was studied 24 h following LPS treatment. TLR-4
expression in LPS-stimulated murine macrophages increased from
undetectable levels compared with untreated macrophages. TLR-4
expression was decreased in the experimental groups treated with
LPS and AS II. In addition, high fluorescence intensity was
observed outside the cell membrane when cells were stimulated with
fluorescent-dye conjugated LPS; however, fluorescence intensity
weakened in the presence of AS II (Fig. 4).
Discussion
The present study examined the potential
anti-inflammatory effects of AS II and investigated whether AS II
regulates the inflammatory response by suppressing signaling
pathways in an LPS-stimulated inflammatory model. NO, which is a
reactive oxygen species, is a frontline immune response effector
molecule that attacks foreign agents but also has strong cytotoxic
effects related with a number of inflammatory diseases (19). Thus, NO is considered an important
inflammation parameter (20).
Excess production of NO and PGE2 following an infection
is caused by expression of iNOS, which is involved in the synthesis
of NO and COX-2, which is the rate-limiting enzyme catalyzing
conversion of arachidonic acid. Therefore, NO and PGE2
are key downstream effectors of iNOS and COX-2 during inflammation
(21). The current study
identified that AS II reduced LPS-induced NO production and
PGE2 expression in murine macrophages. In addition, iNOS
and COX-2 protein and mRNA overexpression decreased following
pretreatment with AS II. These results indicated that the effects
of AS II on NO and PGE2 production may be caused by
suppression of iNOS and COX-2 expression.
IL-6 and TNF-α are important inflammatory factors in
immune responses, including fever and the acute phase response.
Macrophages express the pro-inflammatory cytokines TNF-α and IL-6
during inflammation, in addition to other inflammatory factors,
including NO and PGs, that initiate the transfer of additional
immune cells to the sites of infection or tissue injury (22). As demonstrated in the present
study, LPS stimulation increased TNF-α and IL-6 expression in RAW
264.7 cells but AS II downregulated TNF-α and IL-6 protein and mRNA
expression levels. These results suggest that AS II exhibits
anti-inflammatory activity by inhibiting the expression of
pro-inflammatory mediators.
One of the notable findings of the present study was
the identification of the effect of AS II on the nuclear
translocation of p65, a component of NF-κB induced by LPS. It was
demonstrated that AS II reduced nuclear translocation of NF-κB. A
heterodimer of p65 and p50, another NF-κB component, binds to
inhibitory κB, which is an inhibitor of NF-κB. LPS-induced
phosphorylation of inhibitory κB releases NF-κB and allows NF-κB to
translocate to the nucleus. Due to this p65 transactivation
activity, a key process of NF-κB activation is nuclear
translocation of p65 into the nucleus (23). NF-κB is an important transcription
factor that regulates immune and inflammatory signaling by
promoting the transcription of pro-inflammatory mediators,
including iNOS, COX-2 and cytokines, and translocation of NF-κB has
been described as a rate-limiting step (24). Thus, the inhibitory effect of AS II
on inflammatory mediators may be mediated by blocking the
activation of NF-κB.
TLRs are the first to recognize various microbial
pathogens that invade the body, including LPS. TLRs initiate
downstream pro-inflammatory activities leading to the innate immune
response (25). The immune
response triggered by interactions between pathogens and TLRs
operate an acute and early release of inflammatory mediators. Among
them, TLR-4 is the most important receptor that recognizes LPS. The
TLR-4 signaling pathway is indispensable for LPS-stimulated NO
production in inflammatory cells (26). LPS interacts with TLR-4 by binding
to cell membranes, resulting in downstream inflammatory events,
which may be responsible for inflammatory disorders (27). LPS-stimulated TLR-4 triggers
enhanced NO, TNF-α and IL-6 expression through the TLR-4-NF-κB
signaling pathway, which mediates host damage (28). The effects of AS II on the
interaction between LPS and TLR-4 in RAW 264.7 macrophage cells was
investigated in the current study. The observations from the
present study demonstrated that AS II pretreatment markedly
attenuated LPS binding to TLR-4 on the cell surface, suggesting
that AS II may interfere with TLR-4 clustering. In addition,
pretreatment with AS II markedly inhibited LPS-induced TLR-4
expression in RAW 264.7 cells. These observations suggested that
the subsequently suppressed activation of the NF-κB signaling
pathway by AS II is induced by inhibiting initiation of the
intracellular signaling cascade. Previous studies have indicated
that certain anti-inflammatory agents compete with LPS for TLR-4
binding, resulting in the downregulation of downstream signaling
pathways (29,30). Therefore, the antagonistic function
of AS II against TLR-4 may be responsible for the anti-inflammatory
effects of AS II in LPS-stimulated RAW 264.7 macrophages.
Previous studies have reported that the TLR4-NFκB
signaling pathway is the main mechanism for the inflammatory
response following LPS stimulation (31,32).
The results of the present study demonstrated that AS II exerted
anti-inflammatory actions that may be mediated by inhibition of
LPS-TLR-4 binding in activated RAW 264.7 cells. Other experiments
demonstrated that AS II inhibited NO production in murine
macrophages and attenuated the LPS-induced inflammatory response by
downregulating the NF-κB pathway. It is hypothesized that AS II
inhibits downstream inflammatory mediators (iNOS and COX-2) and
markers (NO, PGE2, IL-6 and TNF-α) through this action.
Therefore, the results of the present study indicated that AS II
may be useful for the prevention of inflammatory diseases.
Acknowledgements
The present study was supported by Wonkwang
University (Jeollabuk, Korea) in 2017.
References
|
1
|
Cho YC, Ju A, Kim BR and Cho S:
Anti-inflammatory effects of Crataeva nurvala Buch. Ham. Are
mediated via inactivation of ERK but not NF-κB. J Ethnopharmacol.
162:140–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chawla A, Nguyen KD and Goh YP:
Macrophage-mediated inflammation in metabolic disease. Nat Rev
Immunol. 11:738–749. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jang JC and Nair MG: Alternatively
activated macrophages Revisited: New insights into the regulation
of immunity, inflammation and metabolic function following parasite
infection. Curr Immunol Rev. 9:147–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma JN, Al-Omran A and Parvathy SS:
Role of nitric oxide in inflammatory diseases.
Inflammopharmacology. 15:252–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou R, Shi X, Gao Y, Cai N, Jiang Z and
Xu X: Anti-inflammatory activity of guluronate oligosaccharides
obtained by oxidative degradation from alginate in
lipopolysaccharide-activated murine macrophage RAW 264.7 cells. J
Agric Food Chem. 63:160–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ci X, Ren R, Xu K, Li H, Yu Q, Song Y,
Wang D, Li R and Deng X: Schisantherin A exhibits anti-inflammatory
properties by down-regulating NF-kappaB and MAPK signaling pathways
in lipopolysaccharide-treated RAW 264.7 cells. Inflammation.
33:126–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mi Jeong Sung, Davaatseren M, Kim W, Sung
Kwang Park, Kim SH, Haeng Jeon Hur, Myung Sunny Kim, Kim YS and Dae
Young Kwon: Vitisin A suppresses LPS-induced NO production by
inhibiting ERK, p38, and NF-kappaB activation in RAW 264.7 cells.
Int Immunopharmacol. 9:319–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun SC, Chang JH and Jin J: Regulation of
nuclear factor-κB in autoimmunity. Trends Immunol. 34:282–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayne ST: Antioxidant nutrients and
chronic disease: Use of biomarkers of exposure and oxidative stress
status in epidemiologic research. J Nutr. 133:(Suppl 3). 933S–940S.
2003.PubMed/NCBI
|
|
10
|
Gratas-Delamarche A, Derbré F, Vincent S
and Cillard J: Physical inactivity, insulin resistance, and the
oxidative-inflammatory loop. Free Radic Res. 48:93–108. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Golbidi S, Badran M and Laher I:
Antioxidant and anti-inflammatory effects of exercise in diabetic
patients. Exp Diabetes Res. 2012:9418682012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JH, Liu XQ, Dai L, Yook CS and Lee KT:
Cytotoxicity and anti-inflammatory effects of root bark extracts of
Acanthopanax henryi. Chin J Nat Med. 12:121–125. 2014.PubMed/NCBI
|
|
13
|
Zhang XD, Liu XQ, Kim YH and Whang WK:
Chemical constituents and their acetyl cholinesterase inhibitory
and antioxidant activities from leaves of Acanthopanax
henryi: Potential complementary source against Alzheimer's
disease. Arch Pharm Res. 37:606–616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Liu XQ, Dai L, Yook CS and Lee KT:
Cytotoxicity and anti-inflammatory effects of root bark extracts of
Acanthopanax henryi. Chin J Nat Med. 12:121–125. 2014.PubMed/NCBI
|
|
15
|
Miyase T, Shiokawa KI, Zhang DM and Ueno
A: Araliasaponins I–XI, triterpene saponins from the roots of
Aralia descaisneana. Phytochemistry. 41:1411–1418. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shao CJ, Ryoji Kasai, Xu JD and Tanaka O:
Saponins from leaves of Acanthopanax senticosus Harms., Ciwujia:
Structures of Ciwujianosides B, C1, C2, C3, C4, D1, D2 and E. Chem
Pharm Bull. 36:601–608. 1988. View Article : Google Scholar
|
|
17
|
Zhang XD, Li Z, Liu GZ, Wang X, Kwon OK,
Lee HK, Whang WK and Liu X: Quantitative determination of 15
bioactive triterpenoid saponins in different parts of
Acanthopanax henryi by HPLC with charged aerosol detection
and confirmation by LC-ESI-TOF-MS. J Sep Sci. 39:2252–2262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koike A, Minamiguchi I, Fujimori K and
Amano F: Nitric oxide is an important regulator of heme oxygenase-1
expression in the lipopolysaccharide and interferon-γ-treated
murine macrophage-like cell line J774.1/JA-4. Biol Pharm Bull.
38:7–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guzik TJ, Korbut R and Adamek-Guzik T:
Nitric oxide and superoxide in inflammation and immune regulation.
J Physiol Pharmacol. 54:469–487. 2003.PubMed/NCBI
|
|
21
|
Chan AT, Ogino S and Fuchs CS: Aspirin and
the risk of colorectal cancer in relation to the expression of
COX-2. N Engl J Med. 356:2131–2142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boscá L, Zeini M, Través PG and Hortelano
S: Nitric oxide and cell viability in inflammatory cells: A role
for NO in macrophage function and fate. Toxicology. 208:249–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo AK, Hou YY, Hirata H, Yamauchi S, Yip
AK, Chiam KH, Tanaka N, Sawada Y and Kawauchi K: Loss of p53
enhances NF-κB-dependent lamellipodia formation. J Cell Physiol.
229:696–704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beg AA, Finco TS, Nantermet PV and Baldwin
AS Jr: Tumor necrosis factor and interleukin-1 lead to
phosphorylation and loss of I kappa B alpha: A mechanism for
NF-kappa B activation. Mol Cell Biol. 13:3301–3310. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brown KL, Cosseau C, Gardy JL and Hancock
RE: Complexities of targeting innate immunity to treat infection.
Trends Immunol. 28:260–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schröder NW, Opitz B, Lamping N, Michelsen
KS, Zähringer U, Göbel UB and Schumann RR: Involvement of
lipopolysaccharide binding protein, CD14, and Toll-like receptors
in the initiation of innate immune responses by Treponema
glycolipids. J Immunol. 165:2683–2693. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koo JE, Park ZY, Kim ND and Lee JY:
Sulforaphane inhibits the engagement of LPS with TLR4/MD2 complex
by preferential binding to Cys133 in MD2. Biochem Biophys Res
Commun. 434:600–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller SI, Ernst RK and Bader MW: LPS,
TLR4 and infectious disease diversity. Nat Rev Microbiol. 3:36–46.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi YH, Kim GY and Lee HH:
Anti-inflammatory effects of cordycepin in
lipopolysaccharide-stimulated RAW 264.7 macrophages through
Toll-like receptor 4-mediated suppression of mitogen-activated
protein kinases and NF-κB signaling pathways. Drug Des Devel Ther.
8:1941–1953. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nijland R, Hofland T and van Strijp JA:
Recognition of LPS by TLR4: Potential for anti-inflammatory
therapies. Mar Drugs. 12:4260–4273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suganami T, Tanimoto-Koyama K, Nishida J,
Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S,
et al: Role of the Toll-like receptor 4/NF-kappaB pathway in
saturated fatty acid-induced inflammatory changes in the
interaction between adipocytes and macrophages. Arterioscler Thromb
Vasc Biol. 27:84–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dou W, Zhang J, Sun A, Zhang E, Ding L,
Mukherjee S, Wei X, Chou G, Wang ZT and Mani S: Protective effect
of naringenin against experimental colitis via suppression of
Toll-like receptor 4/NF-κB signalling. Br J Nutr. 110:599–608.
2013. View Article : Google Scholar : PubMed/NCBI
|