Introduction
Myocardial infarction (MI) is a major cause of
cardiovascular disease, mortality and disability (1). In China, millions die of acute MI
(AMI), which is regarded as a major threat to human health
(2). With the extensive employment
of thrombolysis and cardiac intervention therapy, in addition to
the rapid advancements in drug therapy for treating AMI, myocardium
damage in patients with AMI has been greatly ameliorated and the
prognosis markedly improved (3).
However, there remain patients who cannot be aided with timely
revascularization and so suffer irreversible death of the
myocardium and ventricular reconstruction (4).
Ventricular reconstruction refers to the changes in
the morphological structures of myocardial cells and the
intercellular substance caused by the activation of neurohumoral
regulatory mechanisms, inflammation and cytokines (5). Cardiac structures and functions
become altered according to certain patterns (6). The progressive enlargement and
changes in appearance of the ventriculus sinister include changes
in ventricular volume, shape, ventricular wall thickness and
cardiac structures, which lead to abnormalities of cardiac
structure and hemodynamics, progressive dilation of the left
ventricle and the decrease of systolic functions (7). Finally, heart failure and mortality
may occur (8).
Poly ADP-ribose polymerase (PARP) is widely
expressed in eukaryotic cells. Activated PARP is involved in DNA
repair (9). Ischemia and hypoxia
result in DNA damage and the excessive activation of PARP, can
cause the exhaustion of ATP resources (10). As a result of such dysfunction, the
cells die. The activities of PARP can be inhibited by
3-aminobenzamide, which can also reduce the consumption of ATP
during the process of DNA repair (11). It has been employed for studying
multiple tissue ischemic injuries and possesses potential for
ischemic myocardium treatment (9).
Previous studies have identified that
diethylcarbamazine can be used to inhibit vasoconstriction, reduce
systemic arterial pressures and block hypoxic pulmonary
vasoconstriction (12). As a
diethylcarbamazine, hetrazan has been reported to inhibit the
contraction of isolated vascular circles, decrease systemic
arterial and pulmonary arterial pressure (13). Trials on animal models have
suggested that intravenous injection of diethylcarbamazine can
temporarily change cardiac functions and decrease the heart rate
(14). The aim of the present
study was to investigate the protective effect of
diethylcarbamazine against isoproterenol-induced AMI in a rat model
and investigate a possible mechanism for its protective effect.
Materials and methods
Animals, induction of AMI and
experimental protocol
The present study was performed in accordance with
the Guide for the Care and Use of Laboratory Animals of Cangzhou
Central Hospital. Male albino Wistar rats (n=24; 230–250 g) were
obtained from the Animal Experimental Center of Hebei Medical
University (Shijiazhuang, China) and housed in standard
polypropylene cages under a 12:12 h light:dark cycle at a constant
temperature of 23±2°C and an ambient humidity of 55±5%.
Isoproterenol (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
injected subcutaneously to rats (100 mg/kg/day) to create the AMI
model. All the rats were randomly divided into four groups
consisting of 6 rats each: Sham, diethylcarbamazine, AMI model and
AMI model + diethylcarbamazine group. In the sham and AMI model
groups, normal rats and isoproterenol-induced AMI rats were
injected with distilled water. In the diethylcarbamazine and the
AMI model + diethylcarbamazine groups, diethylcarbamazine (50
mg/kg/day) was administered to the rats by gavage for 12 days,
prior to induction of the AMI with isoproterenol.
Tissue weights and histopathological
examination
The animals were euthanized with 35 mg/kg
pentobarbital 2 days after induction of the AMI model, and the
hearts were removed and weighed. The wet heart weight to body
weight ratio was calculated to assess the degree of myocardial
weight gain. Hearts samples were fixed in 10% buffered formalin
prior to being embedded in paraffin wax and sectioned at 5 µm
thickness. Sections were stained with hematoxylin and eosin.
Measurement of casein kinase (CK),
lactate dehydrogenase (LDH), reactive oxygen species (ROS),
inflammation response and nuclear factor (NF)-κB activation
Serum samples were extracted from the vena cava
following the isoproterenol-induced AMI model. Supernatant was
collected at 5,000 × g for 10 min at 4°C. The CK (A032, Nanjing
Jiancheng Bioengineering Institute, Nanjing, China), LDH
(E-EL-R0338c), tumor necrosis factor (TNF)-α (−EL-R0019c),
interleukin (IL) −6 (E-EL-R0015c, Elabscience) and NF-κB/p65
(E-EL-R0674c) (all from Elabscience Biotechnology, Co., Ltd.,
Bethesda, MD, USA) activities were measured using commercial ELISA
kits according to the manufacturer's protocols.
Western blot analysis
Hearts were removed and then homogenized by a
Wheaton overhead stirrer. Homogenates were centrifuged at 3,000 × g
for 10 min and the supernatant collected. Protein concentrations
were determined with a bicinchoninic acid protein assay kit (Thermo
Scientific Inc., Waltham, MA, USA). The proteins (40 mg) were
separated with 10% sodium dodecyl sulfate-polyacrylamide by gel
electrophoresis and electrophoretically transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Membranes were blocked with 5% nonfat milk in TBS-0.1%
Tween-20 (TBS-T) for 1 h and were incubated at room temperature,
for 2 h, with anti-COX-2 (1:2,000), anti-transforming growth factor
(TGF)-β1 (1:4,000), anti-inducible nitric oxide synthase (iNOS;
1:2,000), anti-PARP (1:3,000) and anti-β-actin (1:4,000) (all from
Abcam, Cambridge, CA, USA). Following washing in TBS-T, the
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit secondary antibody (1:5,000; Abcam, CA, USA) for 1 h
and 30 min at room temperature and visualized with an enhanced
chemiluminescence reagent (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). Intensity of each band was determined using the ImageJ
program version 1.38 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analyses
Data are expressed as the mean ± standard deviation
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to assess differences between the groups,
followed by Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Protective effect of
diethylcarbamazine inhibits cardiac function in
isoproterenol-induced AMI rats
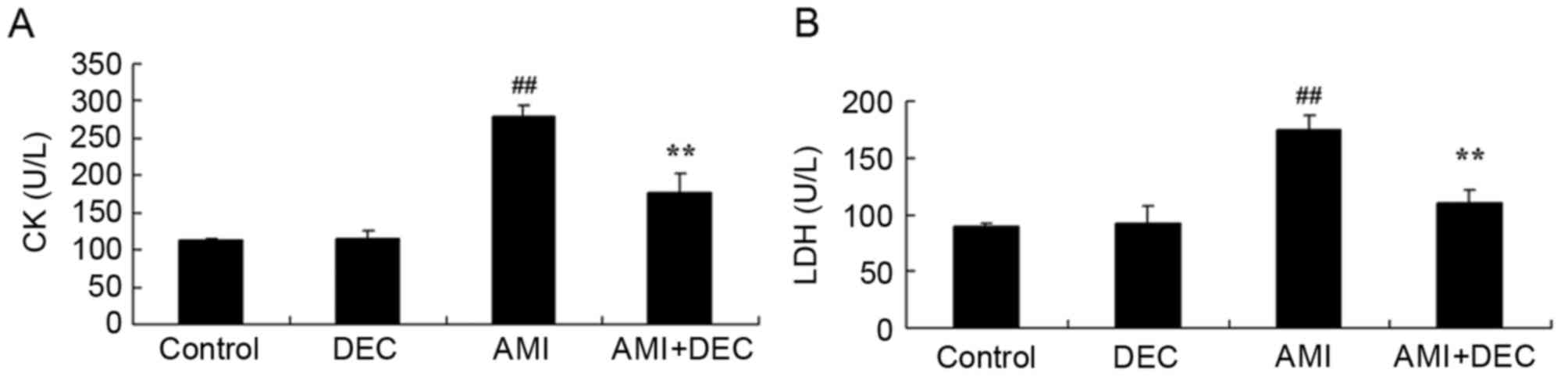
The protective effect of diethylcarbamazine on the
cardiac function of isoproterenol-induced AMI rats was evaluated
(Fig. 1). Following
diethylcarbamazine treatment (50 mg/kg) for 12 days, no significant
inter-group difference in cardiac functions was observed between
the control and the diethylcarbamazine-alone groups (P>0.05).
The levels of CK and LDH in the isoproterenol-induced AMI model
group were higher compared with those of the control group
(P<0.01). However, treatment with diethylcarbamazine
significantly inhibited the AMI-induced CK and LDH levels in AMI
rats (P<0.01).
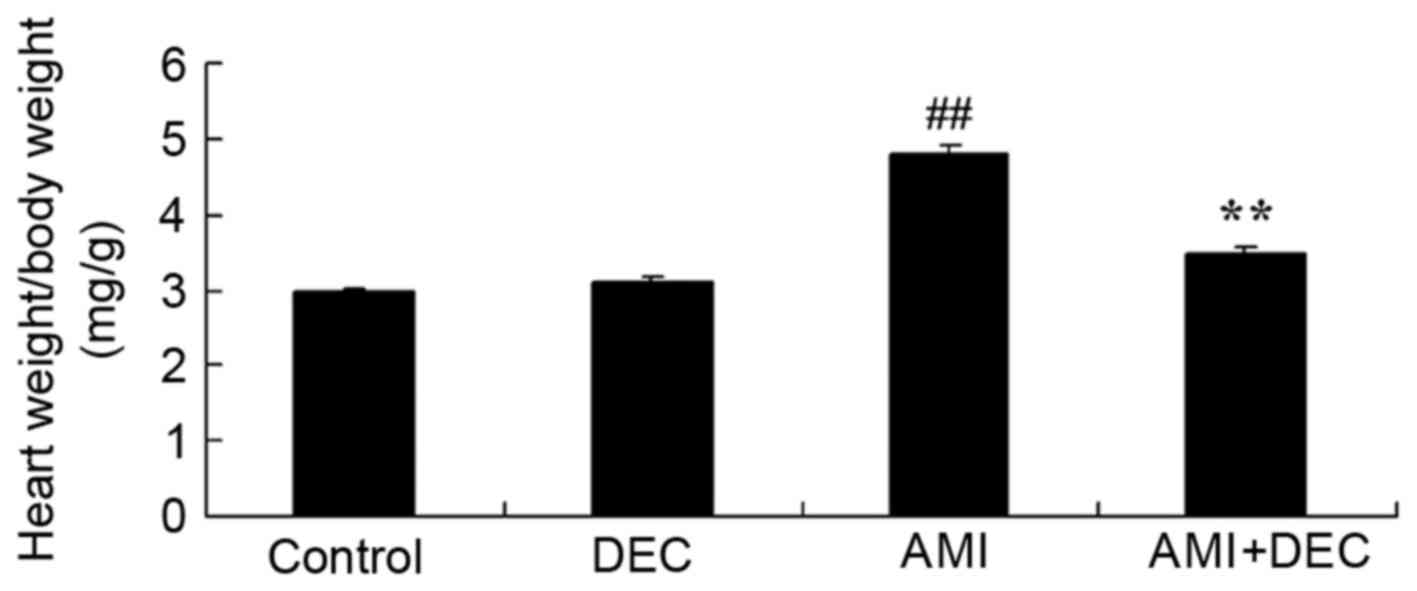
Protective effect of
diethylcarbamazine reduces the wet heart weight to body weight
ratio in AMI rats
Following diethylcarbamazine treatment in AMI rats,
the wet heart weight to body weight ratio of the control group was
similar to the AMI model group. Compared with the control group,
the wet heart weight to body weight ratio of the AMI model group
was significantly increased (P<0.01). Treatment with
diethylcarbamazine significantly reduced the AMI-induced wet heart
weight to body weight ratio in AMI rats (P<0.01; Fig. 2).
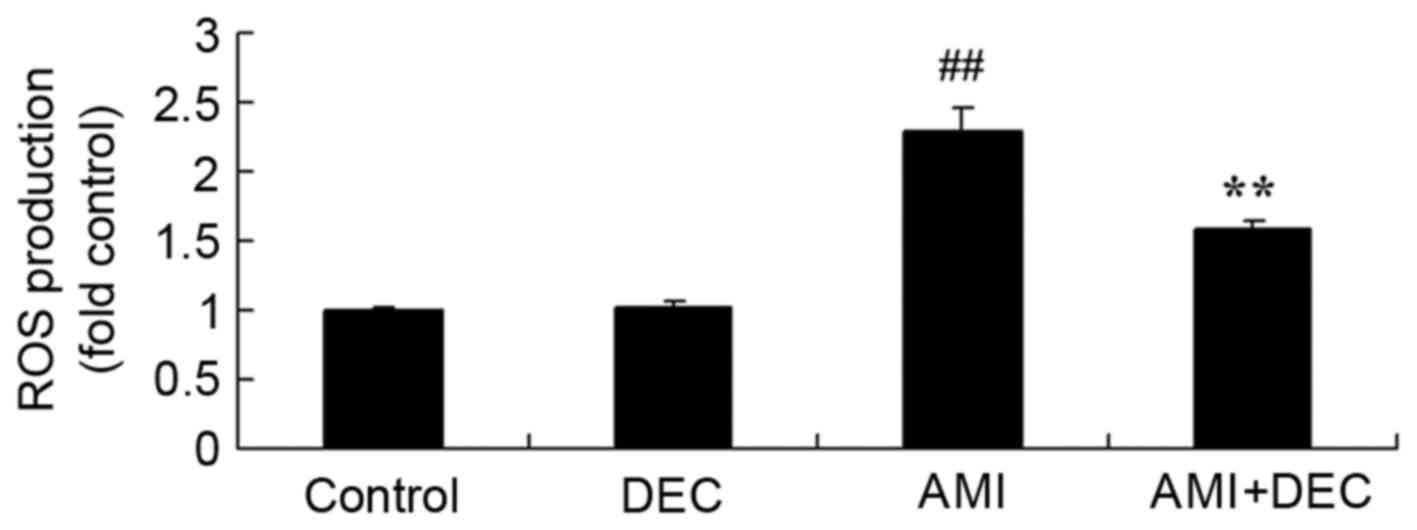
Protective effect of
diethylcarbamazine inhibits ROS production in AMI rats
To evaluate the protective effect of
diethylcarbamazine on oxidative stress in AMI rats, ROS production
was measured to estimate the protective effect of
diethylcarbamazine on AMI (Fig.
3). No significant difference was observed between the control
and diethylcarbamazine-alone groups (P>0.05). In the AMI model
group, ROS production was significantly enhanced compared with the
control group (P<0.01). Diethylcarbamazine treatment
significantly weakened ROS production in AMI rats (P<0.01).
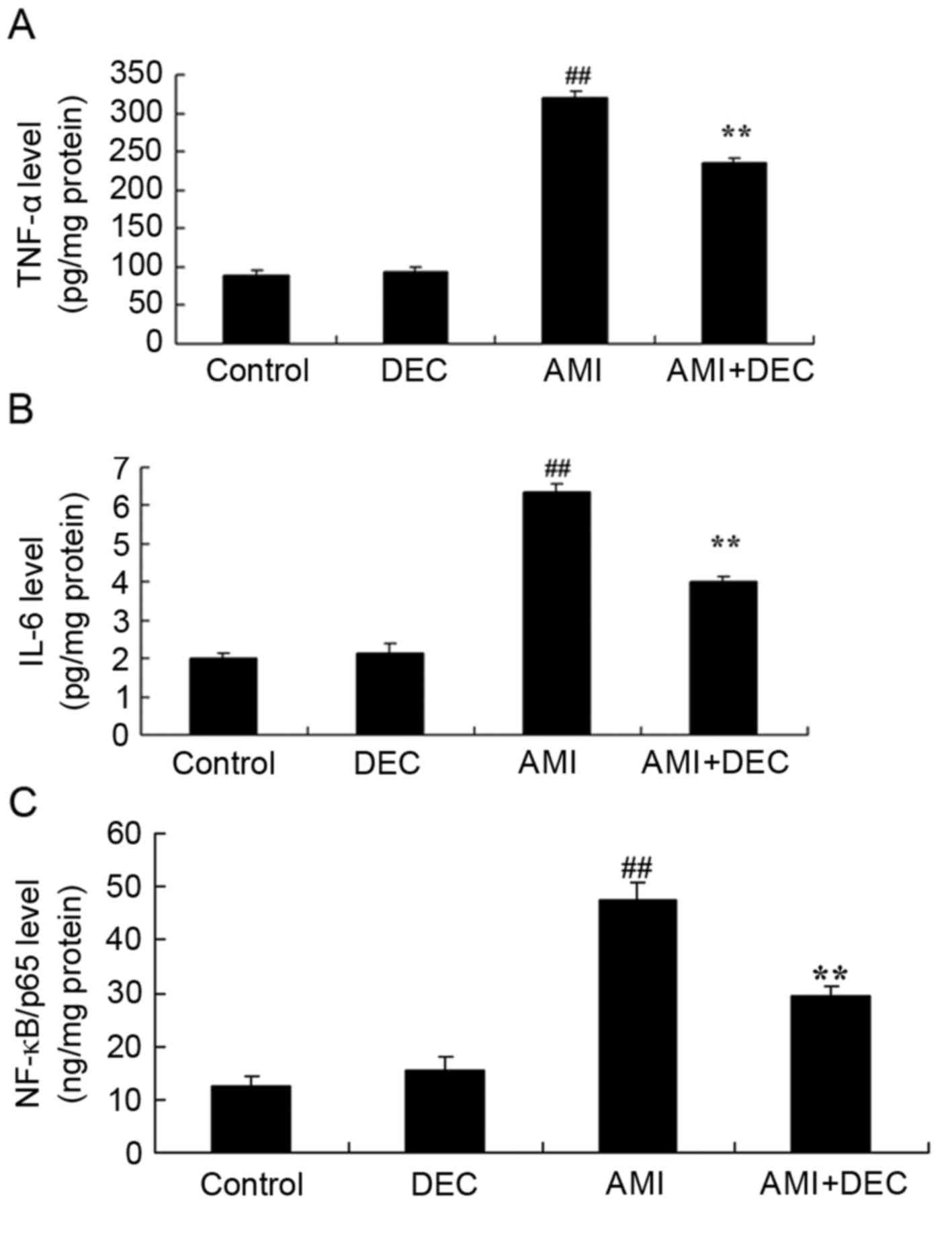
Protective effect of
diethylcarbamazine inhibits inflammation response in
isoproterenol-induced AMI rats
TNF-α, IL-6 and NF-κB/p65 activity was detected
using ELISA kits to evaluate the protective effect of
diethylcarbamazine on AMI (Fig.
4). No significant difference was observed in the level of
TNF-α, IL-6 and NF-κB/p65 between the control and the
diethylcarbamazine-alone groups (P>0.05). Compared with control
group, the level of TNF-α, IL-6 and NF-κB/p65 were significantly
increased in the AMI model group (P<0.01). However, pretreatment
with diethylcarbamazine significantly reduced the AMI-induced
TNF-α, IL-6 and NF-κB/p65 levels (P<0.01).
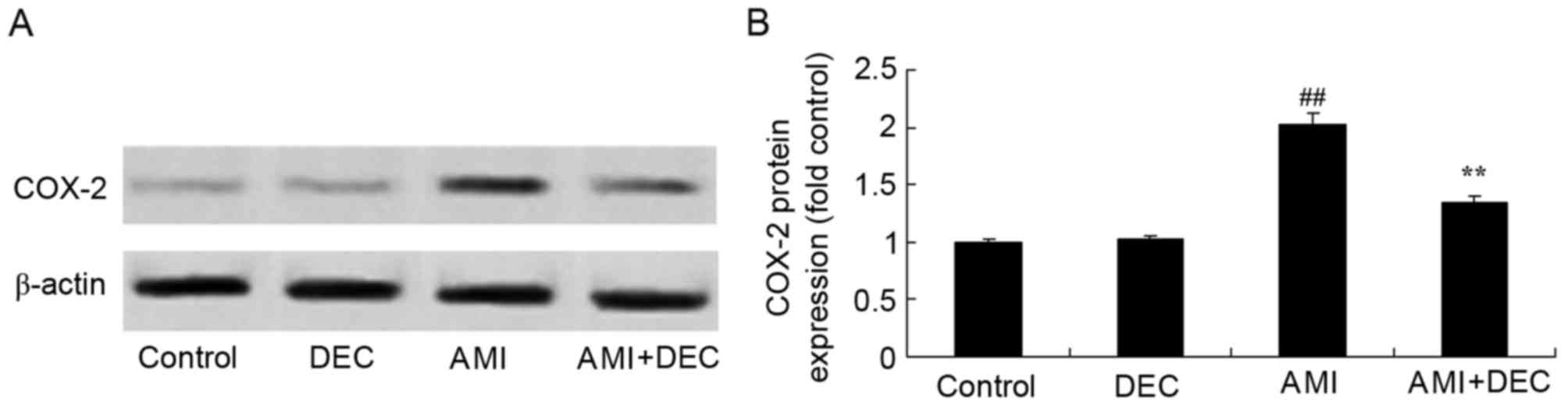
Protective effect of
diethylcarbamazine inhibits COX-2 expression in AMI rats
No significant inter-group difference was identified
between the control and the diethylcarbamazine-alone groups for
COX-2 expression (P>0.05). COX-2 expression in the AMI model
group was higher compared with the control group (P<0.01;
Fig. 5). Compared with the AMI
model group, pretreatment with diethylcarbamazine noticeably
reduced the AMI-induced COX-2 protein expression (P<0.01).
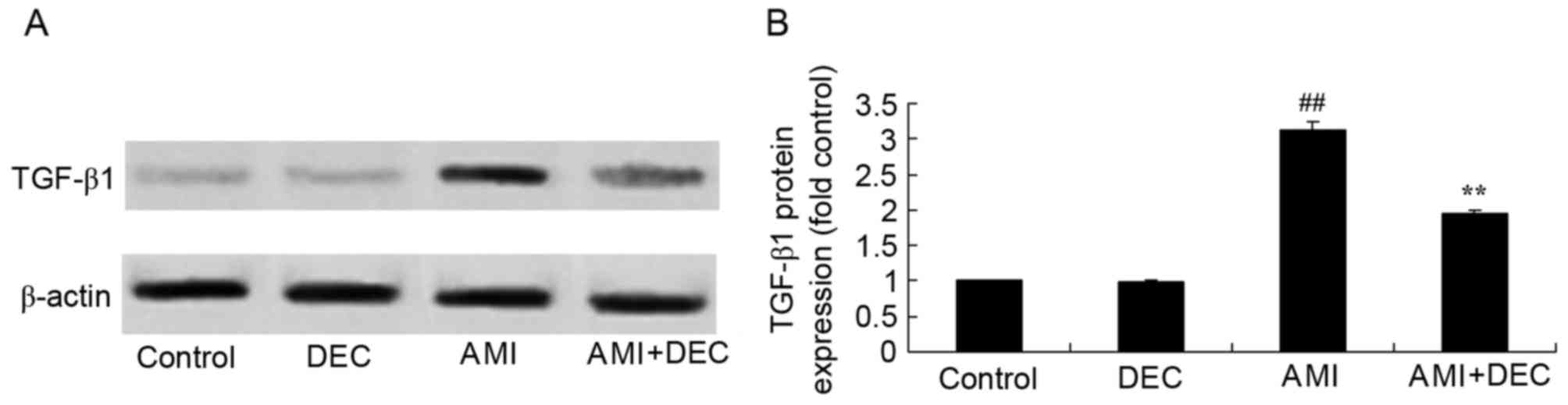
Protective effect of
diethylcarbamazine reduces TGF-β1 expression in AMI rats
No significant changes were observed in protein
expression of TGF-β1 between the control group and the
diethylcarbamazine-alone group. The TGF-β1 protein expression was
notably induced by AMI, compared with the control group
(P<0.01). Diethylcarbamazine significantly suppressed the
AMI-induced TGF-β1 protein expression (P<0.01; Fig. 6).
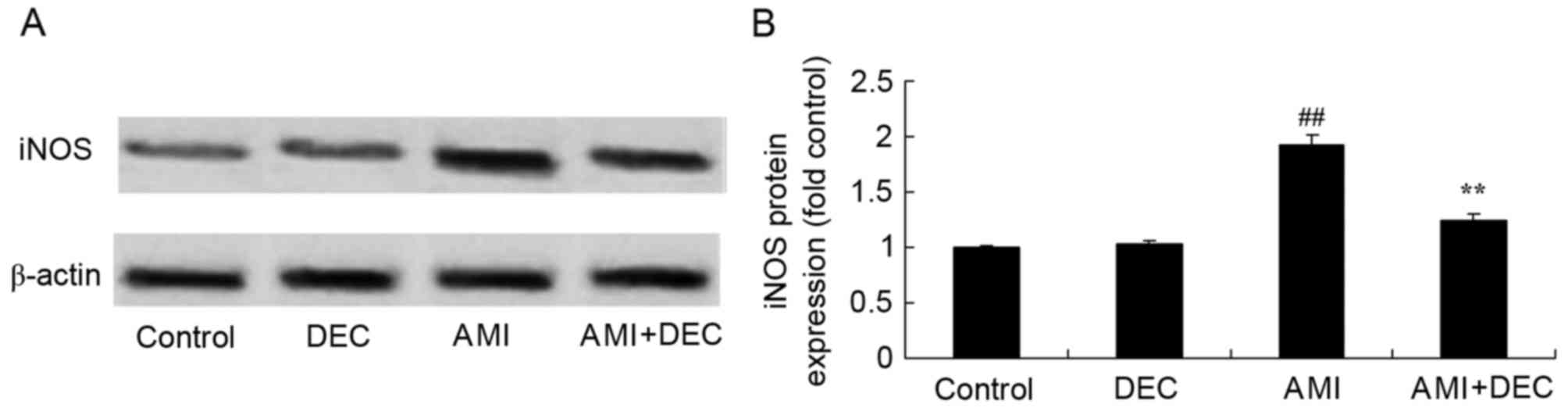
Protective effect of
diethylcarbamazine reduces iNOS expression in AMI rats
To explore the protective effect of
diethylcarbamazine on iNOS expression in AMI rats, iNOS protein
expression was measured using western blotting (Fig. 7). No significant difference was
observed between the control and the diethylcarbamazine-alone
groups. Compared with the control group, iNOS protein expression
was significantly increased in AMI rats (P<0.01). The activation
of iNOS protein expression was significantly attenuated by
diethylcarbamazine in AMI rats (P<0.01).
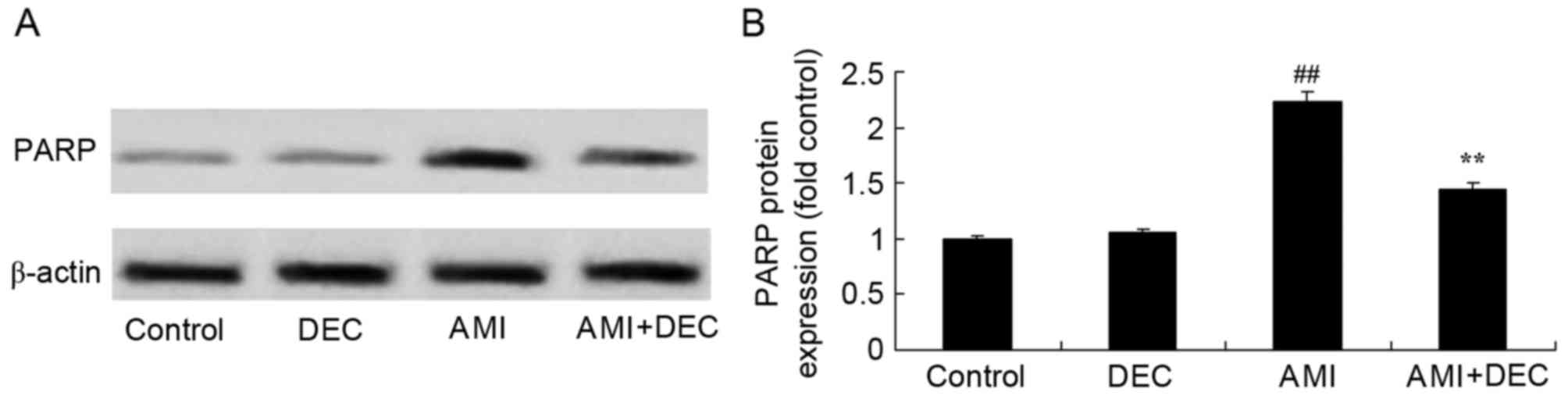
Protective effect of
diethylcarbamazine inhibits PARP expression in AMI rats
To further explore the protective effect of
diethylcarbamazine on PARP expression in AMI rats, PARP protein
expression levels were measured using western blotting (Fig. 8). No significant difference was
observed between the control and diethylcarbamazine-alone groups
for PARP expression (P>0.05). However, AMI significantly
increased PARP expression compared with the control group
(P<0.01). Diethylcarbamazine significantly suppressed the
AMI-induced PARP protein expression in AMI rats, compared with that
of AMI model group (P<0.01).
Discussion
As a common cardiovascular disease, the morbidity of
AMI is a leading health problem. With advances in medical
technologies the mortality rates of AMI have decreased to a certain
extent (7). However, survivors
still experience complications, including recurrent AMI or
refractory cardiac insufficiency (15). Data have demonstrated that
mortality rates of AMI and cardiac failure are ~80% (15). Consequently, assessment of the
myocardial systolic function of the whole and local left ventricle
can accurately confirm the functional status of the ischemic
myocardium; leading to an early diagnose of ischemic heart disease
and useful prognosis (16). The
present study investigated whether treatment with
diethylcarbamazine significantly inhibited AMI-induced CK and LDH
levels, and reduced the AMI-induced wet heart weight to body weight
ratio in AMI rats.
An inflammatory response can be directly triggered
by ROS produced by ischemic tissues (17). When the formation of ROS is much
higher than the load of the endogenous antioxidant defense system,
cellular damage, mediated by free radicals, occurs (18). ROS can trigger cascade reactions of
inflammatory cytokines and chemotactic factors through activation
of the inflammasome of myocardial cells (19). The formation of inflammasome can
promote the production and activation of IL-1 which is a classic
proinflammatory factor promoting the inflammatory mediator
expression in infarcted myocardium (20). The present study identified that
diethylcarbamazine treatment significantly weakened ROS production
in AMI rats.
Inflammatory reactions have an important role in
ventricular remodeling following AMI. Ventricular remodeling
includes the inflammatory cascade triggered by myocyte necrosis at
the infarcted zone, recruitment of inflammatory cells at the
infarcted zone and upregulation of proinflammatory cytokines
(21). Later, necrotic myocardial
cells are replaced by collagenous fibers, resulting in the
formation of glial scars, cardiomyocyte hypertrophy at the
non-infarcted areas, degradation of myocardial matrix and
reconstruction of matrix (22).
This process involves the inflammatory responses and consequently
the repair process following AMI is a complex development with
related pathways mediated by inflammation as an important link
(22). Continuous inflammatory
responses following AMI triggers myocardial damage, fibrosis and
the expansion of AMI, finally resulting in the deterioration of the
cardiac function (22). A
significant inhibition of TNF-α, IL-6 and NF-κB/p65 levels was
observed following treatment with diethylcarbamazine in AMI
rats.
During the inflammatory response process, iNOS is
activated and excessive nitric oxide (NO) is produced, an important
factor in the death of transplanted cells (23). As an important signal transduction
molecule, NO serves an essential role in physiological processes
and has important effects on the progression of a number of
diseases (24). In the present
study, diethylcarbamazine significantly increased iNOS protein
expression in AMI rats. da Silva et al (14) indicated that diethylcarbamazine
prevented alcohol-induced liver injury via NF-κB, COX-2 and iNOS in
C57BL/6 mice.
As major effector cells of myocardial fibrosis,
myofibroblasts can proliferate, synthesize and secrete a number of
bioactivators, resulting in the increase of collagen deposition in
mesenchyme, disproportionality and disordered arrangement, which
are the pathological basis of AMI. The functions of TGF-β1 in this
process have been investigated (25). TGF-β1 has strong chemotaxis to
fibroblasts, which stimulates it to secrete a considerable amount
of extracellular matrix (12).
Studies have suggested that the expression of collagen I and III
would increase in cardiac muscle tissues with TGF-β1 (25,26).
The present study demonstrated that diethylcarbamazine
significantly suppressed the AMI-induced expression of TGF-β1
protein. Rocha et al (27)
confirmed that diethylcarbamazine reduces chronic inflammation and
fibrosis in liver injury by decreasing IL-1β, COX-2, NF-κB,
interferon-γ and TGF-β expression.
When stimulated by hypoxia-ischemia and
pro-inflammatory factors, high levels of arachidonate is released
and prostaglandin (PG) H2 synthesis is catalyzed by COX-1 and
COX-2. Although their catalyzation principles are similar, the
catalytic action rate of COX-2 on arachidonic acid is four times
that of COX-1 (28). Under the
actions of modifying enzymes, PGH2 can be transformed into other
PGs, including PGE2, PGI2, PGD2 and thromboxane A2. PGs exert their
systemic effects via autocrine and paracrine mechanisms with signal
switching conducted in the surrounding environment (29). The upregulation of COXs in
myocardial cells can increase the contents of PGs in cardiac muscle
tissues and can therefore has an important regulatory role in
myocardial cells. A previous study indicated that COX-2 has a
harmful role in ischemic myocardium and the inhibition of COX-2 can
effectively protect the myocardium (30). In the present study,
diethylcarbamazine significantly reduced the AMI-induced COX-2
protein expression in rats. Ribeiro et al (31) demonstrated that diethylcarbamazine
attenuates carrageenan-induced lung injury in rats via COX-2 and
iNOS expression.
A key factor for necrocytosis or apoptosis is the
level of ATP. If there is a store of ATP, injured cells tend to
undergo apoptosis (32). If energy
is depleted, necrocytosis occurs. As a selective inhibitor of PARP,
3-aminobenzamide can reduce the consumption of ATP in injured cells
and transform necrotic cells to normal cells. A PARP inhibitor can
significantly reduce the death of ischemic neurons and protect
against injury caused by reperfusion following focal cerebral
ischemia (9). The increase of PARP
activation increases cell death. When ischemic damage is mild and
DNA injuries are rather limited, DNA repair takes the lead and
cells tolerate ischemia and survive. Following severe ischemic
damage, multiple DNA damage and DNA repair mechanisms are
activated; however, PARP inhibition is initiated by apoptosis
signaling, which can be harmful or beneficial (11). Therefore, using an optimal dose of
PARP inhibitors has a significant value in overcoming these damaged
mechanisms. The present study identified that diethylcarbamazine
significantly suppressed AMI-induced PARP protein expression in
rats. Santos et al (13)
suggested that diethylcarbamazine inhibits NF-κB activation via
PARP in mice with acute carrageenan-induced lung injury.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that the protective
effect of diethylcarbamazine inhibits NF-κB activation in
isoproterenol-induced AMI rats and exerts a protective effect on
AMI through the suppression of inflammation, iNOS, TGF-β1, COX-2
and PARP, revealing the clinical potential of diethylcarbamazine
for therapeutic and clinical applications.
References
|
1
|
Ritsinger V, Malmberg K, Mårtensson A,
Rydén L, Wedel H and Norhammar A: Intensified insulin-based
glycaemic control after myocardial infarction: Mortality during 20
year follow-up of the randomised Diabetes Mellitus Insulin Glucose
Infusion in Acute Myocardial Infarction (DIGAMI 1) trial. Lancet
Diabetes Endocrinol. 2:627–633. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suh JW, Yoon YE, Oh IY, Yoon CH, Cho YS,
Youn TJ, Chae IH and Choi DJ: A single-center prospective
randomized controlled trial evaluating the safety and efficacy of
IntraCoronary Erythropoietin delivery BEfore Reperfusion: Gauging
infarct size in patients with acute ST-segment elevation myocardial
infarction. Study design and rationale of the ‘ICEBERG Trial’.
Contemp Clin Trials. 35:145–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng Y, Fu X, Li W, Geng W, Xing K, Ru L,
Sun J and Zhao Y: Effect of intracoronary anisodamine and diltiazem
administration during primary percutaneous coronary intervention in
acute myocardial infarction. Coron Artery Dis. 25:645–652. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pellaton C, Cayla G, Silvain J, Zeymer U,
Cohen M, Goldstein P, Huber K, Pollack C Jr, Kerneis M, Collet JP,
et al: Incidence and consequence of major bleeding in primary
percutaneous intervention for ST-elevation myocardial infarction in
the era of radial access: An analysis of the international
randomized Acute myocardial infarction Treated with primary
angioplasty and intravenous enoxaparin or unfractionated heparin to
Lower ischemic and bleeding events at short- and Long-term
follow-up trial. Am Heart J. 170:778–786. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi A, Adachi H, Kawahito K, Murata
S and Ino T: Left ventricular reconstruction benefits patients with
dilated ischemic cardiomyopathy. Ann Thorac Surg. 79:456–461. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manson A, Poyade M and Rea P: A
recommended workflow methodology in the creation of an educational
and training application incorporating a digital reconstruction of
the cerebral ventricular system and cerebrospinal fluid circulation
to aid anatomical understanding. BMC Med Imaging. 15:442015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu SS, Fan HG, Zheng Z, Feng W, Wang W,
Song YH, Wang LQ, Yuan X and Zhang SJ: Left ventricular
reconstruction with no-patch technique: Early and late clinical
outcomes. Chin Med J (Engl). 123:3412–3416. 2010.PubMed/NCBI
|
|
8
|
Vargas-Barron J, Antunez-Montes OY, Roldán
FJ, Aranda-Frausto A, González-Pacheco H, Romero-Cardenas Á and
Zabalgoitia M: Myocardial rupture in acute myocardial infarction:
Mechanistic explanation based on the ventricular myocardial band
hypothesis. Rev Invest Clin. 67:318–322. 2015.PubMed/NCBI
|
|
9
|
Yao L, Huang K, Huang D, Wang J, Guo H and
Liao Y: Acute myocardial infarction induced increases in plasma
tumor necrosis factor-alpha and interleukin-10 are associated with
the activation of poly (ADP-ribose) polymerase of circulating
mononuclear cell. Int J Cardiol. 123:366–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Graziani G and Szabó C: Clinical
perspectives of PARP inhibitors. Pharmacol Res. 52:109–118. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szabó C: Pharmacological inhibition of
poly(ADP-ribose) polymerase in cardiovascular disorders: Future
directions. Curr Vasc Pharmacol. 3:301–303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boerma M, Wang J, Sridharan V, Herbert JM
and Hauer-Jensen M: Pharmacological induction of transforming
growth factor-beta1 in rat models enhances radiation injury in the
intestine and the heart. PLoS One. 8:e704792013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santos LA, Ribeiro EL, Barbosa KP, Fragoso
IT, Gomes FO, Donato MA, Silva BS, Silva AK, Rocha SW, França ME,
et al: Diethylcarbamazine inhibits NF-kB activation in acute lung
injury induced by carrageenan in mice. Int Immunopharmacol.
23:153–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
da Silva BS, Rodrigues GB, Rocha SW,
Ribeiro EL, Gomes FO, E Silva AK and Peixoto CA: Inhibition of
NF-kB activation by diethylcarbamazine prevents alcohol-induced
liver injury in C57BL/6 mice. Tissue Cell. 46:363–371. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai D, Sun J, Li Y and He B: Usefulness of
ventricular endocardial electric reconstruction from body surface
potential maps to noninvasively localize ventricular ectopic
activity in patients. Phys Med Biol. 58:3897–3909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menon SC, Cetta F, Dearani JA, Burkhart
HA, Cabalka AK and Hagler DJ: Hybrid intraoperative pulmonary
artery stent placement for congenital heart disease. Am J Cardiol.
102:1737–1741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ka SM, Chao L Kuoping, Lin JC, Chen ST, Li
WT, Lin CN, Cheng JC, Jheng HL, Chen A and Hua KF: A low toxicity
synthetic cinnamaldehyde derivative ameliorates renal inflammation
in mice by inhibiting NLRP3 inflammasome and its related signaling
pathways. Free Radic Biol Med. 91:10–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mezzaroma E, Toldo S, Farkas D, Seropian
IM, van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF and
Abbate A: The inflammasome promotes adverse cardiac remodeling
following acute myocardial infarction in the mouse. Proc Natl Acad
Sci USA. 108:19725–19730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mezzaroma E, Toldo S and Abbate A: Role of
NLRP3 (cryopyrin) in acute myocardial infarction. Cardiovasc Res.
99:225–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Altaf A, Qu P, Zhao Y, Wang H, Lou D and
Niu N: NLRP3 inflammasome in peripheral blood monocytes of acute
coronary syndrome patients and its relationship with statins. Coron
Artery Dis. 26:409–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alestalo K, Miettinen JA, Vuolteenaho O,
Huikuri H and Lehenkari P: Bone marrow mononuclear cell
transplantation restores inflammatory balance of cytokines after ST
segment elevation myocardial infarction. PLoS One. 10:e01450942015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ruparelia N, Digby JE, Jefferson A, Medway
DJ, Neubauer S, Lygate CA and Choudhury RP: Myocardial infarction
causes inflammation and leukocyte recruitment at remote sites in
the myocardium and in the renal glomerulus. Inflamm Res.
62:515–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zaitone SA and Abo-Gresha NM: Rosuvastatin
promotes angiogenesis and reverses isoproterenol-induced acute
myocardial infarction in rats: Role of iNOS and VEGF. Eur J
Pharmacol. 691:134–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das B and Sarkar C: Is preconditioning by
oxytocin administration mediated by iNOS and/or mitochondrial
K(ATP) channel activation in the in vivo anesthetized rabbit heart?
Life Sci. 90:763–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ørn S, Ueland T, Manhenke C, Sandanger Ø,
Godang K, Yndestad A, Mollnes TE, Dickstein K and Aukrust P:
Increased interleukin-1b levels are associated with left
ventricular hypertrophy and remodelling following acute ST segment
elevation myocardial infarction treated by primary percutaneous
coronary intervention. J Intern Med. 272:267–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Fan Q, He S, Tang T, Liao Y and Xie
J: MicroRNA-21 negatively regulates Treg cells through a
TGF-β1/Smad-independent pathway in patients with coronary heart
disease. Cell Physiol Biochem. 37:866–878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rocha SW, de França ME, Rodrigues GB,
Barbosa KP, Nunes AK, Pastor AF, Oliveira AG, Oliveira WH, Luna RL
and Peixoto CA: Diethylcarbamazine reduces chronic inflammation and
fibrosis in carbon tetrachloride- (CCl4-) induced liver injury in
mice. Mediators Inflamm. 2014:6963832014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davies NM, Smith GD, Windmeijer F and
Martin RM: COX-2 selective nonsteroidal anti-inflammatory drugs and
risk of gastrointestinal tract complications and myocardial
infarction: An instrumental variable analysis. Epidemiology.
24:352–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Varas-Lorenzo C, Castellsague J, Stang MR,
Perez-Gutthann S, Aguado J and Rodriguez LA: The use of selective
cyclooxygenase-2 inhibitors and the risk of acute myocardial
infarction in Saskatchewan, Canada. Pharmacoepidemiol Drug Saf.
18:1016–1025. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Metcalfe C, Wheeler BW, Gunnell D and
Martin RM: International regulatory activity restricting COX-2
inhibitor use and deaths due to gastrointestinal haemorrhage and
myocardial infarction. Pharmacoepidemiol Drug Saf. 19:778–785.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ribeiro EL, Barbosa KP, Fragoso IT, Donato
MA, Gomes FO, da Silva BS, e Silva AK Soares, Rocha SW, da Silva
Junior VA and Peixoto CA: Diethylcarbamazine attenuates the
development of carrageenan-induced lung injury in mice. Mediators
Inflamm. 2014:1051202014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong F, Yang XJ, Jiang TB and Chen Y:
Ischemia triggered ATP release through Pannexin-1 channel by
myocardial cells activates sympathetic fibers. Microvasc Res.
104:32–37. 2016. View Article : Google Scholar : PubMed/NCBI
|