Introduction
Cerebral low-grade gliomas (LGGs) are associated
with neurological disability and present a challenge to
neurosurgeons and neuro-oncologists (1). Although they are a relatively
slow-growing brain tumor, LGGs have complex clinical manifestations
(1). LGG frequently occur in
Caucasians, particularly males and typically affect young patients.
Therefore, scientific and clinical advances are required. However,
predictive markers for diagnosis and prognosis of cerebral LGG are
rare and mortality is high.
Clinical outcomes may be improved by identification
of potential molecular biomarkers of LGG. Candidate therapeutic
biomarkers have been identified by high-throughput technologies.
The identification of microRNAs (miRNAs) has revealed novel
insights into diagnosis and prognosis of cancers (2,3).
miRNAs are a class of small, non-coding RNAs which have roles in
cell apoptosis, differentiation, proliferation and stress responses
(4,5). Previous studies have determined that
alteration of miRNA expression level is associated with development
and prognosis of human cancers, including pancreatic, breast,
non-small cell lung and ovarian cancer (6–9). For
example, miRNA-26a is overexpressed in high-grade glioma and
directly targets phosphatase and tensin homolog which suppresses
protein kinase B (Akt) signaling (10). miRNA (miR)-221 has been identified
to be upregulated in glioblastoma and directly targets the tumor
suppressor p27 (11). Conversely,
downregulated miR-7 has been identified to reduce proliferation and
invasiveness in cultured glioma cells by targeting the epidermal
growth factor receptor (12).
Additionally, miR-205 has been identified as a potential prognostic
indicator for human glioma (13).
A previous study has demonstrated that miR-221/222 may be a
predictive marker for increased cell invasion and poor prognosis in
glioma (14). However, miRNA
biomarkers for tumorigenesis and prognosis of LGG have not been
determined and candidate therapeutic targets remain to be
identified.
The present study aimed to gain further insight into
the clinical outcome of LGG by miRNA profile analysis. miRNA
expression and clinical data of LGGs from the Cancer Genome Atlas
(TCGA) database was downloaded, followed by identification of risk
miRNAs using survival and Cox proportional hazard regression
analysis. Functional annotation and protein-protein interaction
(PPI) network construction of targets of miRNAs were performed.
Additionally, sub-pathways were mined for further investigation of
the function of risk miRNAs.
Materials and methods
Data collection
Clinical data and miRNA expression profiles were
obtained from the TCGA database (http://cancergenome.nih.gov/) based on the platform of
BCGSC_IlluminaHiSeq_miRNASeq. On the 11 August, 2014, there were
529 miRNA expression profiles and 411 clinical data from patients
with cerebral LGGs, 408 of which contained associated miRNA
expression profiles.
Survival analysis
The reads per kilobase of exon model per million
mapped (RPKM) value which estimated the expression value for each
gene was calculated to detect present miRNAs. In order to analyze
the association between a queried miRNA and survival, the patients
were grouped according to the median expression of the selected
miRNA (or upper or lower quartile). In order to identify the
genomic factors associated with survival, patient survival
Kaplan-Meier (KM) curves were plotted using the survival (15) and KMsurv (16) packages in R and differences between
curves were evaluated by two-sided log-rank test.
Cox proportional hazard regression
analysis
To identify prognosis-associated miRNAs, the joint
effect of variables with a significant P-value were examined using
the Cox proportional hazard regression model which was built with
the aforementioned two packages. P<0.05 was considered to
indicate a statistically significant difference.
Regulatory network construction of
miRNA-targets
In order to predict target genes of risk miRNA,
which were selected from the miRNecords (17) and MiRWalk (18) databases, or recorded in at least 3
databases of the following databases: miRanda (19), MirTarget2 (20), PicTar (21), PITA (22) and TargetScan (23). A regulatory network of miRNA
targets was constructed using the combined databases and visualized
using Cytoscape software version 2.8 (24).
Functional annotation of miRNA target
genes
In order to annotate functions of miRNA targets, GO
(Gene Ontology) (25) function in
biological process, cellular components, molecular function and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (26) enrichment analysis were performed
using Database for Annotation, Visualization, and Integrated
Discovery (DAVID) (27). P<0.05
was considered to indicate a statistically significant
difference.
PPI network construction for miRNA
targets
In order to determine the interaction of miRNA
targets, a PPI network was built using STRING software version 9.1
(28) and visualized with
Cytoscape for protein-protein pairs, where the combined score was
>0.4.
Sub-pathway analysis for miRNA
targets
In order to identify the risk disease-associated
sub-pathway of miRNA targets, the present study used a k-clique
concept from the SubpathwayMiner package in R (29). P was calculated using a
hypergeometric distribution and P<0.05 was considered to
indicate a statistically significant difference.
Results
Results of survival analysis
The median miRNA intensity value of the 408 patients
was used as the cut-off point in the KM curve analysis. A total of
39 miRNAs were obtained, which significantly affected survival in
the KM curve (data not shown). From the survival analysis, patients
with high expression of these 39 miRNAs had reduced survival
compared with patients with low expression.
Risk miRNA for cerebral LGG
prognosis
The cerebral LGGs risks were estimated as a hazard
ratio (HR) and 95% confidence intervals (CI) using the Cox
proportional hazard regression model. In the Cox proportional
hazard regression analysis, 3 miRNAs including has-miR-1287,
has-miR-326 and has-miR-1275 were considered as risk miRNAs for
cerebral LGGs prognosis (Table
I).
 | Table I.Identification of risk microRNAs in
patients with cerebral low-grade glioma using Cox proportional
hazard regression analysis. |
Table I.
Identification of risk microRNAs in
patients with cerebral low-grade glioma using Cox proportional
hazard regression analysis.
| Name | β | HR | P | Lower CI | Upper CI |
|---|
| hsa-miR-1287 |
0.016169188 | 1.016300616 |
1.69446×10−7 | 1.010161075 | 1.022477472 |
| hsa-miR-326 | −0.008757427 | 0.991280808 | 0.005923248 | 0.985117424 | 0.997482752 |
| hsa-miR-1275 | −0.193926634 | 0.823718335 | 0.035076194 | 0.687784787 | 0.986517742 |
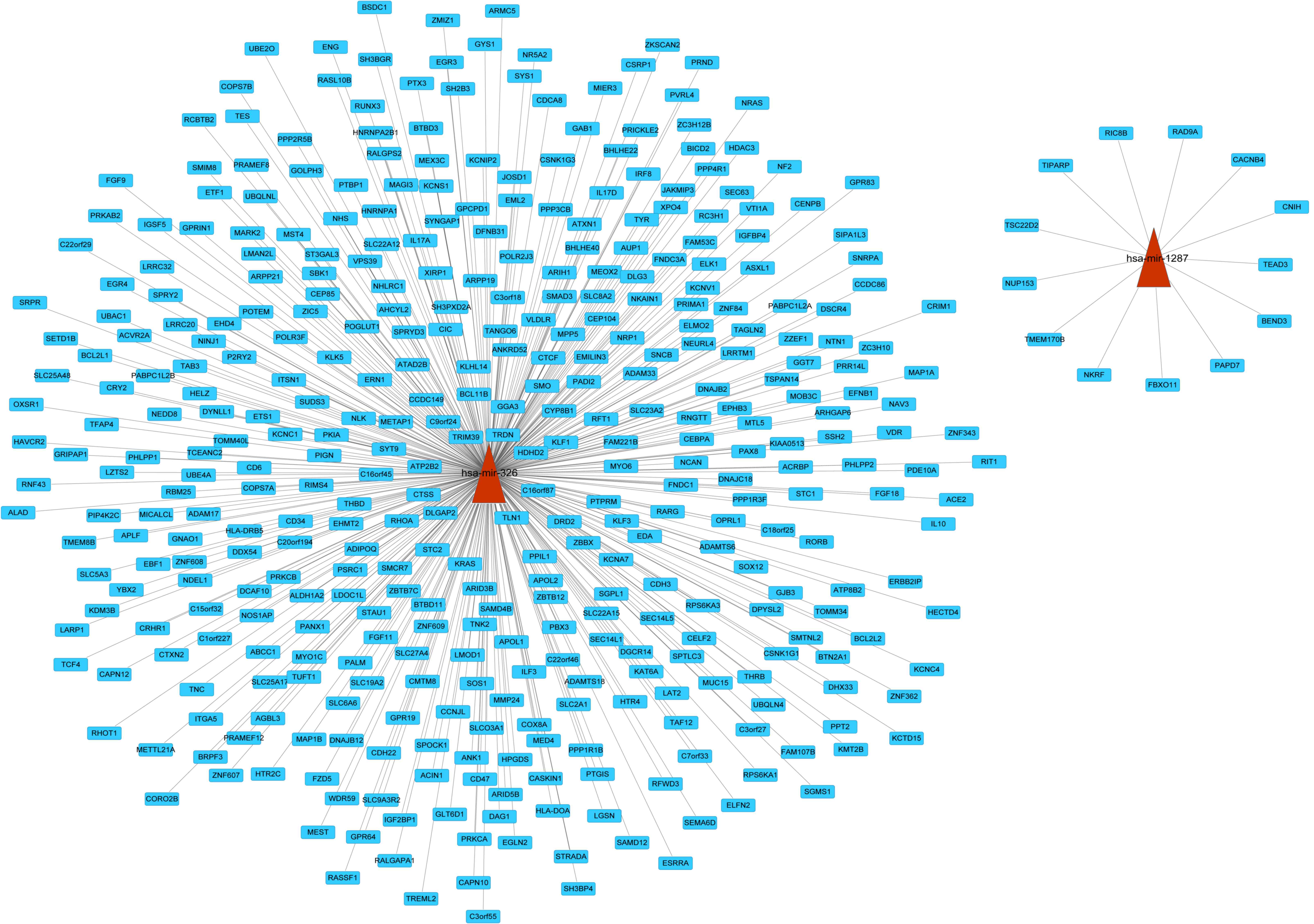
Regulatory network of risk
miRNA-targets
In order to investigate regulatory function of risk
miRNAs, a regulatory network for miRNA-target genes was constructed
(Fig. 1). However, no predicted
target genes were identified for has-miR-1275. The present study
predicted 13 and 397 targets regulated by hsa-miR-1287 and
hsa-miR-326, respectively. In the network, there were 410 links and
412 nodes. In the network, son of sevenless homolog 1
(SOS1), neuroblastoma RAS viral (v-ras) oncogene homolog
(NRAS), and vitamin D (1,25-dihydroxyvitamin D3) receptor
(VDR) were targets of miR-326.
Functional enrichment analysis of
miRNA targets
In order to determine the regulatory functions of
has-miR-326, GO function and pathway enrichment analysis were
performed for the target genes. The top 5 GO terms and pathways are
presented in Table II. The
findings revealed that targets of miR-326 were significantly
enriched in various functions, including neuron development, neuron
differentiation and regulation of cell proliferation. Additionally,
targets of miR-326 were significantly enriched in cancer pathways,
and the epithelial growth factor receptor (EGFR) and nerve growth
factor (NGF) signaling pathways.
 | Table II.Functional annotation of hsa-miR-326
targets. |
Table II.
Functional annotation of hsa-miR-326
targets.
| A, Biological
processes |
|---|
|
|---|
| Term | Function | Count | P-value |
|---|
| GO:0048666 | Neuron
development | 20 | 1.10×10-4 |
| GO:0030182 | Neuron
differentiation | 22 |
4.20×10−4 |
| GO:0042127 | Regulation of cell
proliferation | 32 | 6.13×10-4 |
| GO:0045197 | Establishment or
maintenance of epithelial cell apical/basal polarity | 4 |
7.15×10−4 |
| GO:0044057 | Regulation of
system process | 17 | 9.04×10-4 |
|
| B, Cellular
components |
|
| Term | Function | Count | P-value |
| GO:0044459 | Plasma membrane
part | 72 |
3.26×10−5 |
| GO:0005886 | Plasma
membrane | 107 | 7.61×10-5 |
| GO:0005911 | Cell-cell
junction | 12 |
1.82×10−3 |
| GO:0031965 | Nuclear
membrane | 7 | 3.72×10-3 |
| GO:0030054 | Cell junction | 21 |
4.78×10−3 |
|
| C, Molecular
function |
|
| Term | Function | Count | P-value |
| GO:0019904 | Protein domain
specific binding | 18 | 1.00×10-3 |
| GO:0003707 | Steroid hormone
receptor activity | 6 |
4.20×10−3 |
| GO:0004879 | Ligand-dependent
nuclear receptor activity | 6 | 8.61×10-3 |
| GO:0016247 | Channel regulator
activity | 6 |
9.24×10−3 |
| GO:0005249 | Voltage-gated
potassium channel activity | 7 | 2.24×10-2 |
|
| D, KEGG
pathways |
|
| Term | Function | Count | P-value |
| hsa05200 | Pathways in
cancer | 19 |
5.37×10−4 |
| hsa04360 | Axon guidance | 10 | 3.02×10-3 |
| hsa04720 | Long-term
potentiation | 7 |
4.86×10−3 |
| hsa05211 | Renal cell
carcinoma | 7 | 5.60×10-3 |
| hsa05223 | Non-small cell lung
cancer | 6 |
8.18×10−3 |
|
| E, REACTOME
pathways |
|
| Term | Function | Count | P-value |
| REACT_9417 | Signaling by
epidermal growth factor receptor | 6 | 4.27×10-3 |
| REACT_11061 | Signaling by nerve
growth factor | 11 |
6.89×10−3 |
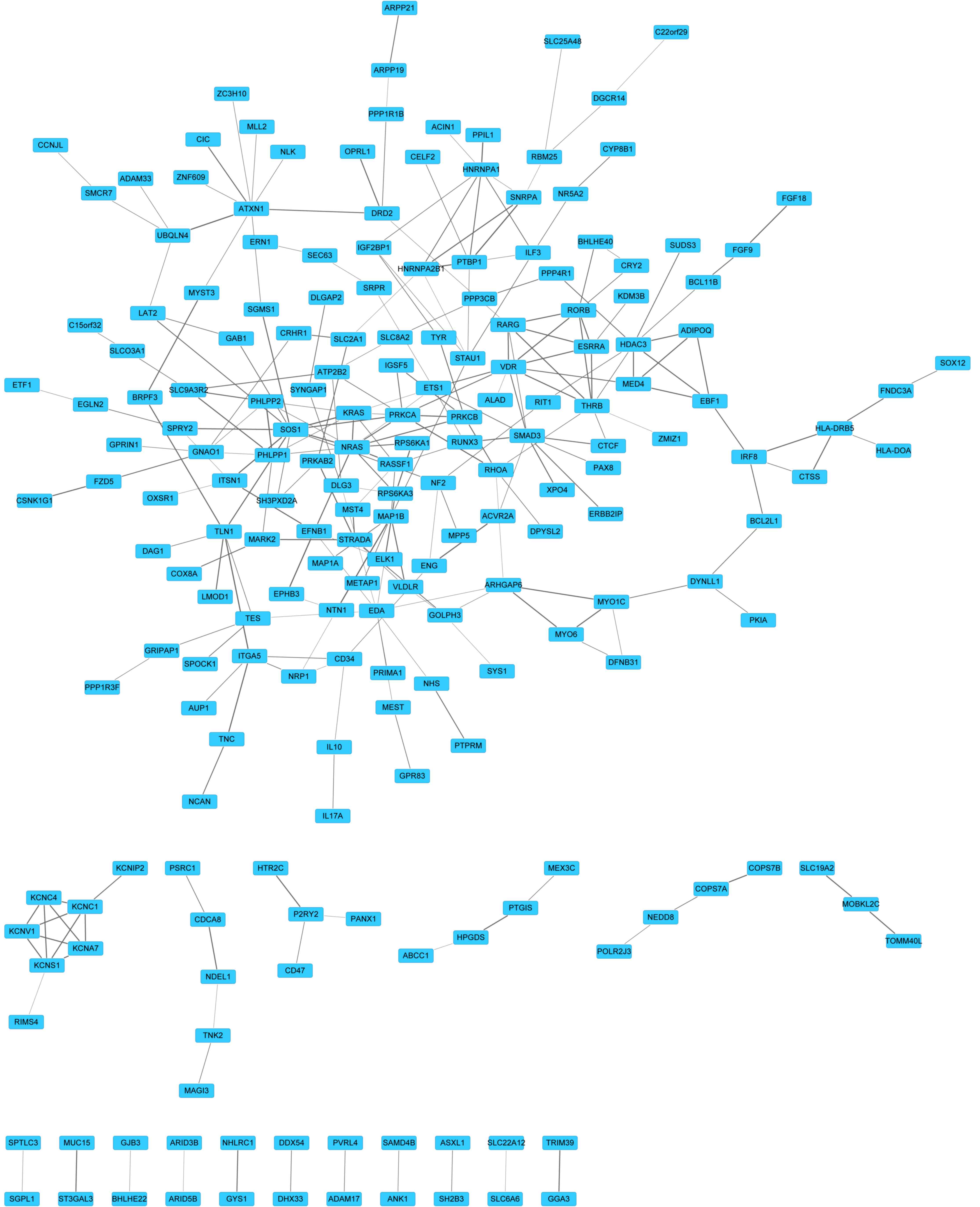
PPI interaction network
A PPI network was constructed to predict novel
interactions for targets of has-miR-326. It was determined that 203
proteins shared 262 links (Fig.
2). According to the degree of targets, the top 10 proteins
were with highest degree (Table
III) were SOS1, NRAS, VDR and mothers against decapentaplegic
family member 3 (SMAD3). Along with the combined score, the top
five protein pairs were polypyrimidine tract binding protein
1-small nuclear ribonucleoprotein polypeptide A (PTBP1-SNRPA;
0.999), PH domain and leucine rich repeat protein phosphatase 1–2
(PHLPP1-PHLPP2; 0.992), microtubule-associated protein 1B-netrin 1
(MAP1B-NTN1; 0.985), ubiquilin 4-ataxin 1 (UBQLN4-ATXN1; 0.983) and
talin 1-integrin, α 5 (TLN1-ITGA5; 0.982).
 | Table III.Degree of the top 10 targets of
miR-326 in the protein-protein interaction network. |
Table III.
Degree of the top 10 targets of
miR-326 in the protein-protein interaction network.
| Gene | Degree |
|---|
| SOS1 | 11 |
| NRAS | 10 |
| VDR | 9 |
| SMAD3 | 9 |
| ATXN1 | 9 |
| THRB | 8 |
| PRKCA | 8 |
| KRAS | 8 |
| HDAC3 | 8 |
| EDA | 8 |
Identification of risk
sub-pathway
In order to determine the association between the
enriched pathways of miR-326 targets and cerebral LGGs, risk
sub-pathways were identified for targets of miR-326 (Table IV). A total of 4 sub-pathways were
obtained, involving 8 targets of miR-326. Sphingomyelin synthase 1
(SGMS1), serine palmitoyltransferase, long chain base
subunit 3 (SPTLC3) and sphingosine-1-phosphate lyase 1
(SGPL1) were significantly enriched in the sphingolipid
metabolism pathway. Hematopoietic prostaglandin D synthase
(HPGDS) and prostaglandin I2 (prostacyclin) synthase
(PTGIS) were involved in the arachidonic acid metabolism.
adenosylhomocysteinase-like 2 (AHCYL2) and
gamma-glutamyltransferase 7 (GGT7) were primarily involved
in the selenoamino acid metabolism. Additionally, aldehyde
dehydrogenase 1 family, member A2 (ALDH1A2) was
significantly enriched in the retinol metabolism sub-pathway.
 | Table IV.Enriched sub-pathways of miR-326
targets. |
Table IV.
Enriched sub-pathways of miR-326
targets.
| Pathway ID | Pathway name | P | Gene |
|---|
| path:00600_3 | Sphingolipid
metabolism | 0.01024568 | SGMS1; SPTLC3;
SGPL1 |
| path:00590_9 | Arachidonic acid
metabolism | 0.01632627 | HPGDS; PTGIS |
| path:00450_3 | Selenoamino acid
metabolism | 0.01935802 | AHCYL2; GGT7 |
| path:00830_2 | Retinol
metabolism | 0.03609776 | ALDH1A2 |
Discussion
Abnormal miRNA expression and alterations are
frequently associated with progression and prognosis of cancers
(2,3). Specific miRNAs may be classified as
tumor suppressors or oncogenes. However, a further analysis of
their functions in LGGs is necessary. In the present study, three
risk miRNAs, including has-miR-326 were identified by means of
survival analysis and Cox proportional hazard regression model.
Additionally, the PPI network revealed that SOS1, NRAS, VDR and
SMAD3 were with a higher degree. Additionally, 8 target genes of
miR-326 including SGMS1, SPTLC2, HPGDS and
PTGIS were significantly enriched in metabolic
sub-pathways.
Hsa-miR-326 has been downregulated in gliomas by
suppression of the Notch signaling pathway, and is in turn
inhibited by Notch (30). The
Notch signaling pathway is a candidate pathway which may contribute
to glioma progression (31).
Additionally, miR-326 may be a potential tumor suppressor in glioma
cells, and transfection of miR-326 into glioma cells may reduce
tumorigenicity (30). As one grade
of glioma, LGGs may be affected by miR-326. From the results of
survival analysis, patients with high expression of miR-326 had
reduced survival compared with those with low expression of it.
Accordingly, miR-326 may suppress some oncogenes and lead to tumor
development. Targets of miR-326 were regulated, including
SOS1, NRAS, VDR, SMAD3 and
SGMS1.
SOS1, as a dual guanine nucleotide exchange factor
for Ras and Rac1, may convert inactive Ras-guanosine diphosphate
into active Ras-guanosine triphosphate in various cells (32). Additionally, Ras was stimulated by
EGFR and its close relative, erb-b2 receptor tyrosine kinase 2
(33). EGFR signaling has been
determined to be involved in cell survival, tumorigenesis and
metastasis (34). Notably, EGFR is
one of the targets of several therapeutic agents in colorectal and
non-small-cell lung cancers (34).
Additionally, NGF may stimulate SOS1 and activate Ras signaling to
exert various functions in cell proliferation (35). A previous study indicated that NGF
may lead to proliferation and migration of endothelial cells and
had a vital role in angiogenesis associated with tumors and
cardiovascular diseases (35).
NRAS, a member of the Ras family, is widely expressed in several
cell types. Therefore, activation of NRAS may be stimulated by SOS1
and be involved in EGFR and NGF signaling. Consistent with a
previous study (35), in the
present study, SOS1 and NRAS were enriched in the
EGFR and NGF signaling pathway. Furthermore, NRAS
participated in cell proliferation in GO function analysis.
Therefore, SOS1 and NRAS may regulate cell
proliferation and angiogenesis via the EGFR and NGF signaling
pathway in LGGs.
VDR is a transcription factor expressed in the brain
(36) and mediates the effects of
1,25(OH)2D3. The vitamin D metabolite
1,25(OH)2D3 has been demonstrated to protect
against cancer by inducing apoptosis and inhibiting cell
proliferation and angiogenesis (37). SMAD3, the effector of transforming
growth factor β (TGF-β), may directly bind Akt and inhibit
TGF-β-induced apoptosis (38).
SMAD3 may additionally interact with phosphatase and tensin
homologue, which is a tumor suppressor in glioblastomas, to
downregulate TGF-β signaling and decrease TGF-β-mediated tumor
invasion (39). Consistent with
this, the function annotation in the presents study revealed that
VDR and SMAD3 were significantly enriched in the cell
proliferation GO term. Therefore, it is possible that targets of
miR-326, VDR and SMAD3 may regulate tumor cell
proliferation in LGGs to increase tumor growth and invasion.
SGMS1, a sphingomyelin synthase, may produce
sphingomyelin in the Golgi apparatus. The sphingomyelin levels have
been previously reported to reduce the variety of tumor cells and
reduce sphingomyelin by negatively regulating SGMS1 induction of
cell proliferation of cancer cells (40). A previous study has reported that
sphingolipids metabolism may influence cell cycle progression and
cell migration (41).
Additionally, ceramide and sphingosine-1-phosphate, two major
sphingolipid metabolites, have been determined to be involved in
process of apoptosis, cell proliferation and differentiation
(42). Therefore, the miR-326
target SGMS1 may regulate cerebral LGG cell proliferation and
apoptosis via the sphingolipids metabolism signaling pathway.
HPGDS, a prostaglandin D (PGD) synthase, catalyzes
the synthesis of PDG2 from endogenous arachidonic acid.
Additionally, arachidonic acid may be metabolized by
cyclooxygenases (COX), cytochrome P450 and lipoxygenases (LOX)
(43). Previous studies have
revealed that COX and LOX inhibition induces apoptosis in several
tumor cells (44–46). These were consistent with the
current finding that HPGDS was primarily enriched in the
arachidonic acid metabolism signaling pathway. Accordingly,
HPGDS may regulate LGG cell apoptosis via this pathway.
In conclusion, the present study determined that
hsa-miR-326 may be a potential risk miRNA for diagnosis and
prognosis of LGG. Hsa-miR-326 may regulate cell proliferation and
apoptosis of cancer cells by targeting certain genes including
SOS1, NRAS, VDR, SMAD3, SGMS1
and HPGDS. However, further empirical investigations are
required to confirm these findings.
References
|
1
|
Cavaliere R, Lopes MB and Schiff D:
Low-grade gliomas: An update on pathology and therapy. Lancet
Neurol. 4:760–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giovannetti E, van der Velde A, Funel N,
Vasile E, Perrone V, Leon LG, de Lio N, Avan A, Caponi S, Pollina
LE, et al: High-throughput microRNA (miRNAs) arrays unravel the
prognostic role of MiR-211 in pancreatic cancer. PLoS One.
7:e491452012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Markou A, Tsaroucha EG, Kaklamanis L,
Fotinou M, Georgoulias V and Lianidou ES: Prognostic value of
mature microRNA-21 and microRNA-205 overexpression in non-small
cell lung cancer by quantitative real-time RT-PCR. Clin Chem.
54:1696–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu X, Macdonald DM, Huettner PC, Feng Z,
El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN and Wang X:
A miR-200 microRNA cluster as prognostic marker in advanced ovarian
cancer. Gynecol Oncol. 114:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huse JT, Brennan C, Hambardzumyan D, Wee
B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T
and Holland EC: The PTEN-regulating microRNA miR-26a is amplified
in high-grade glioma and facilitates gliomagenesis in vivo. Genes
Dev. 23:1327–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
le Sage C, Nagel R, Egan DA, Schrier M,
Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA, et
al: Regulation of the p27(Kip1) tumor suppressor by miR-221 and
miR-222 promotes cancer cell proliferation. EMBO J. 26:3699–3708.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kefas B, Godlewski J, Comeau L, Li Y,
Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S and
Purow B: microRNA-7 inhibits the epidermal growth factor receptor
and the Akt pathway and is down-regulated in glioblastoma. Cancer
Res. 68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou SX, Ding BJ, Li HZ, Wang L, Xia F, Du
F, Liu LJ, Liu YH, Liu XD, Jia JF, et al: Identification of
microRNA-205 as a potential prognostic indicator for human glioma.
J Clin Neurosci. 20:933–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Zhang J, Hao J, Shi Z, Wang Y,
Han L, Yu S, You Y, Jiang T, Wang J, et al: High level of
miR-221/222 confers increased cell invasion and poor prognosis in
glioma. J Transl Med. 10:1192012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Therneau TM and Grambsch PM: Modeling
survival data: Extending the Cox model. Springer; 2000, View Article : Google Scholar
|
|
16
|
Original by Klein, Moeschberger and
modifications by. Jun Yan: 2012.KMsurv: Data sets from Klein and
Moeschberger (1997), Survival Analysis. R package version
0.1–5.
|
|
17
|
Xiao F, Zuo Z, Cai G, Kang S, Gao X and Li
T: miRecords: An integrated resource for microRNA-target
interactions. Nucleic Acids Res. 37:D105–D110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X and El Naqa IM: Prediction of both
conserved and nonconserved microRNA targets in animals.
Bioinformatics. 24:325–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M
and Rajewsky N: Combinatorial microRNA target predictions. Nat
Genet. 37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nat Genet. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li C: iSubpathwayMiner: The package can
implement the graph-based reconstruction and analyses of the KEGG
pathways. R package version 3.0. 2012.
|
|
30
|
Kefas B, Comeau L, Floyd DH, Seleverstov
O, Godlewski J, Schmittgen T, Jiang J, diPierro CG, Li Y, Chiocca
EA, et al: The neuronal microRNA miR-326 acts in a feedback loop
with notch and has therapeutic potential against brain tumors. J
Neurosci. 29:15161–15168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong JW: MicroRNA-induced silencing of
glioma progression. J Neurosci. 30:3868–3869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gureasko J, Galush WJ, Boykevisch S,
Sondermann H, Bar-Sagi D, Groves JT and Kuriyan J:
Membrane-dependent signal integration by the Ras activator Son of
sevenless. Nat Struct Mol Biol. 15:452–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goffin JR and Zbuk K: Epidermal growth
factor receptor: Pathway, therapies, and pipeline. Clin Ther.
35:1282–1303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nico B, Mangieri D, Benagiano V,
Crivellato E and Ribatti D: Nerve growth factor as an angiogenic
factor. Microvasc Res. 75:135–141. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eyles DW, Smith S, Kinobe R, Hewison M and
McGrath JJ: Distribution of the vitamin D receptor and 1
alpha-hydroxylase in human brain. J Chem Neuroanat. 29:21–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hansen CM, Binderup L, Hamberg KJ and
Carlberg C: Vitamin D and cancer: Effects of 1,25(OH)2D3 and its
analogs on growth control and tumorigenesis. Front Biosci.
6:D820–D848. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Conery AR, Cao Y, Thompson EA, Townsend CM
Jr, Ko TC and Luo K: Akt interacts directly with Smad3 to regulate
the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol.
6:366–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hjelmeland AB, Hjelmeland MD, Shi Q, Hart
JL, Bigner DD, Wang XF, Kontos CD and Rich JN: Loss of phosphatase
and tensin homologue increases transforming growth factor
beta-mediated invasion with enhanced SMAD3 transcriptional
activity. Cancer Res. 65:11276–11281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tafesse FG, Ternes P and Holthuis JC: The
multigenic sphingomyelin synthase family. J Biol Chem.
281:29421–29425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zeidan YH and Hannun YA: Translational
aspects of sphingolipid metabolism. Trends Mol Med. 13:327–336.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oskouian B and Saba JD: Cancer treatment
strategies targeting sphingolipid metabolismSphingolipids as
Signaling and Regulatory Molecules. Springer; pp. 185–205. 2010,
View Article : Google Scholar
|
|
43
|
Pidgeon GP, Lysaght J, Krishnamoorthy S,
Reynolds JV, O'Byrne K, Nie D and Honn KV: Lipoxygenase metabolism:
Roles in tumor progression and survival. Cancer Metastasis Rev.
26:503–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang WG, Douglas-Jones A and Mansel RE:
Levels of expression of lipoxygenases and cyclooxygenase-2 in human
breast cancer. Prostaglandins Leukot Essent Fatty Acids.
69:275–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hoque A, Lippman SM, Wu TT, Xu Y, Liang
ZD, Swisher S, Zhang H, Cao L, Ajani JA and Xu XC: Increased
5-lipoxygenase expression and induction of apoptosis by its
inhibitors in esophageal cancer: A potential target for prevention.
Carcinogenesis. 26:785–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leung HW, Yang WH, Lai MY, Lin CJ and Lee
HZ: Inhibition of 12-lipoxygenase during baicalein-induced human
lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol.
45:403–411. 2007. View Article : Google Scholar : PubMed/NCBI
|