Introduction
Melanoma is the fastest-growing malignant tumor and
therefore one of the most dangerous types of skin cancer. Globally,
there were 232,000 newly diagnosed cases of melanoma and 55,000
estimated deaths in 2012 (1). The
primary cause of melanoma is excessive exposure to ultraviolet
light (UV), which leads to the uncontrollable growth of
melanocytes. Early-stage melanoma may be treated via surgical
resection. However, for patients with lymph node metastasis,
radical lymph node dissection with adjuvant approaches including
radiation, chemo, and immunotherapy are recommended (2). Radiotherapy may improve regional
lymph node basin control however does not alter long-term survival
(3), and chemotherapy drugs may
induce adverse effects including neutropenia, neurotoxicity,
fatigue, thrombocytopenia, delayed myelosuppression and
gastrointestinal toxicities (4,5).
Melanoma is one of the most immunogenic types of cancer, therefore
immunotherapy attempting to harness the immune system against
tumors may potentially act as a successful curative in the
future.
Dendritic cells (DCs), are effective
antigen-presenting cells (APCs) that exist in the majority of human
tumors and function to recognize, acquire and present antigens to
naive T cells for the initiation of an antigen-specific adaptive
immune response (6,7). These properties result in the use of
DCs as an ideal vaccine and immunotherapeutic strategy against
cancers, as they are capable of capturing tumor antigens and
presenting them to T cells in tumor-draining lymphoid tissues,
which subsequently triggers the generation of tumor-specific
cytotoxic T lymphocytes (CTLs) that reduce tumor mass (8). The efficacy of DC vaccines as the
adjuvant treatment against metastatic melanoma has been explored in
numerous clinical trials (9–13).
However, tumors may exhibit the ability to escape immune
recognition and rejection by inhibiting the maturation and
differentiation of DCs which prevents the antigen presentation
mediated by DCs (14). Therefore,
attempts to manipulate DC signaling in order to create a defense
against tumor-induced DC defects are required for the successful
immunotherapy of cancer. It has been reported that regulation of
the p38 mitogen activated protein kinase (MAPK) signaling pathway
influences the differentiation of immature DCs and T cells
(15,16), and therefore p38 may act as an
effective target to optimize the DC-mediated immunotherapy.
Multiple microRNAs (miRNAs, miRs) may regulate the
expression of p38 and function in tumor growth, differentiation,
apoptosis and metastasis. Lawson et al (17) previously demonstrated that p38α is
post-transcriptionally repressed by miR-128, which is a
well-recognized tumor inhibitor (18–20)
in HEK293 cells. However, the role of miR-128 in DC-mediated
immunotherapy for melanoma has not yet been investigated. The
present study analyzed the effect of miR-128 on p38 expression in
DCs, and the curative effects of miR-128 and p38 were further
assessed using a melanoma-bearing mouse model.
Materials and methods
Cell lines and animals
Murine B16 melanoma cells were purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA). A total
of 80 C57BL/6 male mice (3–5 weeks, 20 g in weight), were obtained
from the Academy of Military Science of Chinese people's Liberation
Army (Beijing, China) and allocated into 12 cages, 5 mice per cage.
All mice were allowed to acclimate for 1 week in a specific
pathogen-free animal room at a temperature of 25°C and relative
humidity of 60% prior to experiments. The artificial feed was
sterilized by radioactive irradiation (60Co) and
provided daily, and the drinking water was filtered at level four
and underwent ozone and ultraviolet disinfection. The light/dark
cycles were 12 h, from 7.30 am to 7.30 pm. All animal studies were
approved by the Animal Care and Utilization Committee of the Second
Hospital of Tianjin Medical University. (Tianjin, China).
Preparation and transfection of mouse
bone marrow-derived DCs
The DCs used in the present study were derived from
bone marrow cells (21,22). Briefly, 4-week-old C57BL/6 mice
were sacrificed by cervical dislocation. The femurs were dissected
and each end of femurs cut off. The bone marrow was flushed out
with RPMI-1640 (Nissui, Tokyo, Japan). Following centrifugation at
600 × g for 10 min at 4°C, cells were resuspended in 2 ml ammonium
chloride/potassium carbonate/EDTA (ACK buffer) and cultured at room
temperature for 5 min to lyse red blood cells. Then, cells were
washed in RPMI-1640 complete medium supplemented with 10% fetal
bovine serum (FBS) and 100 U/ml penicillin and streptomycin (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Differentiation
of progenitor cells to immature DCs was induced by culturing in
media containing 10 ng/ml granulocyte/macrophage colony-stimulating
factor (GM-CSF; BD Biosciences, San Jose, CA, USA) for 5–6
days.
MiR-128 mimic (5′-UCACAGUGAACCGGUCUCUUU−3′), miR-128
inhibitor, p38 inhibitor or the negative control oligonucleotide
were bought from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
MiR-128 mimic, miR-128 inhibitor and p38 inhibitor were transfected
into DCs using Entransfer™-R-4000 (Engreen Biosystem
Ltd., Beijing, China) according to the manufacturer's protocol, at
concentrations of 50 and 100 nm. The transfected cells were used
for the following reverse transcription-quantitative polymerase
chain reaction (RT-qPCR), western blotting and ELISA analyses.
Treatment of DCs with B16 cell
suspension
B16 melanoma cells (1×106) were cultured
in RPMI-1640 complete medium in 6-well plates, and subsequently
cultured in an incubator (37°C, 5% CO2). Cells at the
logarithmic phase were harvested to extract the cell suspension.
The mouse bone marrow-derived DCs were co-cultured with the
suspension of B16 cells for 24 h at 37°C. Then, the expression
levels of miR-128, p38 mRNA and p38 protein in DCs were
determined by using RT-qPCR and western blot analysis.
RT-qPCR
Total RNA was isolated from DCs using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 1
µg RNA was added into the reverse transcription system containing
M-MLV (Takara Biotechnology Co., Ltd., Dalian, China) according to
the manufacturer's protocol. SYBR Green (Roche Applied Science,
Penzberg, Germany) Real-time RT-qPCR was performed using an Applied
Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the following steps:
denaturation at 94°C for 5 min, followed by 40 amplification cycles
of 94°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec. Primers
used for the PCR method are listed in Table I. Relative expression level of gene
or miRNA was calculated using the 2−ΔΔCq method, where
ΔCq means Cq (tested)-Cq(internal control)
(23). GAPDH and U6 were
used as the internal control for genes and miRNAs,
respectively.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primers | Sequence
(5′-3′) |
|---|
| RT |
| U6 |
AACGCTTCACGAATTTGCGT |
|
miR-128 |
GTCGTATCCAGTGCAGGGTCCGA |
|
|
GGTATTCGCACTGGATACGACAAAGAG |
| RT-qPCR |
|
| U6 |
|
Forward |
CTCGCTTCGGCAGCACA |
|
Reverse |
AACGCTTCACGAATTTGCGT |
| miR-128 |
|
Forward |
GGCTCACAGTGAACCGG |
|
Reverse |
GTGCAGGGTCCGAGGT |
| GAPDH |
|
Forward |
TGCACCACCAACTGCTTAG |
|
Reverse |
GATGCAGGGATGATGTTC |
| P38 |
|
Forward |
CCTGATGATGAGCCTGTTGC |
|
Reverse |
GAGAAGGTCTTCCCCTCACA |
| IL-6 |
|
Forward |
AGTGGCTAAGGACCAAGACC |
|
Reverse |
ACCACAGTGAGGAATGTCCA |
| IL-10 |
|
Forward |
TGGACTCCAGGACCTAGACA |
|
Reverse |
GTCCCCAATGGAAACAGCTT |
| IL-12 |
|
Forward |
CATCCTGTGTCACATTGACACTTGTG |
|
Reverse |
GCTTTGAGTCAAATCCAGAACATGC |
Western blot analysis
Firstly, murine B16 melanoma cells were lysed using
a lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China). Lysate concentration were measured using a Bradford assay.
Following centrifugation at 4,800 × g for 5 min at 4°C, the lysates
were boiled in sodium dodecyl sulfate (SDS) loading buffer for 5
min. Proteins (50 µg) were separated by 10% SDS-PAGE, and then
transferred to a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked with 5% Tris-buffered
saline (TBS), 0.3% Tween-20, 5% non-fat milk for 2 h at 37°C, and
incubated at 4°C overnight with primary antibody of rabbit
polyclonal anti-p38 (1:5,000; catalog no. 9212; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA).
Following washing with 0.1% Tween-TBS three times,
the membrane was further incubated with the horseradish peroxidase
conjugated secondary antibody goat anti-rabbit IgG-H&L
(1:10,000; catalog no. A21076, Bioworld Technology, Inc., St. Louis
Park, MN, USA) for 1 h at 25°C. Protein signals were visualized by
using the enhanced chemiluminescence western blot detection system
(EMD Millipore), and β-actin was used as the internal control.
ELISA
The transfected immature DC cells were stimulated
with 1 µg/ml lipopolysaccharide (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to induce activation and maturation. Cell
supernatants were collected at 6, 12, 24 and 48 h time points and
the secretion of cytokines including interleukin (IL)-6, −10 and
−12 were measured using commercially available ELISA kits (catalog
no. DY726; R&D Systems, Inc., Minneapolis, MN, USA).
Animal model of melanoma
B16 melanoma cells were maintained under the
aforementioned conditions. The cells were harvested by
centrifugation at 600 × g for 5 min, resuspended in PBS at a
density of 1×106, and subcutaneously inoculated into the
right flank of the C57BL/6 mice (100 µl/mice). The tumor growth of
each mouse was monitored every 2 days. The model of melanoma was
considered as successfully established if the tumor size was able
to be measured on the 12th day following injection. Then, mice
bearing B16 melanoma were randomly allocated into 5 groups, and
there were 6 mice in each group: i) untreated, ii) negative control
DCs treated, iii) miR-128 mimic treated, iv) miR-128 inhibitor
treated and v) p38 treated groups. DCs harboring miR-128 mimic or
inhibitor were injected intratumorally (1×106/mice) once
every week. During this period, a subcutaneous Buprenorphine
(Temgesic; 0.05 mg/kg) treatment was applied to minimize the
suffering of mice. The tumor size was determined every two days.
The mice were sacrificed using the cervical dislocation method when
the tumor size reached 3,000 mm3. Tumor volume was
expressed as width2 × length × π/6. Euthanasia was
conducted if the mouse exhibited a moribund state including severe
mobility loss, hunched back, piloerection, ruffled fur and weight
loss. The survival time was defined as the period between tumor
inoculation and this endpoint.
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using SPSS software, version 19.0 (IBM SPSS,
Armonk, NY, USA). Differences were analyzed using the unpaired
Student's t-test for comparisons between two groups, and one-way
analysis of variance followed by Bonferroni's multiple comparison
test, for comparison among three or more groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
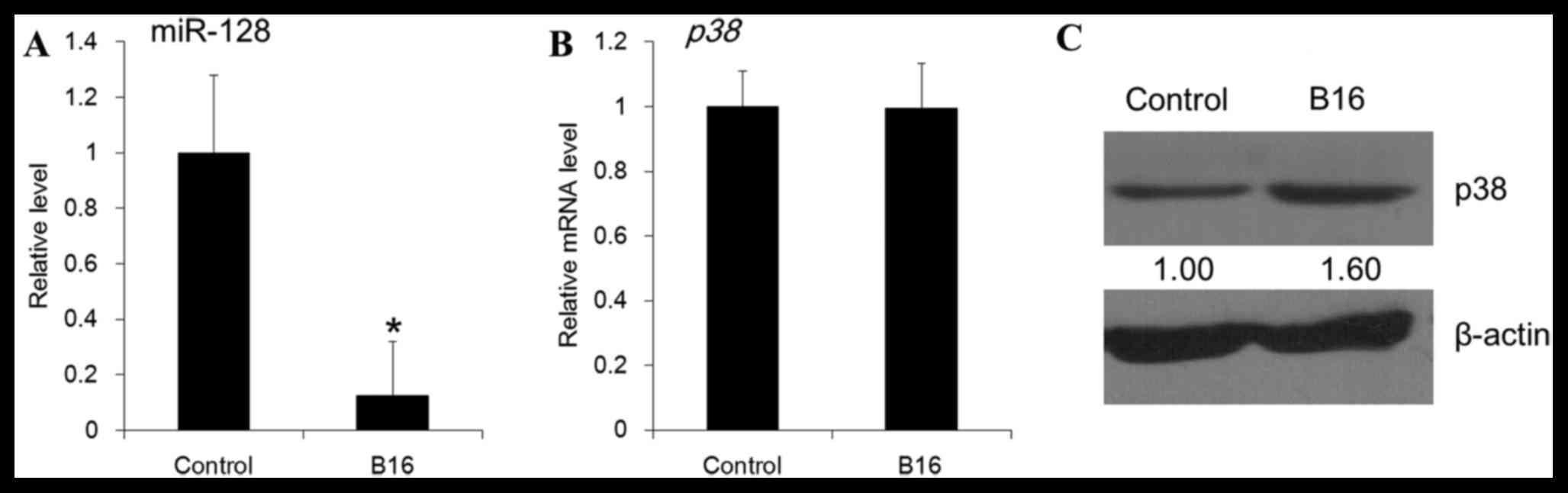
miR-128 and p38 levels vary in DCs as
a response to B16 stimulation
To explicate the roles of miR-128 and p38, the
present study firstly determined the corresponding levels in DCs,
as a response to B16 cells. Co-culturing with the B16 cell
suspension resulted in a decreased level of miR-128 in the DCs
(P=0.02; Fig. 1A). In addition,
the mRNA abundance of p38 in DCs was not significantly
altered following B16 stimulation (P=0.35; Fig. 1B), whereas the p38 protein level
was demonstrated to be increased (Fig.
1C).
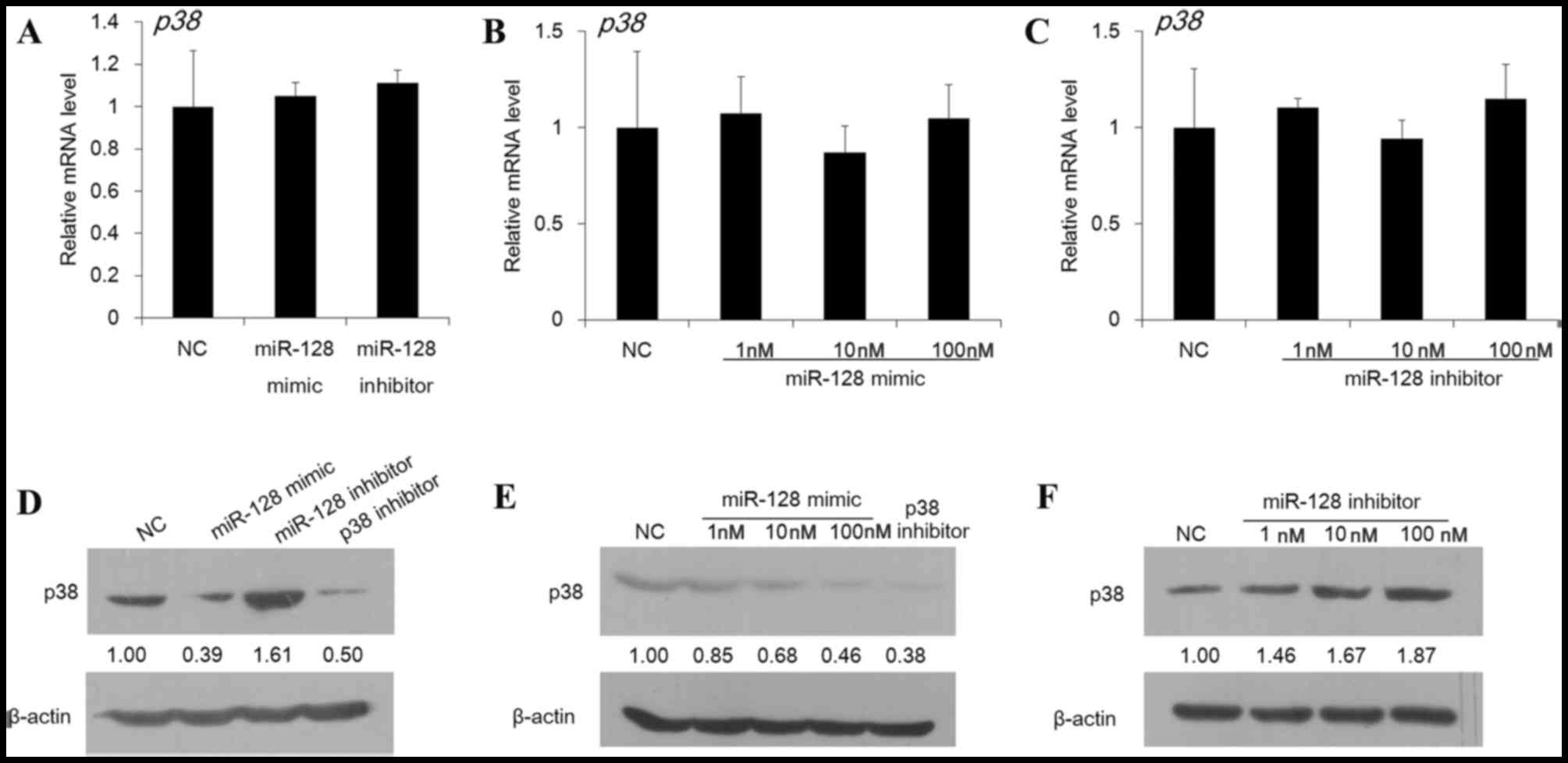
miR-128 downregulates p38 protein
level in DCs
The effect of miR-128 on p38 expression in DC cells
was next assessed at the mRNA and protein level. As presented in
Fig. 2A-C, no significant
difference was observed in the p38 mRNA abundance in DCs
following treatment with miR-128 mimic or inhibitor at a dose of 1,
10 or 100 nM (P>0.05). However, the protein expression of p38
was markedly suppressed by miR-128 mimic, whereas the miR-128
inhibitor enhanced the protein level (Fig. 2D). As presented in Fig. 2E and F, effects of the miR-128
mimic and inhibitor were observed to be exhibited in a dose
dependent manner. These findings therefore demonstrated a
post-transcriptional regulation of p38 by miR-128.
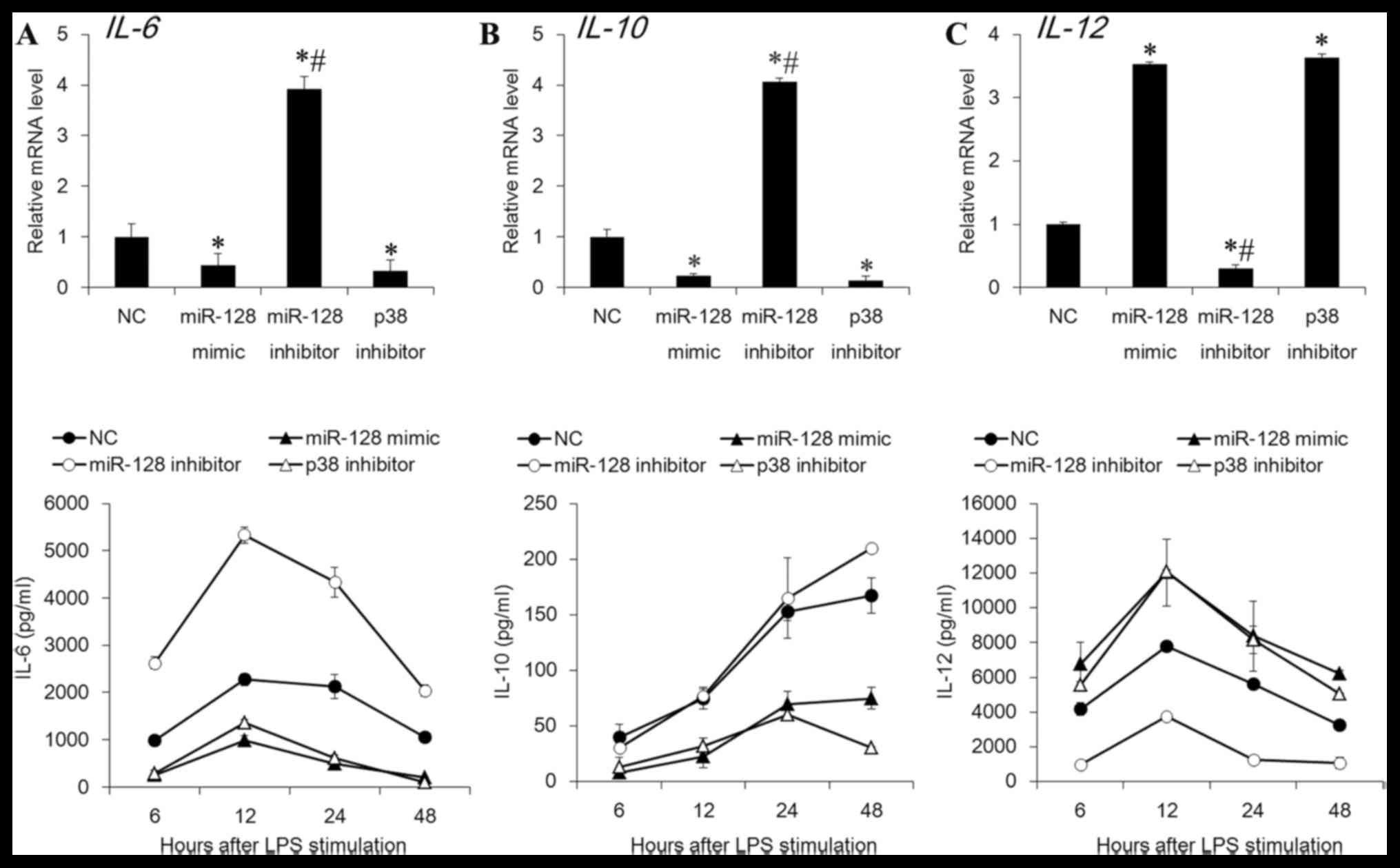
miR-128 regulates the expression of
cytokines in DCs
p38 has previously been demonstrated to be important
in the regulation of the expression of cytokines (24,25).
miR-128 inhibited the p38 expression in DCs, therefore the present
study further detected if the downstream cytokines of p38 were
modulated by miR-128. As presented in Fig. 3, the gene expression and secretion
levels of IL-6 and IL-10 were significantly suppressed by miR-128
mimic or p38 inhibitor, whereas the levels of IL-12 were increased
(P<0.05). Conversely, the miR-128 inhibitor demonstrated the
opposite effects (P<0.05). These data indicated that miR-12
regulated the expression of cytokines via inhibition of the p38
MAPK signaling pathway.
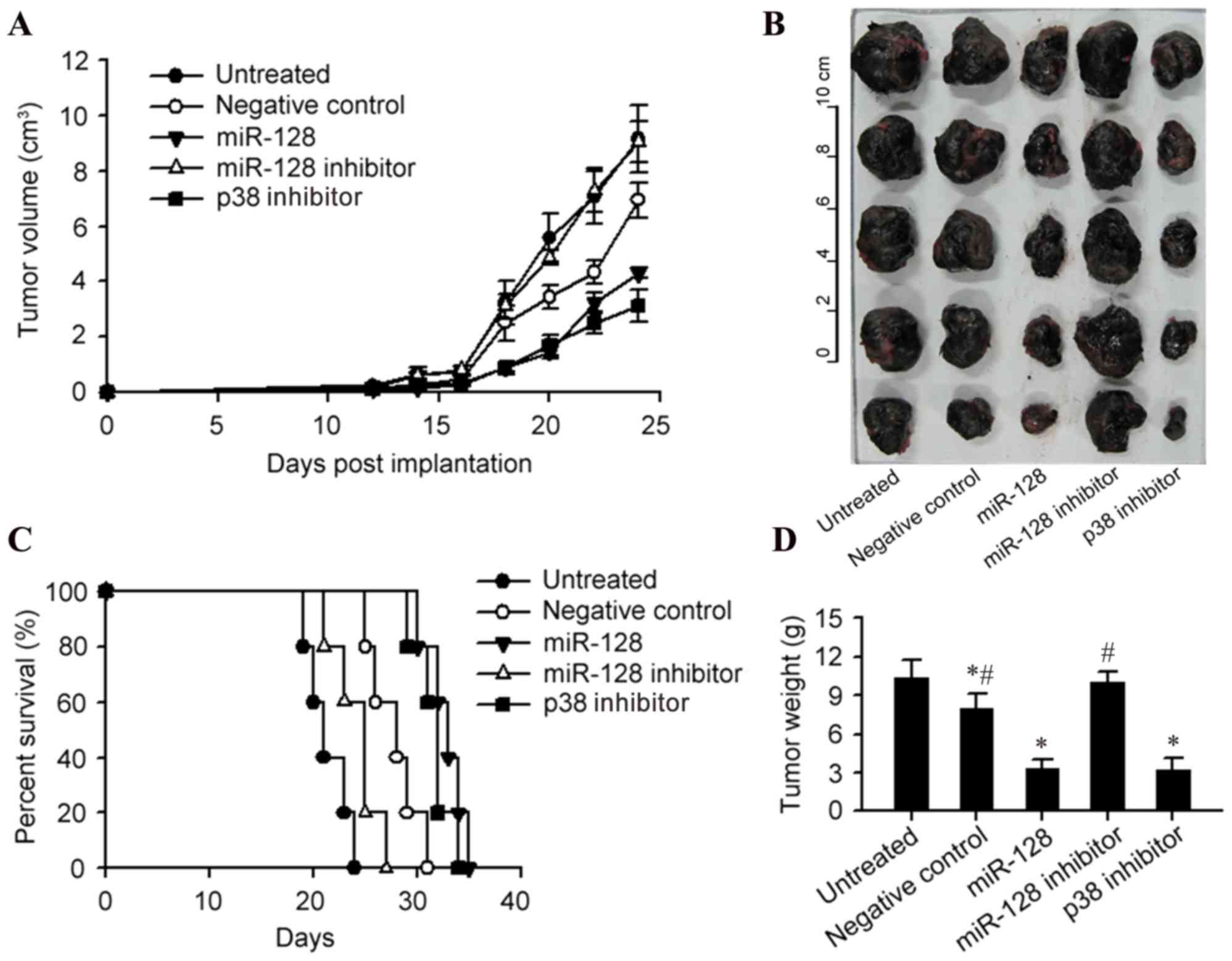
miR-128 enhances the anti-tumor effect
of DCs
The present study then detected the influence of
miR-128 and p38 on tumor growth in the C57BL/6 mice bearing B16
melanoma. Injection of DCs harboring miR-128 mimic or p38 inhibitor
significantly improved the therapeutic effect of DCs on melanoma,
compared with the negative control DCs, which was reflected by
retarded tumor growth, decreased tumor size and weight and a
prolonged survival time (P<0.05; Fig. 4A-D). Conversely, the miR-128
inhibitor increased the tumor size and weight compared with the
negative control (P<0.05; Fig.
4D).
Discussion
DC vaccines are currently the primary,
investigational therapeutic approach against solid tumors. In the
tumor environment, immature DCs have been revealed to accumulate
whereas the functional mature DC total is decreased. The immature
DCs are able to present tumor-derived antigens, however are unable
to express co-stimulatory molecules, including cluster of
differentiation (CD) 80 and CD86, adequate levels of major
histocompatibility complex (MHC) molecules and appropriate
cytokines that are required to active T cells (14). Therefore, the use of genetically
modified DCs is of great research interest. The present study
attempted to identify an improved immunotherapeutic strategy to
target cancer, using DCs.
miRNAs are a class of non-coding RNAs that regulate
genes by binding to their 3′-untranslated regions. Various miRNAs
have previously been demonstrated to be important in the actions of
the immune system. miR-128, a well-recognized tumor inhibitor, is
capable of inhibiting Th2 differentiation and facilitating the Th1
response (26,27), suggesting its potential application
in immunosuppressive therapy. However, its role in DC-mediated
anti-tumor immunity remains to be elucidated. In the present study,
it was observed that the expression level of miR-128 was
significantly decreased in DCs following stimulation with the
supernatant of B16 cells. As DCs are recommended as
immunotherapeutic strategy against cancer, the alteration of
miR-128 in DCs after B16 stimulation suggests that expression of
miR-128 may be associated with an immune reaction in melanoma.
Furthermore, the miR-128 mimic or inhibitor was
introduced into mouse bone marrow-derived DCs, and subsequently
injected into mice bearing B16 melanoma. The results revealed that
miR-128 induced objective tumor shrinkage and prolonged the
survival time, verifying its previously established tumor
inhibitory effect.
p38 is the target gene of miR-128, which was
verified with the observation that miR-128 inhibited the protein
level of p38 in DCs without affecting the mRNA abundance. In
addition, the p38 protein expression levels were enhanced in DCs
following B16 stimulation. Therefore, it was hypothesized that the
miR-128/p38 regulation pattern may be important in the DC
anti-tumor functionality. p38 exerts regulatory effects on
cytokines which have pivotal effects on anti-tumor immunity,
therefore the expression of genes encoding various cytokines and
the secretion of these cytokines were tested following an
introduction of miR-128 mimic, miR-128 inhibitor or p38 inhibitor
into DCs. IL-6 may alter the differentiation route of monocytes to
macrophages rather than DCs, which would block the priming of
tumor-specific T cells by DCs (28). IL-6 additionally functions in
maintaining immature DCs and obstructing the maturation of DCs via
triggering the activation of signal transducer and activator of
transcription (STAT) 3 (29),
which underlines an inhibitory pathway of p38/IL-6/STAT3 in DC
activation. In addition to IL-6, IL-10 is a critical cytokine
blocking the maturation of DCs. By secreting the immunosuppressive
cytokine IL-10, tumors may inhibit the maturation of DCs or convert
DCs into macrophage-like cells (30,31).
Direct addition of anti-IL-10-neutralizing Ab to immature DCs,
augments the expression of MHC and co-stimulatory molecules and the
release of IL-12 (32). High
expressions of MHC class II molecules and co-stimulatory molecules
are associated with the maturation of DCs. The mature DCs have the
ability to acquire C-C motif chemokine receptor 7 that allows the
migration of mature DCs into the draining lymph node (33,34).
Furthermore, the generated mature DCs acquire the ability to induce
the differentiation of CD4+ and CD8+ T cells
into antigen-specific T cells, and therefore activate the CTL
response to destroy the tumor cells (8). It has previously been demonstrated
that DC cells derived from mice overexpressing IL-10 markedly
restrain the T cell and CTL responses and IL-12 production
(35). The results of the present
study demonstrated that IL-6 and IL-10 production were attenuated
by miR-128 mimic and p38 inhibitor, whereas the IL-12 level was
promoted. The miR-128 inhibitor demonstrated the opposite effect on
the cytokine production levels. Jarnicki et al (36) suggests that inhibition of p38
signaling suppresses IL-10 and enhances IL-12 production in
lipopolysaccharide-activated DCs, which increases the
immunotherapeutic efficacy of DCs (36). Hence, combined with the fact that
p38 is the target of miR-128, the inhibitory effect of
miR-128 overexpressed DCs on tumor growth may be attributed to the
generation of appropriate cytokines.
In conclusion, the results of the present study
suggested that miR-128 post-transcriptionally inhibited p38
expression in DCs and suppressed the downstream levels of cytokines
secreted by DCs via decreasing the expression levels of their
encoding genes, which consequently enhanced the anti-tumor immune
response and inhibited tumor growth. The facilitation of
DC-mediated anti-tumor immunity via miR-128 in the tumor
microenvironment provides a novel strategy of immunotherapeutic
value against various malignancies, including melanoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31470876, 91029736
and 31200676), ISF-NSFC program (grant no. 31461143010), the
Ministry of Science and Technology (863 program, grant no.
2008AA02Z129), the National Key Scientific Program (grant no.
2011CB964902), the Program for Changjiang Scholars and Innovative
Research Team in University (grant no. IRT13023) and the
China Postdoctoral Science Foundation funded project (grant no.
2015M571272).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo J, Qin S, Liang J, Lin T, Si L, Chen
X, Chi Z, Cui C, Du N, Fan Y, et al: Chinese guidelines on the
diagnosis and treatment of melanoma (2015 Edition). Ann Transl Med.
3:3222015.PubMed/NCBI
|
|
3
|
Henderson MA, Burmeister B, Ainslie J,
Fisher R, Di Iulio J, Smithers BM, Hong A, Shannon KF, Scolyer RA,
Carruthers S, et al: Adjuvant radiotherapy after lymphadenectomy in
melanoma patients: Final results of an intergroup randomized trial
(ANZMTG 0.1. 02/TROG 02.01). J Clin Oncol. 31 Suppl:S90012013.
|
|
4
|
Walker L, Schalch H, King DM, Dietrich L,
Eastman M, Kwak M, Kim K and Albertini MR: Phase II trial of weekly
paclitaxel in patients with advanced melanoma. Melanoma Res.
15:453–459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avril MF, Aamdal S, Grob JJ, Hauschild A,
Mohr P, Bonerandi JJ, Weichenthal M, Neuber K, Bieber T, Gilde K,
et al: Fotemustine compared with dacarbazine in patients with
disseminated malignant melanoma: A phase III study. J Clin Oncol.
22:1118–1125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mackensen A, Herbst B, Chen JL, Köhler G,
Noppen C, Herr W, Spagnoli GC, Cerundolo V and Lindemann A: Phase I
study in melanoma patients of a vaccine with peptide-pulsed
dendritic cells generated in vitro from CD34(+) hematopoietic
progenitor cells. Int J Cancer. 86:385–392. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oshita C, Takikawa M, Kume A, Miyata H,
Ashizawa T, Iizuka A, Kiyohara Y, Yoshikawa S, Tanosaki R, Yamazaki
N, et al: Dendritic cell-based vaccination in metastatic melanoma
patients: Phase II clinical trial. Oncol Rep. 28:1131–1138.
2012.PubMed/NCBI
|
|
12
|
Engell-Noerregaard L, Hansen TH, Andersen
MH, Straten P Thor and Svane IM: Review of clinical studies on
dendritic cell-based vaccination of patients with malignant
melanoma: Assessment of correlation between clinical response and
vaccine parameters. Cancer Immunol Immunother. 58:1–14. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bol KF, Aarntzen EH, Hout FE, Schreibelt
G, Creemers JH, Lesterhuis WJ, Gerritsen WR, Grunhagen DJ, Verhoef
C, Punt CJ, et al: Favorable overall survival in stage III melanoma
patients after adjuvant dendritic cell vaccination. Oncoimmunology.
5:e10576732016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gabrilovich D: Mechanisms and functional
significance of tumour-induced dendritic-cell defects. Nat Rev
Immunol. 4:941–952. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie J, Qian J, Yang J, Wang S, Freeman ME
III and Yi Q: Critical roles of Raf/MEK/ERK and PI3K/AKT signaling
and inactivation of p38 MAP kinase in the differentiation and
survival of monocyte-derived immature dendritic cells. Exp Hematol.
33:564–572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Hong S, Yang J, Qian J, Zhang X,
Shpall E, Kwak LW and Yi Q: Optimizing immunotherapy in multiple
myeloma: Restoring the function of patients' monocyte-derived
dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and
neutralizing interleukin-6 in progenitor cells. Blood.
108:4071–4077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lawson SK, Dobrikova EY, Shveygert M and
Gromeier M: p38α mitogen-activated protein kinase depletion and
repression of signal transduction to translation machinery by
miR-124 and −128 in neurons. Mol Cell Biol. 33:127–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi ZM, Wang J, Yan Z, You YP, Li CY, Qian
X, Yin Y, Zhao P, Wang YY, Wang XF, et al: MiR-128 inhibits tumor
growth and angiogenesis by targeting p70S6K1. PLoS One.
7:e327092012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Cheng Y, Li Y, Jin Z, Pan Y, Liu G,
Fu S, Zhang Y, Feng K and Feng Y: microRNA-128 plays a critical
role in human non-small cell lung cancer tumourigenesis,
angiogenesis and lymphangiogenesis by directly targeting vascular
endothelial growth factor-C. Eur J Cancer. 50:2336–2350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li M, Fu W, Wo L, Shu X, Liu F and Li C:
miR-128 and its target genes in tumorigenesis and metastasis. Exp
Cell Res. 319:3059–3064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Liu Q, Che Y, Yuan X, Dai L, Zeng
B, Jiao G, Zhang Y, Wu X, Yu Y, et al: Antigen presentation by
dendritic cells in tumors is disrupted by altered metabolism that
involves pyruvate kinase M2 and its interaction with SOCS3. Cancer
Res. 70:89–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min S, Liang X, Zhang M, Zhang Y, Mei S,
Liu J, Su X, Cao S, Zhong X, Li Y, et al: Multiple tumor-associated
microRNAs modulate the survival and longevity of dendritic cells by
targeting YWHAZ and Bcl2 signaling pathways. J Immunol.
190:2437–2446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anderson P: Post-transcriptional control
of cytokine production. Nat Immunol. 9:353–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Godlewski J, Nowicki MO, Bronisz A,
Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca
EA and Lawler S: Targeting of the Bmi-1 oncogene/stem cell renewal
factor by microRNA-128 inhibits glioma proliferation and
self-renewal. Cancer Res. 68:9125–9130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guerau-de-Arellano M, Smith KM, Godlewski
J, Liu Y, Winger R, Lawler SE, Whitacre CC, Racke MK and
Lovett-Racke AE: Micro-RNA dysregulation in multiple sclerosis
favours pro-inflammatory T-cell-mediated autoimmunity. Brain.
134:3578–3589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chomarat P, Banchereau J, Davoust J and
Palucka AK: IL-6 switches the differentiation of monocytes from
dendritic cells to macrophages. Nat Immunol. 1:510–514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park SJ, Nakagawa T, Kitamura H, Atsumi T,
Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, et al:
IL-6 regulates in vivo dendritic cell differentiation through STAT3
activation. J Immunol. 173:3844–3854. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McBride JM, Jung T, de Vries JE and Aversa
G: IL-10 alters DC function via modulation of cell surface
molecules resulting in impaired T-cell responses. Cell Immunol.
215:162–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fortsch D, Röllinghoff M and Stenger S:
IL-10 converts human dendritic cells into macrophage-like cells
with increased antibacterial activity against virulent
Mycobacterium tuberculosis. J Immunol. 165:978–987. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Corinti S, Albanesi C, la Sala A, Pastore
S and Girolomoni G: Regulatory activity of autocrine IL-10 on
dendritic cell functions. J Immunol. 166:4312–4318. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trombetta ES and Mellman I: Cell biology
of antigen processing in vitro and in vivo. Annu Rev Immunol.
23:975–1028. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clatworthy MR, Aronin CE, Mathews RJ,
Morgan NY, Smith KG and Germain RN: Immune complexes stimulate
CCR7-dependent dendritic cell migration to lymph nodes. Nat Med.
20:1458–1463. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sharma S, Stolina M, Lin Y, Gardner B,
Miller PW, Kronenberg M and Dubinett SM: T cell-derived IL-10
promotes lung cancer growth by suppressing both T cell and APC
function. J Immunol. 163:5020–5028. 1999.PubMed/NCBI
|
|
36
|
Jarnicki AG, Conroy H, Brereton C,
Donnelly G, Toomey D, Walsh K, Sweeney C, Leavy O, Fletcher J,
Lavelle EC, et al: Attenuating regulatory T cell induction by TLR
agonists through inhibition of p38 MAPK signaling in dendritic
cells enhances their efficacy as vaccine adjuvants and cancer
immunotherapeutics. J Immunol. 180:3797–3806. 2008. View Article : Google Scholar : PubMed/NCBI
|