Introduction
Neuropathic pain is a major chronic condition
arising from injury or disease affecting the peripheral or central
nervous system (1). It is
characterized by hyperalgesia, allodynia and spontaneous pain.
Nowadays, neuropathic pain has become a significant public health
problem, affecting ~10–40% of the general population (2). Despite immense advances in treatment
strategies, the effective treatment of patients suffering from
neuropathic pain remains challenging (3,4).
Thus, it is urgent to investigate effective and nontoxic analgesics
for the management of neuropathic pain.
Accumulating evidence has demonstrated that nerve
injury-induced inflammatory cytokines and reactive oxygen species
(ROS) serve important roles in the progress of neuropathic pain
(5–7). Nerve damage causes the upregulation
of inflammatory mediators, including tumor necrosis factor (TNF)-α
and interleukin (IL)-1β (8,9).
Nuclear factor (NF)-κB, a critical regulator of inflammatory
process, has also been demonstrated to be activated in neuropathic
pain (10). Therefore, inhibition
of these cytokines attenuates nerve injury-induced allodynia.
Diosgenin is a steroidal saponin extract from
numerous plants, including Solanum and Dioscorea
species. Increasing evidences have reported that diosgenin has
multiple pharmacological activities, including anti-inflammatory,
anti-oxidant and anti-cancer properties (11–13).
In addition, diosgenin has been reported to exert neuroprotective
activity. For example, diosgenin significantly improved memory
function and reduced axonal degeneration in an Alzheimer's disease
mouse model (14). However, the
role of diosgenin in neuropathic pain remains unclear. The present
study examined the effects of diosgenin on allodynia, and the
levels of inflammatory mediators in rats following neuropathic pain
evoked by chronic constriction injury (CCI). In addition, the
underlying molecular mechanisms involved in the diosgenin-induced
suppression of neuropathic pain were investigated.
Materials and methods
Animals
Male Sprague-Dawley rats (n=25) weighing 180–200 g
were supplied by the Experimental Animal Centre of Zhengzhou
Central Hospital Affiliated to Zhengzhou University (Zhengzhou,
China). The animals were housed in a room maintained at 22±1°C with
an alternating 12-h light/dark cycle, and provided food and water
ad libitum. The animal experimental procedures were approved and
reviewed by the Institutional Animal Care and Use Committee of
Zhengzhou Central Hospital Affiliated to Zhengzhou University.
Induction of neuropathic pain
Neuropathic pain was induced in experimental animals
by CCI of the sciatic nerve which was performed as previously
described (15). In brief, rats
were anesthetized intraperitoneally with 40 mg/kg sodium
pentobarbital (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Four
ligatures (silk 4–0) were tied loosely around proximal bifurcation
part of the nerve with 1 mm spacing between each ligature, until a
brisk twitch of the right hind limb was observed. Sham surgery was
performed with the sciatic nerve exposed but not ligated in control
rats (n=3 per group).
Drug treatment
Diosgenin in doses of 10, 20 and 40 mg/kg were
administered intraperitoneally to neuropathic rats once a day for
two weeks, starting from the first day following the induction of
neuropathic pain; the sham-operated rats received normal saline (20
µl) alone, following the same treatment procedure. The rats were
sacrificed by spinal dislocation 24 h after the last
administration.
Evaluation of mechanical allodynia and
thermal hyperalgesia
Mechanical allodynia was evaluated as indicated by
the paw withdrawal threshold in response to von Frey filaments
using the up-down method according to previously described protocol
(16). In brief, rats were placed
in an inverted clear plexiglass cage (23×18×13 cm) on a 3-mm-thick
glass plate, and were allowed to acclimatize for 30 min before
testing. The plantar surface of each hind paw was applied with
pressure from below with the electronic Von Frey filament via the
mesh floor. The force applied at the time of paw withdrawal was
recorded.
Heat hypersensitivity was tested using a plantar
test (cat. no. 7370; Ugo Basile Srl, Varese, Italy) according to a
method described previously (17).
In brief, the heat source was positioned under the glass floor
directly beneath the hind paw. The heat intensity was set to last
for ~10 sec to produce paw withdrawal latency, and the cut-off was
set at 20 sec to avoid tissue damage. Each paw was measured
alternatively after >5 min.
Western blot analysis
At day 14, the rats were sacrificed by spinal
dislocation. Then, the lumbar spinal cord tissues (L4/5) were
rapidly removed. Proteins were extracted from the lumbar spinal
cord tissues (L4/5) using RIPA Cell Lysis Buffer (Takara
Biotechnology, Dalian, China). Lysates were sonicated for 5 sec on
ice and centrifuged at 6,000 × g for 10 min at 4°C. Supernatants
were collected and the protein concentration was quantified using a
Pierce Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Equal amounts of protein (30
µg) were separated by 10% SDS-PAGE and subsequently transferred to
polyvinylidene difluoride membranes. The membrane was blocked with
5% non-fat dry milk in Tris-buffered saline with 0.1% Tween-20
(TBST) for 1 h at room temperature. The membrane was then incubated
with a 1:1,000 dilution of the following primary antibodies, all
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA):
rabbit anti-mouse phosphorylated (p)-p38 mitogen activated protein
kinase (MAPK) antibody (sc-101759; 1:3,000), rabbit anti-mouse p38
MAPK antibody (sc-535; 1:2,500), rabbit anti-mouse p-NF-κB p65
antibody (sc-33020; 1:3,000;) and rabbit anti-mouse GAPDH antibody
(sc-25778; 1:2,500) overnight at 4°C. Following three washes with
TBST buffer, the membrane was washed and incubated with a goat
anti-rabbit IgG horseradish peroxidase-conjugated secondary
antibody (sc-2030; 1:2,500) for 1 h at 37°C. Proteins were
subsequently detected by Enhanced Chemiluminescence (GE Healthcare
Life Sciences, Chalfont, UK) and quantified using Gel-Pro Analyzer
version 4.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Enzyme linked immunosorbent assay
(ELISA)
The levels of TNF-α, IL-1β and IL-2 in the lumbar
spinal cords were measured using commercially available rat TNF-α
(RAB0479), IL-1β (RAB0277) and IL-2 (RAB0288) ELISA kits
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Plates were read using an ELISA reader (Omega Bio-Tek,
Inc., Norcross, GA, USA) at a wavelength of 450 nm.
The levels of malondialdehyde (MDA) and glutathione
peroxidase (GSH-PX) in the lumbar spinal cords were estimated by
using MDA and GSH-PX kits from the Biological Engineering Research
Institute (Nanjing, China).
Statistical analysis
Analysis was performed using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA). All data are presented as the mean
± standard deviation. The data of behavioral tests were analyzed by
two-way analysis of variance, while the data of cytokine assays
were analyzed by one-way analysis of variance, followed by
Newman-Keuls post hoc test. P<0.05 were considered to indicate a
statistically significant difference.
Results
Effect of diosgenin on mechanical
allodynia and thermal hyperalgesia
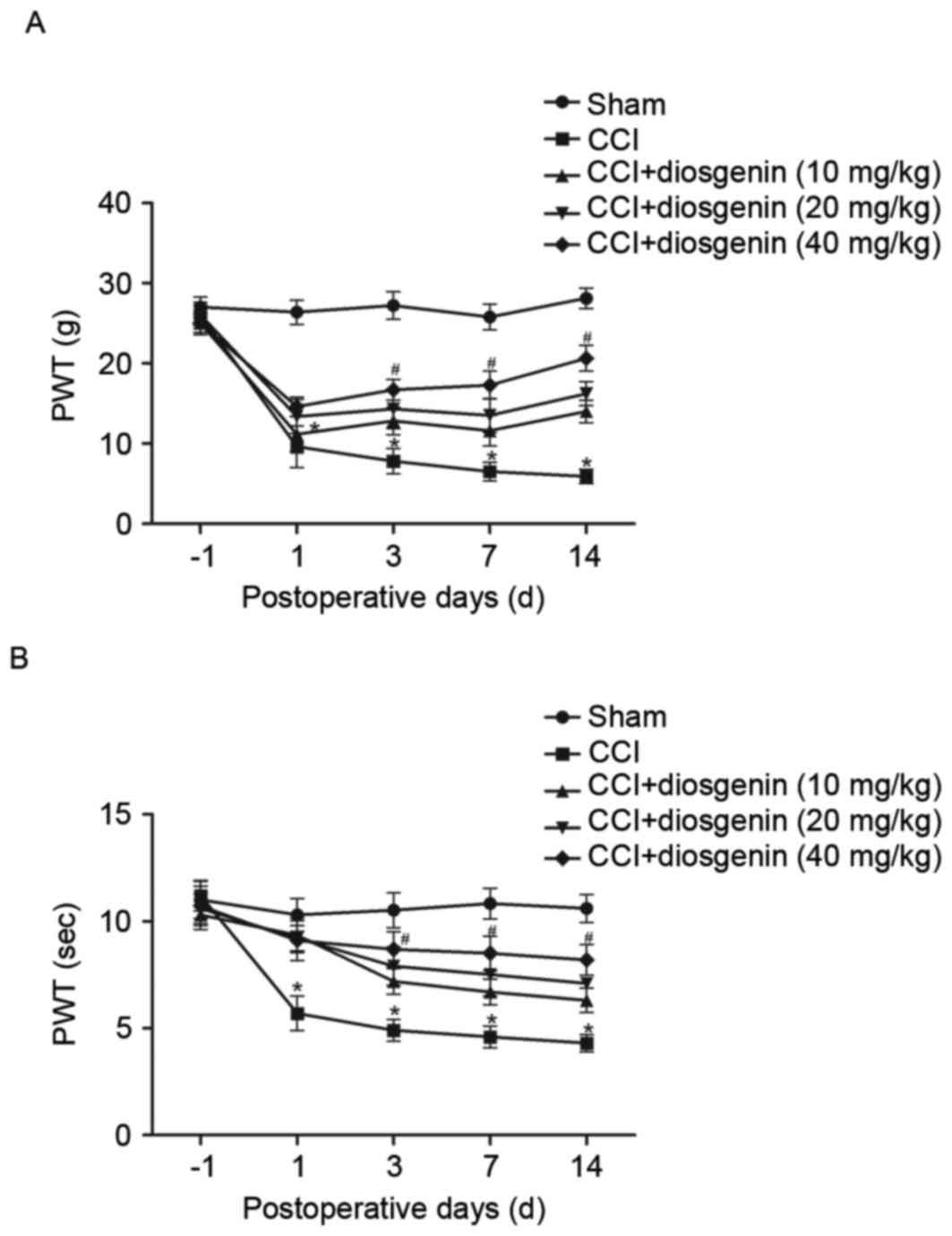
The effects of diosgenin on mechanical allodynia and
thermal hyperalgesia were examined. CCI resulted in significant
development of mechanical allodynia (Fig. 1A) and thermal hyperalgesia
(Fig. 1B), as compared with the
sham group as assessed on day 1, 7 and 14. However, diosgenin
treatment reversed CCI-induced mechanical allodynia and thermal
hyperalgesia in a dose-dependent manner.
Effect of diosgenin on
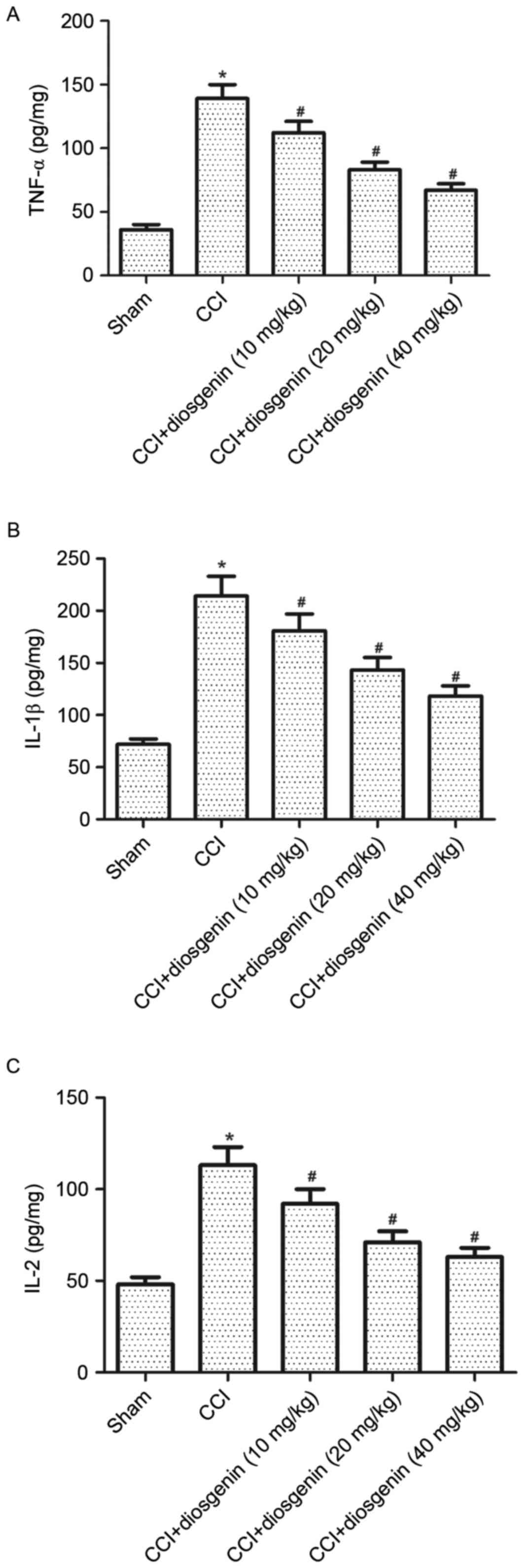
pro-inflammatory cytokine levels in the spinal cord
There is strong evidence that pro-inflammatory
cytokines have important roles in the pathology of neuropathic
pain. Thus, the present study examined the effects of diosgenin on
pro-inflammatory cytokine levels in spinal cord by ELISA. The
levels of TNF-α (Fig. 2A), IL-1β
(Fig. 2B) and IL-2 (Fig. 2C) were significantly increased in
the spinal cord of CCI rats compared with the sham group. However,
diosgenin reversed CCI-increased levels of TNF-α, IL-1β and IL-2 in
a dose-dependent manner.
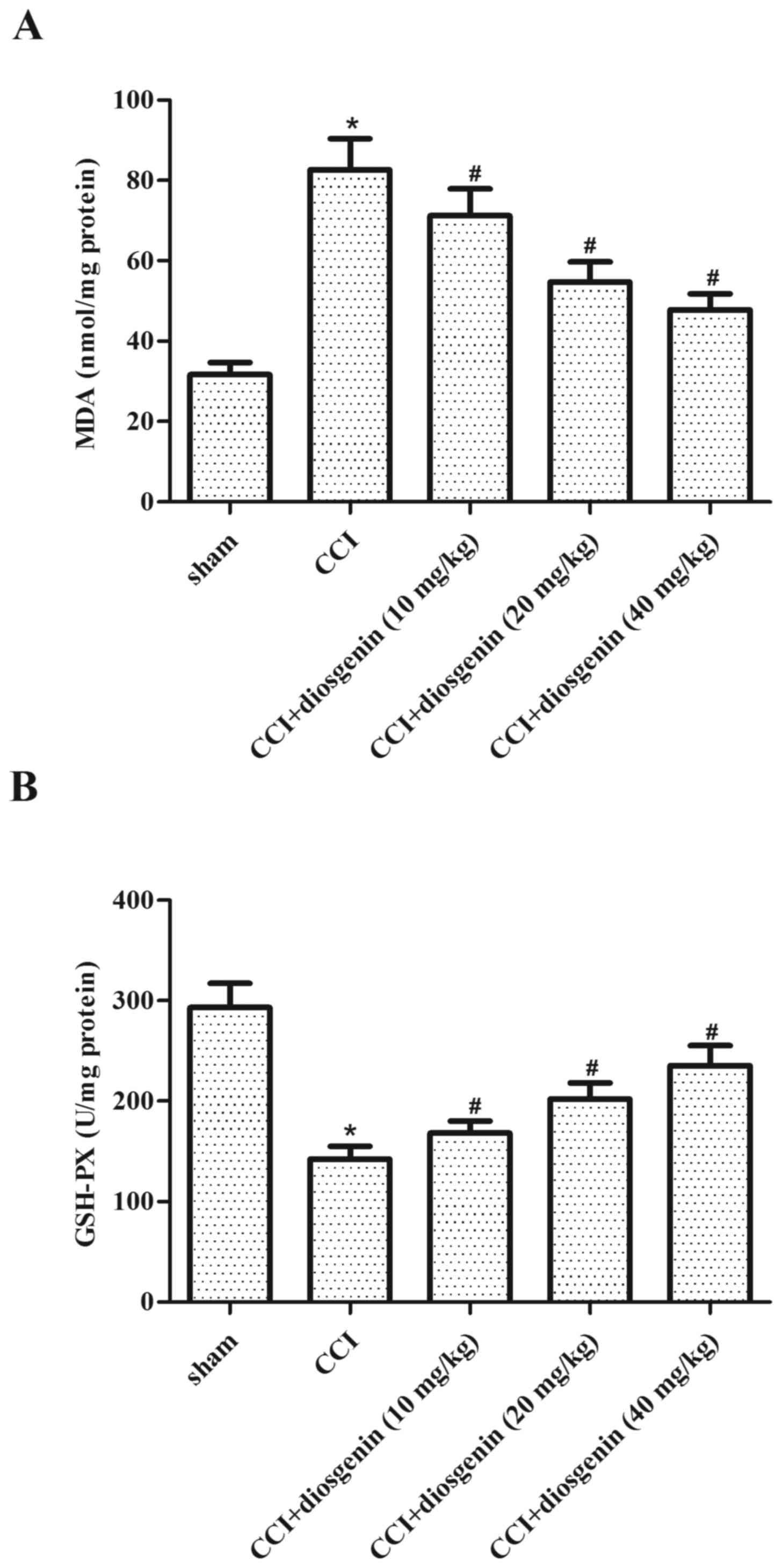
Effect of diosgenin on oxidative
stress in the spinal cord, following CCI
The effects of diosgenin on oxidative stress in
spinal cord were examined by ELISA. Rats in the CCI group exhibited
a significant increase in the production of MDA (Fig. 3A) and decrease in the content of
GSH-PX (Fig. 3B), compared with
the sham group. Diosgenin treatment obviously reversed CCI-induced
oxidative stress in spinal cord in a dose-dependent manner.
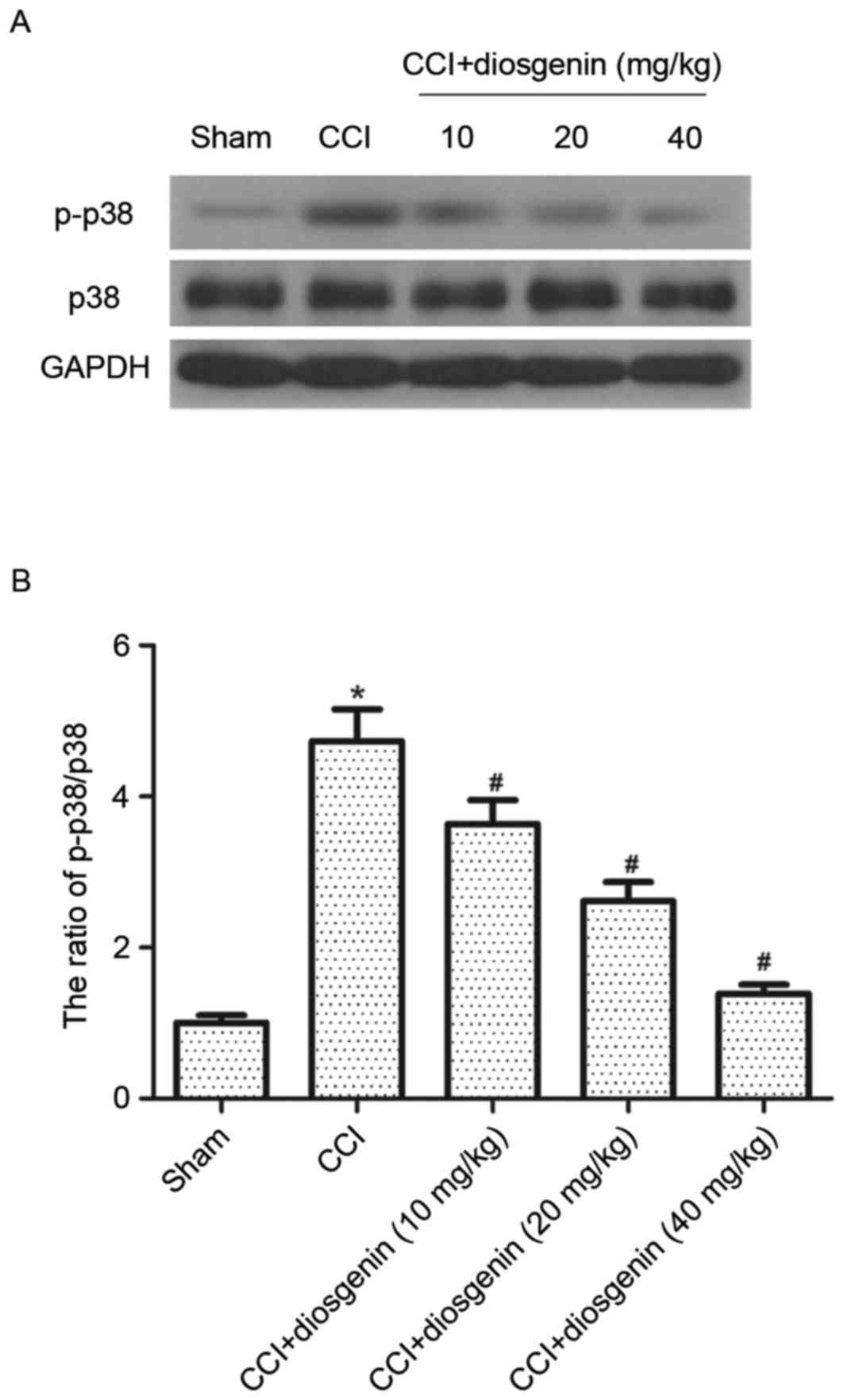
Effect of diosgenin on p-p38 MAPK in
the spinal cord, following CCI
It has been reported that activation of p-p38 MAPK
contributes to the development of inflammatory and neuropathic pain
induced by nerve injury. Therefore, the effects of diosgenin on
phosphorylation of p38 MAPK in spinal cord were investigated. As
presented in Fig. 4, protein
expression levels of p-p38 MAPK were greatly increased by CCI,
compared with the sham group. However, diosgenin treatment
significantly inhibited the expression level of p-p38 MAPK in the
spinal cord of CCI rats.
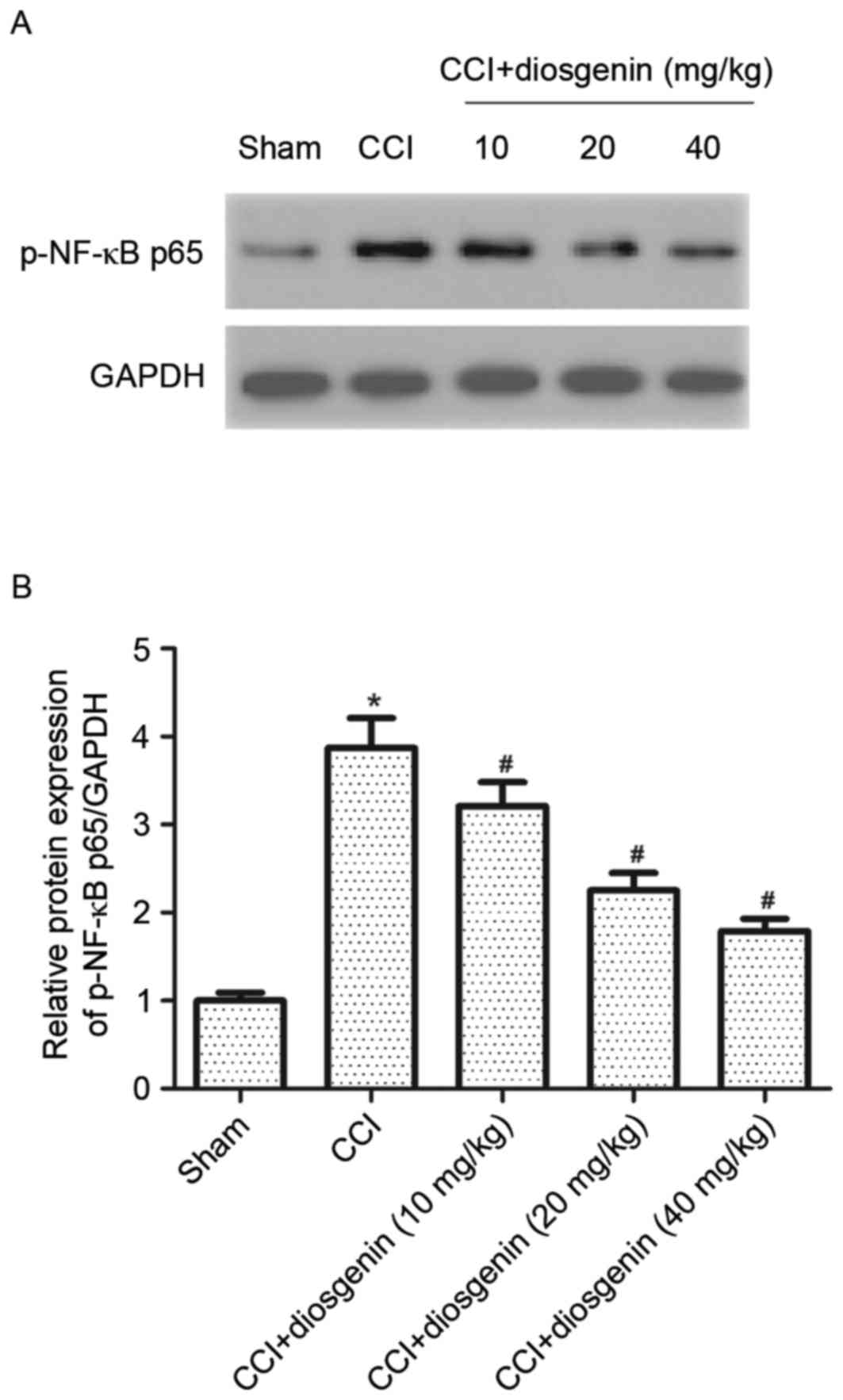
Effect of diosgenin on NF-κB
activation in the spinal cord, following CCI
The NF-κB signaling pathway serves a key role in
regulating the expression of pro-inflammatory and pain mediators.
To investigate the underlying mechanism of diosgenin in CCI-induced
neuropathic pain, protein expression levels of p-NF-κB p65 in
spinal cord of CCI rats were detected. Western blot analysis
demonstrated that the CCI group had significantly increased levels
of p-NF-κB p65, compared with the sham group. However, diosgenin
markedly decreased the expression of p-NF-κB p65 in the spinal cord
of CCI rats, in a dose-dependent manner (Fig. 5).
Discussion
The present study demonstrated that diosgenin
reversed CCI-decreased mechanical withdrawal threshold and thermal
withdrawal latency. Diosgenin inhibited CCI-induced increased
levels of the pro-inflammatory cytokines TNF-α, IL-1β and IL-2, and
suppressed oxidative stress induced by CCI in the spinal cord.
Furthermore, diosgenin significantly inhibited protein expression
levels of p-p38 MAPK and NF-κB in the spinal cord induced by
CCI.
The CCI model is the most commonly employed
neuropathic pain model of nerve damage-induced
allodynia/hyperalgesia (18). The
present study constructed the CCI model to investigate the effects
of diosgenin on allodynia/hyperalgesia and the levels of
inflammatory mediators in rats following neuropathic pain. It was
observed that CCI led to significant development of mechanical
allodynia and heat hyperalgesia following surgery. However,
diosgenin reversed CCI-induced mechanical allodynia and thermal
hyperalgesia in a dose-dependent manner. These data suggested that
diosgenin may attenuate neuropathic pain in a CCI model.
Increasing evidence suggests that peripheral nerve
injury contributes to neuropathic pain via upregulation of
pro-inflammatory cytokines (19–21).
TNF-α is a predominant pro-inflammatory cytokine contributing to
pain hypersensitivity following nerve damage; intrathecal injection
of a TNF-α inhibitor prior to nerve injury reduces neuropathology
and pain-associated behaviors (22). In addition, IL-1β levels increase
significantly in the sciatic nerve following CCI (23). Consistent with previous studies,
the present study demonstrated that the levels of TNF-α, IL-1β and
IL-2 were significantly increased in the spinal cord of CCI rats,
compared sham-operated rats. However, diosgenin treatment reversed
this effect in a dose-dependent manner. These results suggested
that the beneficial effects of diosgenin in CCI-induced neuropathic
pain are mediated via its attenuating effect on pro-inflammatory
mediators.
Previous studies have indicated that CCI produces
significant oxidative damage in the sciatic nerve due to the
increase in lipid peroxidation and ROS concentration (7,24).
Administration of natural and synthetic ROS scavengers reduces
allodynia and hyperalgesia in a number of neuropathic pain models
(25,26). The present study revealed that CCI
resulted in a significant increase in the production of MDA, and a
decrease in the content of GSH-PX. However, diosgenin treatment
reversed the CCI-induced oxidative stress in the spinal cord. These
results suggested that the beneficial effects of diosgenin in
CCI-induced neuropathic pain are mediated via its attenuating
effect on oxidative stress.
Previous studies have demonstrated that p-p38 MAPK
in spinal cord glial cells after peripheral nerve injury are
involved in the development of neuropathic pain (27–29).
Tsuda et al (30) reported
that administration of a p38 MAPK inhibitor attenuates the
development of nerve injury-induced tactile allodynia. Furthermore,
the NF-κB signaling pathway has been implicated in the mediation of
neuropathic pain (31–33). Intrathecal infusion of the NF-κB
inhibitor ammonium pyrrolidine dithiocarbamate improved mechanical
allodynia and downregulated the overexpression of TNF-α induced by
peri-sciatic administration of TNF (34). The present study revealed that
diosgenin significantly inhibited CCI-induced upregulated
expression levels of p-p38 MAPK and p-NF-κB p65 in the spinal cord.
These data suggested that diosgenin attenuates neuropathic pain in
CCI rats by inhibiting activation of the p38 MAPK and NF-κB
signaling pathways.
In conclusion, the present study demonstrated that
diosgenin may be effective to reduce neuropathic pain by inhibition
of activation of the p38 MAPK and NF-κB signaling pathways. These
results implicate diosgenin in the treatment of neuropathic pain,
which merits further clinical investigation.
References
|
1
|
Rowbotham MC: Mechanisms of neuropathic
pain and their implications for the design of clinical trials.
Neurology. 65 12 Suppl 4:S66–S73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neville A, Peleg R, Singer Y, Sherf M and
Shvartzman P: Chronic pain: A population-based study. Isr Med Assoc
J. 10:676–680. 2008.PubMed/NCBI
|
|
3
|
Baastrup C and Finnerup NB:
Pharmacological management of neuropathic pain following spinal
cord injury. CNS Drugs. 22:455–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finnerup NB, Otto M, McQuay H, Jensen TS
and Sindrup SH: Algorithm for neuropathic pain treatment: An
evidence based proposal. Pain. 118:289–305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dray A: Inflammatory mediators of pain.
Brit J Anaesth. 75:125–131. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DeLeo JA and Yezierski RP: The role of
neuroinflammation and neuroimmune activation in persistent pain.
Pain. 90:1–6. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HK, Park SK, Zhou JL, Taglialatela G,
Chung K, Coggeshall RE and Chung JM: Reactive oxygen species (ROS)
play an important role in a rat model of neuropathic pain. Pain.
111:116–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohtori S, Takahashi K, Moriya H and Myers
RR: TNF-alpha and TNF-alpha receptor type 1 upregulation in glia
and neurons after peripheral nerve injury: Studies in murine DRG
and spinal cord. Spine (Phila Pa 1976). 29:1082–1088. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taves S, Berta T, Chen G and Ji RR:
Microglia and spinal cord synaptic plasticity in persistent pain.
Neural Plast. 2013:7536562013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma W and Bisby MA: Increased activation of
nuclear factor kappa B in rat lumbar dorsal root ganglion neurons
following partial sciatic nerve injuries. Brain Res. 797:243–254.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung DH, Park HJ, Byun HE, Park YM, Kim
TW, Kim BO, Um SH and Pyo S: Diosgenin inhibits macrophage-derived
inflammatory mediators through downregulation of CK2, JNK,
NF-kappaB and AP-1 activation. Int Immunopharmacol. 10:1047–1054.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Son IS, Kim JH, Sohn HY, Son KH, Kim JS
and Kwon CS: Antioxidative and hypolipidemic effects of diosgenin,
a steroidal saponin of yam (Dioscorea spp.), on high-cholesterol
fed rats. Biosci Biotechnol Biochem. 71:3063–3071. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moalic S, Liagre B, Corbière C, Bianchi A,
Dauça M, Bordji K and Beneytout JL: A plant steroid, diosgenin,
induces apoptosis, cell cycle arrest and COX activity in
osteosarcoma cells. FEBS Lett. 506:225–230. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tohda C, Urano T, Umezaki M, Nemere I and
Kuboyama T: Diosgenin is an exogenous activator of 1,
25D3-MARRS/Pdia3/ERp57 and improves Alzheimer's disease pathologies
in 5XFAD mice. Sci Rep. 2:5352012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaplan SR, Bach FW, Pogrel JM, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaggi AS, Jain V and Singh N: Animal
models of neuropathic pain. Fund Clin Pharmacol. 25:1–28. 2011.
View Article : Google Scholar
|
|
19
|
Nadeau S, Filali M, Zhang J, Kerr BJ,
Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW and
Lacroix S: Functional recovery after peripheral nerve injury is
dependent on the pro-inflammatory cytokines IL-1β and TNF:
Implications for neuropathic pain. J Neurosci. 31:12533–12542.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Detloff MR, Fisher LC, McGaughy V,
Longbrake EE, Popovich PG and Basso DM: Remote activation of
microglia and pro-inflammatory cytokines predict the onset and
severity of below-level neuropathic pain after spinal cord injury
in rats. Exp Neurol. 212:337–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang JM and An J: Cytokines,
inflammation, and pain. Int Anesthesiol Clin. 45:27–37. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zanella JM, Burright EN, Hildebrand K,
Hobot C, Cox M, Christoferson L and McKay WF: Effect of etanercept,
a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the
rat chronic constriction injury model. Spine (Phila Pa 1976).
33:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
del Rey A, Yau HJ, Randolf A, Centeno MV,
Wildmann J, Martina M, Besedovsky HO and Apkarian AV: Chronic
neuropathic pain-like behavior correlates with IL-1β expression and
disrupts cytokine interactions in the hippocampus. Pain.
152:2827–2835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park ES, Gao X, Chung JM and Chung K:
Levels of mitochondrial reactive oxygen species increase in rat
neuropathic spinal dorsal horn neurons. Neurosci Lett. 391:108–111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Navarro SA, Serafim KG, Mizokami SS,
Hohmann MS, Casagrande R and Verri WA Jr: Analgesic activity of
piracetam: Effect on cytokine production and oxidative stress.
Pharmacol Biochem Behav. 105:183–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Twining CM, Sloane EM, Milligan ED, Chacur
M, Martin D, Poole S, Marsh H, Maier SF and Watkins LR:
Peri-sciatic proinflammatory cytokines, reactive oxygen species and
complement induce mirror-image neuropathic pain in rats. Pain.
110:299–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ji RR and Suter MR: p38 MAPK, microglial
signaling, and neuropathic pain. Mol Pain. 3:332007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhuang ZY, Kawasaki Y, Tan PH, Wen YR,
Huang J and Ji RR: Role of the CX3CR1/p38 MAPK pathway in spinal
microglia for the development of neuropathic pain following nerve
injury-induced cleavage of fractalkine. Brain Behav Immun.
21:642–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hua XY, Svensson CI, Matsui T, Fitzsimmons
B, Yaksh TL and Webb M: Intrathecal minocycline attenuates
peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK
in spinal microglia. Eur J Neurosci. 22:2431–2440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsuda M, Mizokoshi A, Shigemoto-Mogami Y,
Koizumi S and Inoue K: Activation of p38 mitogen-activated protein
kinase in spinal hyperactive microglia contributes to pain
hypersensitivity following peripheral nerve injury. Glia. 45:89–95.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM and
Yao SL: Alleviation of neuropathic pain by intrathecal injection of
antisense oligonucleotides to p65 subunit of NF-kappaB. Brit J
Anaesth. 97:553–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tegeder I, Niederberger E, Schmidt R, Kunz
S, Gühring H, Ritzeler O, Michaelis M and Geisslinger G: Specific
inhibition of IkappaB kinase reduces hyperalgesia in inflammatory
and neuropathic pain models in rats. J Neurosci. 24:1637–1645.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KM, Jeon SM and Cho HJ: Tumor necrosis
factor receptor 1 induces interleukin-6 upregulation through
NF-kappaB in a rat neuropathic pain model. Eur J Pain. 13:794–806.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei XH, Yang T, Wu Q, Xin WJ, Wu JL, Wang
YQ, Zang Y, Wang J, Li YY and Liu XG: Peri-sciatic administration
of recombinant rat IL-1β induces mechanical allodynia by activation
of src-family kinases in spinal microglia in rats. Exp Neurol.
234:389–397. 2012. View Article : Google Scholar : PubMed/NCBI
|