Introduction
It has been previously reported that the combination
of several kinds of anesthesia may damage brain function (1), and accumulating studies have reported
impairment in learning or memory following anesthesia (2–4).
Studies indicate that impairments caused by anesthesia application
performed on brain neurons were observed in various types of
animals, including mammals (5,6), and
indicated that general anesthesia exhibits an age-dependent effect
on the brain (7).
As a common inhaled general anesthetic, isoflurane
is considered to contribute to long-term memory deficit (8). For example, isoflurane application
induced neuron apoptosis in a dose-dependent manner in 7-day-old
rats, and the co-application of isoflurane, imidazole valium and
nitrous oxide further increased apoptosis (9). Stratmann et al (10) reported that isoflurane application
induced neuron apoptosis and cognitive impairment following 8
months of isoflurane treatment in rats. Due to the importance of
potential anesthesia-induced neuron damage and the complex
mechanism of action, an increasing number of studies have focused
on investigating the mechanisms behind these effects (11–13).
microRNAs (miRNAs) are endogenous non-coding RNAs
that are 20–22 nucleotides in length and function in various
biological processes at the transcriptional or post-transcriptional
level by targeting the 3′-untranslated regions of genes (14). Studies have indicated important
roles for certain miRNAs in the pathology of anesthesia-induced
neuron damage. McAdams et al (15) screened 9 differentially expressed
miRNAs, including miR-204-5p, miR-455-3p, miR-448-5p and
miR-574-3p, in the hippocampal tissue of morphine-exposed mice.
Additionally, Luo et al (16) reported that the knockdown of let-7d
acts as a contributor for isoflurane-induced learning and memory
impairment. The roles of miR-448 in cell apoptosis in various
diseases have also been reported (17,18),
however, to the best of our knowledge, this has not previously been
investigated in isoflurane-induced learning and memory
impairment.
In the current study, miR-448 expression in
isoflurane-treated rats was detected and the effects of miR-448
expression on learning and memory in the hippocampal tissue of
isoflurane-treated rats were investigated by downregulating the
expression of miR-448. The present study aimed to investigate the
potential mechanism underlying the effect of miR-448 on learning
and memory impairment in isoflurane-treated rats. The present study
may provide a theoretical basis for the molecular mechanism of
isoflurane in clinical treatment of neuron damage.
Materials and methods
Model construction
The procedure was approved by the local committee of
The First Affiliated Hospital, Wenzhou Medical University (Zhejiang
325000, P.R. China) and all animals were treated according to the
Guide for the Care and Use of Laboratory Animals of the Institute
for Laboratory Animal Research (19). Sprague-Dawley male rats (n=32; age,
18 months; weight, 450–550 g) were obtained from the Institute of
Experimental Animals of the Medical Scientific Academy in Wenzhou
Medical University (Wenzhou, China). The rats were housed in a
standard animal-grade room with free access to standard rodent
pellet diet and water. The temperature was maintained at 23±2°C,
the relative humidity was 55±10% and a 12 h:12 h light:dark cycle.
Following 1 week acclimation period in the laboratory, rats were
randomly assigned to two groups; The control group and anesthesia
group (n=16 per group). The experimental rats were exposed to 2%
isoflurane (Baxter, Deerfield, IL, USA) for 4 h, whereas rats in
the control group received air/oxygen at identical flow rates (0.7
l/min) in identical chambers. The rats were used for the
experiments immediately after the anesthesia. Isoflurane
concentration in the chamber was monitored with a vaporizer. The
rectal temperature was maintained at 37.0±0.5°C. All rats were
visually inspected for respiratory effort and skin color. Pulse
oximeter oxygen saturation was routinely monitored during
anesthesia. Mean arterial blood pressure was recorded using
non-invasive sphygmomanometers.
Morris water maze test
Cognitive function was analyzed between 9 am and 3
pm using the Morris water maze test system, as previously described
(20). Briefly, the maze (depth,
80 cm; diameter, 100 cm) was separated into four quadrants of equal
size on the monitor screen of a computer, and was filled to a depth
of 30 cm with water. The temperature of the water was maintained at
24±0.5°C. Swimming paths of rats were recorded using a video camera
and analyzed with VideoMot software version 2.4.50923 (TSE Systems
GmbH, Bad Homburg, Germany) regarding the following parameters:
Swimming speed, escape latency and time in original quadrant. The
test was conducted on 4 consecutive days to observe escape latency
and time spent in the quadrant of rats in the Morris water maze.
Rats were placed in the maze from four random points of the tank
and were allowed to search for the platform for 2 min. If this was
not achieved, the rat was gently placed on the platform and left
for 20 sec.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) as
previously described (21), and
was treated with RQ1 RNase-free DNase I (Promega Corporation,
Madison, WI, USA). The concentration and purity of the isolated RNA
were measured with SMA 400 UV–VIS (Merinton, Shanghai, China).
Purified RNA (0.5 µg/µl) with nuclease-free water was used for cDNA
synthesis with the PrimeScript 1st Strand cDNA Synthesis kit
(6110A; Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. Target gene expression was detected in an
Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) using the SYBR
ExScript RT-PCR kit (DRR053S; Takara Biotechnology Co., Ltd.). PCR
was run under the following parameters: Initial denaturation cycle
of 1 min at 95°C, 35 cycles of denaturation at 94°C for 30 sec,
annealing at 60°C for 30 sec, extension at 72°C for 2 min and a
final extension at 72°C for 7 min. MiR expression was quantified by
the comparative 2−(∆∆Cq) method and normalized to U6
expression (22). The primers for
miR-448 were: Forward: 5′-TTATTGCGATGTGTTCCTTATG-3′, Reverse
primer: 5′-ATGCATGCCACGGGCATATACACT-3′; and U6 were: Forward:
5′-CTCGCTTCGGCAGCACA-3′ Reverse: 5′-AACGCTTCACGAATTTGCGT-3′.
Experiments were performed at least three times.
Cell culture and cell
transfection
Hippocampal cultures were prepared as described
previously (23). Briefly, the
tissue was dissected and digested in 2 ml of 2 mg/ml papain for 30
min at 37°C and was subsequently inactivated with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The tissue was
triturated by a pipette and was passed through a cell strainer to
remove undissociated tissue. Cells were subsequently centrifuged at
400 × g for 5 min at 4°C. The supernatant was discarded and the
cell pellet was resuspended in Dulbecco's modified Eagle's medium
(DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 1X
antibiotics (penicillin-streptomycin) and 5% FBS. Cells were plated
on poly-L-lysine (Invitrogen; Thermo Fisher Scientific, Inc.)
coated plates or coverslips at a density of 1×105 cells/ml. The
medium was replaced by Neurobasal medium (Invitrogen; Thermo Fisher
Scientific, Inc.) with 2% B27 (Sigma-Aldrich; Merck KGaA) after
plating overnight at 37°C in a 5% CO2 incubator. For
cell transfection, miR-448 inhibitor (150 nM; Sangon Biotech Co.,
Ltd., Shanghai, China) was transfected into hippocampal cells for
24 h at 37°C with Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. To
produce stable miR-448-depleted transfectants, miR-448 inhibitor
sequences were amplified from miRZip-448 construct (System
Biosciences, Palo Alto, CA, USA) and subcloned into pSilencer4.1
polyclone sites with HindIII and BamHI sites. The miR-448 inhibitor
sequence was 5′-AUGGGACAUCCUACAUAUGCAA-3′ and the scramble RNA
sequence was 5′-CUAAGUCUUGGUAGUCACGUUC-3′. Cells transfected with
the scramble RNA (150 nM) or without any transfection were
considered as the control.
Western blotting
After 4 h of treatment with mock or isoflurane, the
rats were sacrificed and total protein was extracted from the
tissue using 1X RIPA buffer (89900; Piece; Thermo Fisher
Scientific, Inc.). Cells were lysed with radioimmunoprecipitation
assay buffer (Sangon Biotech Co., Ltd., Shanghai, China) containing
phenylmethanesulfonyl fluoride (Sigma-Aldrich; Merck KGaA) and the
lysates were centrifuged at 6,400 × g for 10 min at 4°C. The
supernatants were collected and the protein concentration was
determined using a Pierce™ BCA Protein Assay kit (23227; Pierce;
Thermo Fisher Scientific, Inc.). The proteins (20 µg per lane) were
separated by 10% SDS-PAGE followed by transfer onto a
polyvinylidene fluoride (PVDF) membrane (24). The membranes were blocked in 1%
TBS-Tween-20 (TBST) containing 5% non-fat milk for 1 h at room
temperature and then incubated with rabbit anti-rat antibodies
against microtubule-associated protein tau (Tau; 44–734G),
amyloid-β precursor protein (APP; PA1-072), Bcl-x (PA5-21676), and
caspase-3 (PA5-16335) at a 1:100 dilution (all from Invitrogen;
Thermo Fisher Scientific, Inc.) overnight at 4°C. Subsequently, the
membranes were incubated with a horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (PA1-27236; 1:1,000;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. Finally, the PVDF membranes were washed 3 times with
1X TBST buffer for 10 min each. The signals were detected after the
membranes were incubated with a chromogenic substrate using the
enhanced chemiluminescence western blotting substrate (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. GAPDH (MA5-15738-BTIN; 1:1,000; Invitrogen; Thermo Fisher
Scientific, Inc.) served as the internal control. The intensity of
protein bands was quantified by densitometry using ImageJ software
version 1.46 (National Institutes of Health, Bethesda, MD,
USA).
Cell apoptosis assay
After 36 h of transfection, cells were incubated
with the replacement of fresh cell culture medium containing
serum-free medium. The cell apoptosis was analyzed by using the
Annexin V-FITC Fluorescence Microscopy kit kit (550911; BD
Biosciences, San Jose, CA, USA). Total cells were harvested, fixed
with 3.7% formaldehyde for 15 min at room temperature,
permeabilized with 0.1% Triton X-100 for 5 min at 37°C, and washed
three times with PBS buffer, cells were then resuspended in the
1XBinding Buffer. Subsequently, 5 µl Annexin V-fluorescein
isothiocyanate and 5 µl propidium iodide were mixed with the cells.
Following culture at room temperature for 10 min, mixtures were
analyzed using a FACScan flow cytometer (BD Biosciences). Annexin
V-positive and propidium iodide-negative cells were considered to
be apoptotic cells.
Statistical analysis
All experiments were performed independently three
times. Data are presented as the mean ± standard deviation. Data
were calculated using GraphPad Prism version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Significant differences among
groups were analyzed using one-way analysis of variance followed by
Tukey-Kramer's Post-hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Morris water maze test
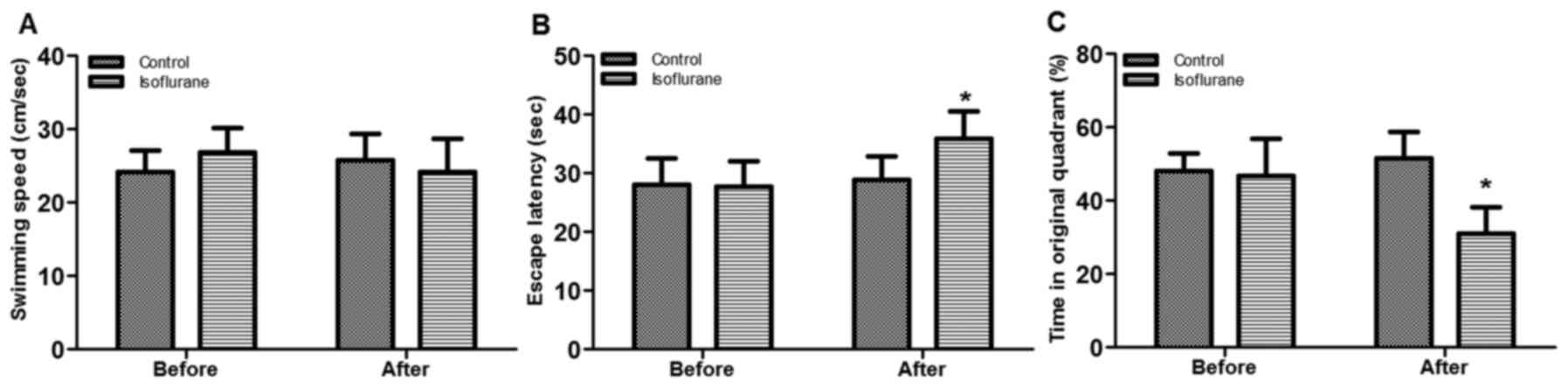
Morris water maze test was conducted to assess the
indexes for rats regarding the swimming speed, escape latency and
time in original quadrant pre- and post-anesthesia (Fig. 1). The results demonstrated that no
significant difference was observed for swimming speed between the
control and anesthetic rats either prior or subsequent to
anesthesia (Fig. 1A). However, the
escape latency was significantly increased by isoflurane treatment
(P<0.05; Fig. 1B), whereas the
time in original quadrant was significantly reduced in the
isoflurane group compared with the control (P<0.05; Fig. 1C), indicating that the isoflurane
treatment may impair learning and memory in rats.
Expression of apoptotic proteins in
isoflurane-treated neurons
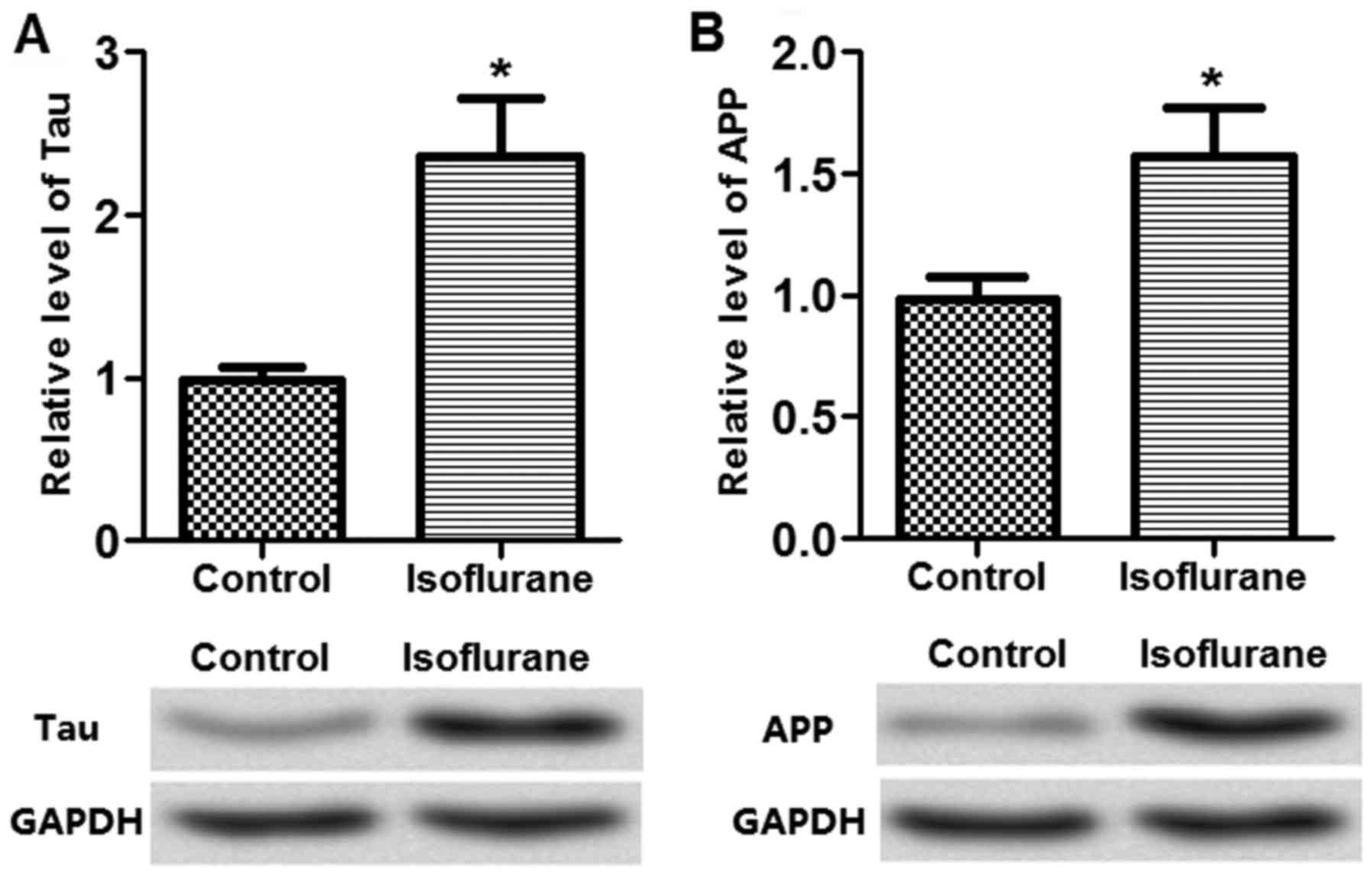
The dementia-associated neuron apoptotic proteins in
the tissues, including Tau and APP, were measured to determine the
effects of isoflurane on neuron apoptosis. The results demonstrated
that Tau and APP expression were significantly upregulated by the
isoflurane treatment (Fig. 2),
indicating that isoflurane treatment may be associated with neuron
cell apoptosis.
Expression of miR-448 in
isoflurane-treated hippocampus tissues
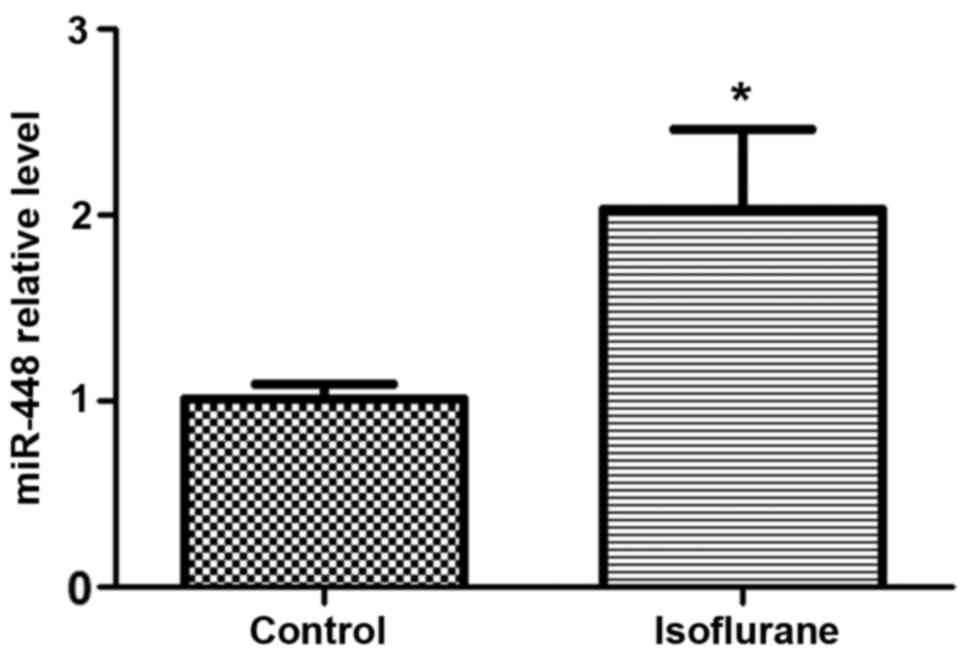
Following isoflurane treatment, the rat hippocampus
was isolated for the detection of miR-448 expression. The results
demonstrated that the relative miR-448 expression level was
significantly increased in the isoflurane-treated hippocampus
compared with control rats (Fig.
3), which indicates that miR-448 expression may be associated
with impaired learning and memory caused by isoflurane treatment in
rats.
miR-448 induced hippocampus neuron
apoptosis
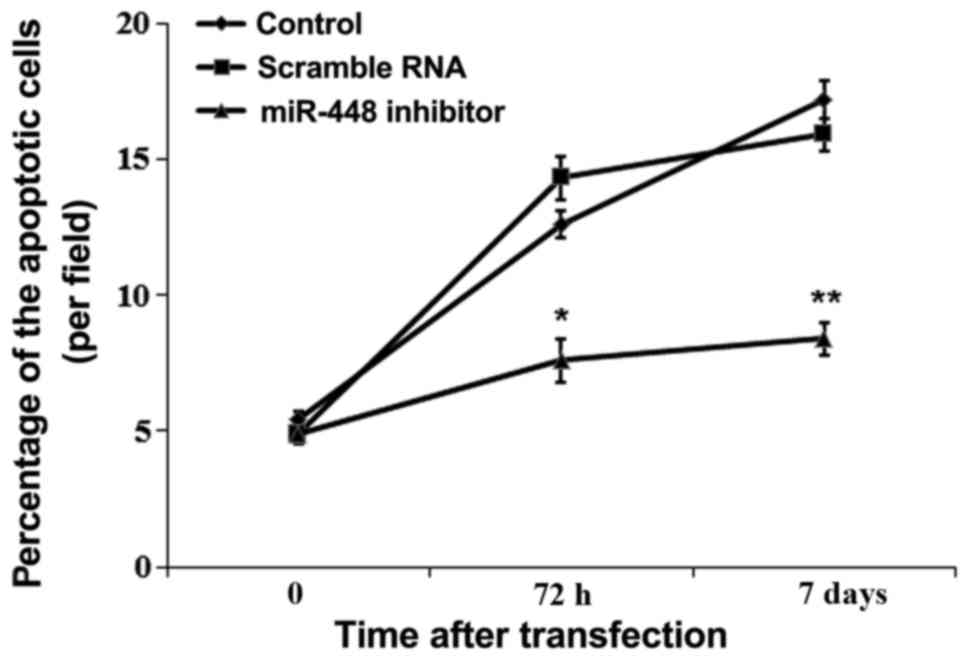
The influence of miR-448 on neuron apoptosis was
detected in the hippocampus of rats (Fig. 4). The percentage of apoptotic
neurons was significantly decreased by miR-448 inhibitor
transfection compared with controls (P<0.05).
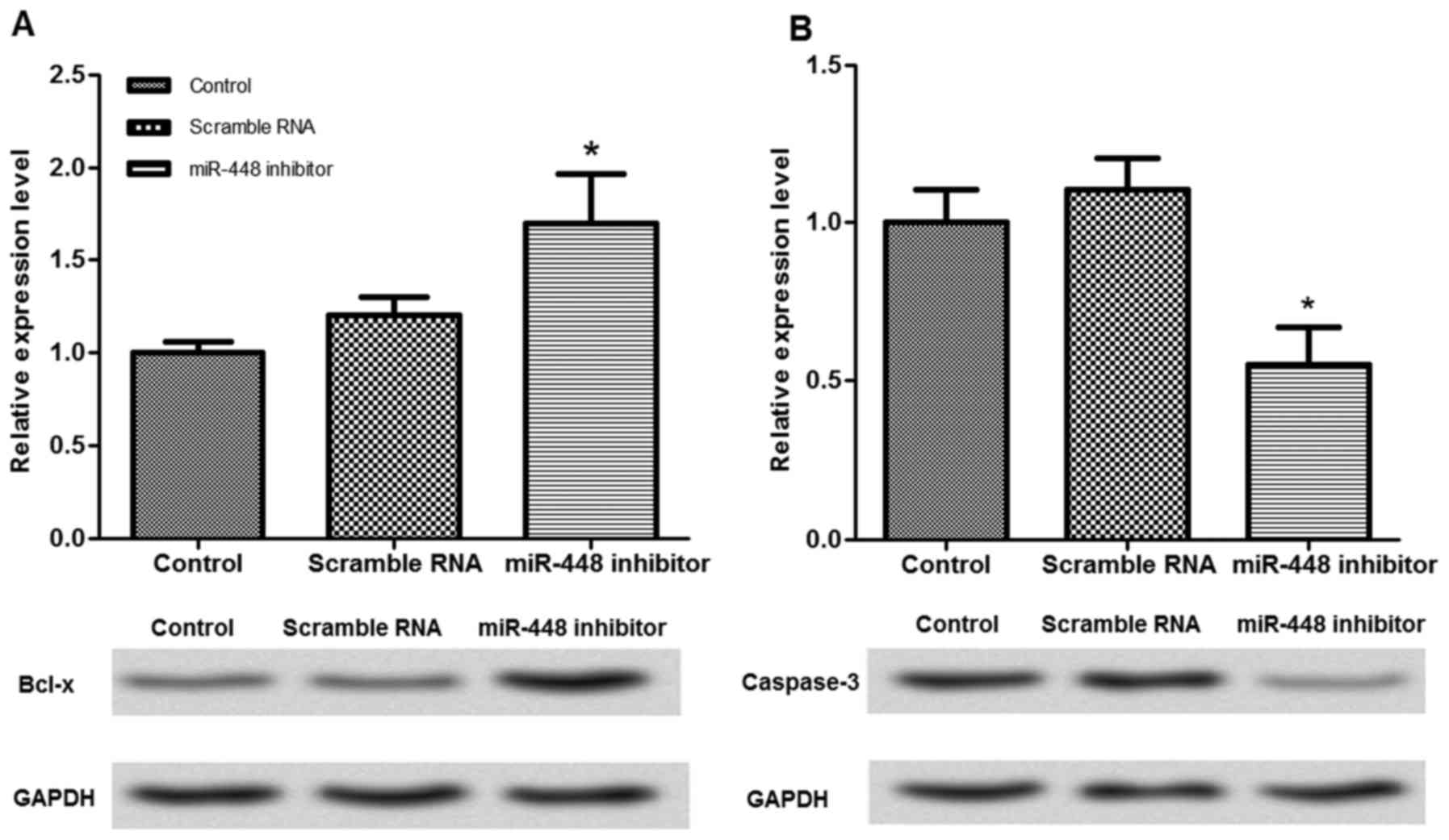
Effects of miR-448 expression on the
expression of cell apoptosis-associated proteins
The expression of cell apoptosis-associated proteins
was measured to investigate the potential mechanism for miR-448 in
neuron apoptosis (Fig. 5). When
miR-448 expression was suppressed in the neurons, the relative
protein expression of Bcl-x was significantly increased, while
caspase-3 was significantly decreased (P<0.05), compared with
the untransfected cells.
Discussion
Interest in the effect of general anesthetic on
long-term memory is on the increase. The effect of anesthesia,
including isoflurane, on learning and memory has been previously
reported (25–27), however, a full understanding of the
underlying mechanism remains to be elucidated. Additionally,
previous studies have demonstrated that neuron apoptosis may be one
of most important mechanisms involved in anesthesia-induced brain
damage (28,29), however, the mechanism remains to be
established. The present study analyzed the expression of miR-448
in the hippocampal tissue of isoflurane-treated rats and
investigated the effect of miR-448 expression on learning and
memory impairment using the knockdown method. Consistent with
previous reports (30,31), isoflurane treatment induced
learning and memory damage, which was indicated by increased escape
latency and reduced time spent in original quadrant during the
Morris water maze test (Fig.
1).
Subsequently, the present study detected the
expression of cell apoptosis markers, including Tau and APP, in
isoflurane-treated rats and the results demonstrated that Tau and
APP levels were highly expressed in the isoflurane-treated rats
(Fig. 2). Tau protein is a
microtubule-associated protein that is expressed abundantly in
neuronal axons (32), whereas APP
protein functions as a cell surface receptor and performs
physiological functions on the surface of neurons relevant to
neurite growth, neuronal adhesion and axonogenesis (33). Li et al (34) demonstrated that Tau protein level
was significantly increased by isoflurane treatment in cognitive
dysfunction in transgenic APP695 mice. Similar results for APP were
observed in transgenic mice in a study conducted by Zhang et
al (35). Based on the results
of the current study, it was hypothesized that isoflurane treatment
impaired learning and memory in rats.
Meanwhile, the present study analyzed the expression
of miR-448 in the hippocampal tissue of isoflurane-treated rats and
the results demonstrated that miR-448 was upregulated following
isoflurane treatment in rats (Fig.
3). Pivotal roles for miRNAs have been identified in various
diseases, including cancer and cardiovascular diseases, via
involvement in biological processes such as cell apoptosis
(36,37). Additionally, the results of the
current study demonstrated that neuron apoptosis was significantly
suppressed by miR-448 inhibitor application (Fig. 4), indicating that miR-448 may have
certain roles in isoflurane-induced learning and memory damage via
cell apoptosis. Consequently, the present study further measured
the expression of cell apoptosis-associated proteins in
vitro. It is suggested that Bcl-x is a bcl-2 family member that
is involved in the regulation of apoptosis (38), while caspase-3 is an apoptotic
executor in various diseases (39). The association between miR-448 and
caspase-3 in neuron apoptosis caused by isoflurane remains to be
fully elucidated. However, Noh et al (40) demonstrated that caspase-3 was
highly expressed following miR-448 overexpression in mice with
Alzheimer's disease. In the present study, caspase-3 protein was
downregulated while Bcl-x was upregulated by the miR-448 inhibitor
in isoflurane-treated neurons (Fig.
5), indicating that miR-448 downregulation may block neuron
apoptosis via reducing caspase-3 and increasing Bcl-x
expression.
In conclusion, the results presented in the present
study indicate that miR-448 downregulation may contribute to
improving the learning and memory impairment induced by isoflurane
application by suppressing neuron apoptosis. The current study may
provide a theoretical basis for the investigation of the mechanism
underlying the effect of isoflurane on memory. Further experimental
studies are required to investigate the underlying mechanism in
depth and to explore the effects of isoflurane treatment on
learning and memory.
References
|
1
|
Young C, Jevtovic-Todorovic V, Qin YQ,
Tenkova T, Wang H, Labruyere J and Olney JW: Potential of ketamine
and midazolam, individually or in combination, to induce apoptotic
neurodegeneration in the infant mouse brain. Br J Pharmacol.
146:189–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith RA: Enhancement of impaired motor
and mental functions, using dextromethorphan and oxidase enzyme
inhibitor US Patent US20070191411 A1. October 7–2005, issued August
16, 2007.
|
|
3
|
Bolger C, Arms SW, Townsend CP and Smith
KR: Method and apparatus for monitoring eye tremor US Patent US
8500282 B2. September 22–2011, issued August 6. 2013
|
|
4
|
Rahaghi F and Botero J: Endotracheal tube
apparatus and method for use US 20070221229 A1. 25–October. 2006,
issued September 27 2007.
|
|
5
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
Benshoff ND, Dikranian K, Zorumski CF, Olney JW and Wozniak DF:
Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.PubMed/NCBI
|
|
6
|
Olney JW, Tenkova T, Dikranian K, Qin YQ,
Labruyere J and Ikonomidou C: Ethanol-induced apoptotic
neurodegeneration in the developing C57BL/6 mouse brain. Brain Res
Dev Brain Res. 133:115–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viberg H, Pontén E, Eriksson P, Gordh T
and Fredriksson A: Neonatal ketamine exposure results in changes in
biochemical substrates of neuronal growth and synaptogenesis, and
alters adult behavior irreversibly. Toxicology. 249:153–159. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ballesteros KA, Sikorski A, Orfila JE and
Martinez JL Jr: Effects of inhaled anesthetic isoflurane on
long-term potentiation of CA3 pyramidal cell afferents in vivo. Int
J Gen Med. 5:935–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson SA, Chainllie Y and Olney JW:
Isoflurane-induced neuroapoptosis in the developing brain of
nonhypoglycemic mice. J Neurosurg Anesthesiol. 20:21–28. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stratmann G, Sall JW, May LD, Bell JS,
Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT and Dai R:
Isoflurane differentially affects neurogenesis and long-term
neurocognitive function in 60-day-old and 7-day-old rats.
Anesthesiology. 110:834–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ocmen E, Derbent A, Micilli SC, Cankurt U,
Aksu I, Dayi A, Yilmaz O and Gokmen N: Erythropoietin diminishes
isoflurane-induced apoptosis in rat frontal cortex. Paediatr
Anaesth. 26:444–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee BH, Chan JT, Kraeva E, Peterson K and
Sall JW: Isoflurane exposure in newborn rats induces long-term
cognitive dysfunction in males but not females. Neuropharmacology.
83:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by preserving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lima SA and Pasquinelli AE: Identification
of miRNAs and Their Targets in C. elegans. Adv Exp Med Biol.
825:431–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McAdams RM, McPherson RJ, Beyer RP,
Bammler TK, Farin FM and Juul SE: Dose-dependent effects of
morphine exposure on mRNA and microRNA (miR) expression in
hippocampus of stressed neonatal mice. PLoS One. 10:e01230472015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo T, Yin S, Shi R, Xu C, Wang Y, Cai J,
Yue Y and Wu A: miRNA expression profile and involvement of
Let-7d-APP in aged rats with isoflurane-induced learning and memory
impairment. PLoS One. 10:e01193362015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garofalo M, Condorelli G and Croce CM:
MicroRNAs in diseases and drug response. Curr Opin Pharmacol.
8:661–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Millan MJ: MicroRNA in the regulation and
expression of serotonergic transmission in the brain and other
tissues. Curr Opin Pharmacol. 11:11–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press; Washington (DC): 2010
|
|
20
|
Yang C, Zhu B, Ding J and Wang ZG:
Isoflurane anesthesia aggravates cognitive impairment in
streptozotocin-induced diabetic rats. Int J Clin Exp Med.
7:903–910. 2014.PubMed/NCBI
|
|
21
|
Hummon AB, Lim SR, Difilippantonio MJ and
Ried T: Isolation and solubilization of proteins after TRIzol
extraction of RNA and DNA from patient material following prolonged
storage. Biotechniques. 42:467–470. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Kuramitsu Y, Takashima M, Yokoyama
Y, Iizuka N, Tamesa T, Sakaida I, Oka M and Nakamura K:
Identification of four isoforms of aldolase B down-regulated in
hepatocellular carcinoma tissues by means of two-dimensional
Western blotting. In Vivo. 25:881–886. 2011.PubMed/NCBI
|
|
24
|
Qin XY, Cheng Y, Murthy SR, Selvaraj P and
Loh YP: Carboxypeptidase E-ΔN, a neuroprotein transiently expressed
during development protects embryonic neurons against glutamate
neurotoxicity. PLoS One. 9:e1129962014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loepke AW, Istaphanous GK, McAuliffe JJ
III, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams
MT, Vorhees CV and Danzer SC: The effects of neonatal isoflurane
exposure in mice on brain cell viability, adult behavior, learning,
and memory. Anesth Analg. 108:90–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y and Xie Z: Anesthetics isoflurane
and desflurane differently affect mitochondrial function, learning,
and memory. Ann Neurol. 72:6302012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Liang G, Wang S, Meng Q, Wang Q and
Wei H: Effects of fetal exposure to isoflurane on postnatal memory
and learning in rats. Neuropharmacology. 53:942–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen G, Shi J, Hu Z and Hang C: Inhibitory
effect on cerebral inflammatory response following traumatic brain
injury in rats: A potential neuroprotective mechanism of
N-acetylcysteine. Mediators Inflamm. 2008:7164582008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Filbert MG and Ballough GPH: Method of
reducing brain damage resulting from seizures US Patent US 6211230
B1. January 19–2000, issued April 3, 2001.
|
|
30
|
Zhu C, Gao J, Karlsson N, Li Q, Zhang Y,
Huang Z, Li H, Kuhn HG and Blomgren K: Isoflurane anesthesia
induced persistent, progressive memory impairment, caused a loss of
neural stem cells, and reduced neurogenesis in young, but not
adult, rodents. J Cereb Blood Flow Metab. 30:1017–1030. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Culley DJ, Baxter MG, Crosby CA,
Yukhananov R and Crosby G: Impaired acquisition of spatial memory 2
weeks after isoflurane and isoflurane-nitrous oxide anesthesia in
aged rats. Anesth Analg. 99:1393–1397; table of contents. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mandell JW and Banker GA: A spatial
gradient of tau protein phosphorylation in nascent axons. J
Neurosci. 16:5727–5740. 1996.PubMed/NCBI
|
|
33
|
Akinlolu RD, Nam M and Wei Q: Competition
between fibrillation and induction of vesicle fusion for the
membrane-associated 40-residue β-amyloid peptides. Biochemistry.
54:3416–3419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li C, Liu S, Xing Y and Tao F: The role of
hippocampal tau protein phosphorylation in isoflurane-induced
cognitive dysfunction in transgenic APP695 mice. Anesth Analg.
119:413–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang CY, Mei-Hua P, Liu N, Anestic DO,
Hospital TF and University J: Effects of trehalose on learning and
memory impairment induced by isoflurane in APP transgenic mice.
Chin J Gerontol. 2015.(In Chinese).
|
|
36
|
Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen
YY, Wang WJ, Chen Q, Tang F, Liu XP and Xu ZD: Involvement of
NF-κB/miR-448 regulatory feedback loop in chemotherapy-induced
epithelial-mesenchymal transition of breast cancer cells. Cell
Death Differ. 18:16–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kyrychenko S, Kyrychenko V, Badr MA, Ikeda
Y, Sadoshima J and Shirokova N: Pivotal role of miR-448 in the
development of ROS-induced cardiomyopathy. Cardiovasc Res.
108:324–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li L, Han W, Gu Y, Qiu S, Lu Q, Jin J, Luo
J and Hu X: Honokiol induces a necrotic cell death through the
mitochondrial permeability transition pore. Cancer Res.
67:4894–4903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Varghese J, Khandre NS and Sarin A:
Caspase-3 activation is an early event and initiates apoptotic
damage in a human leukemia cell line. Apoptosis. 8:363–370. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noh H, Park C, Park S, Lee YS, Cho SY and
Seo H: Prediction of miRNA-mRNA associations in Alzheimer's disease
mice using network topology. Bmc Genomics. 15:6442014. View Article : Google Scholar : PubMed/NCBI
|