Introduction
Esophageal carcinoma (EC) is among the most common
types of malignancy worldwide, with the 8th highest incidence, and
is the 6th leading cause of cancer-associated mortality (1–3).
Despite the development of a combination of surgery, radiotherapy
and chemotherapy, the prognosis for EC remains poor due to the
insidious symptomatology, late clinical presentation and rapid
progression (4,5). Therefore, it is necessary to
understand the pathophysiological mechanisms underlying the
progression of EC and to elucidate novel targets which are able to
inhibit the malignant processes, so as to provide an improved
prognosis for patients with EC.
Vasohibin-1 (VASH1) is a member of the vasohibin
family. Previous studies have reported that VASH1 is selectively
expressed in endothelial cells, and is able to inhibit angiogenesis
by regulating endothelial cell death and biological functions
(6–8). Cumulative evidence has indicated that
VASH1 in the tumor mesenchyme may function as a molecular marker
for prognosis in numerous types of cancer, including breast cancer
(9), renal carcinoma (10), hepatocellular cancer (11) and prostate cancer (12). Previous studies have demonstrated
that VASH1 is not restricted to endothelial cells, and is
additionally expressed in other types of cells, including tumor
cells (13,14). However, the expression of VASH1 in
EC cells and the relevance of this to clinical parameters and
prognosis remains unknown. The direct effect of VASH1 on the
biological behaviors of EC cells has not been reported.
The present study demonstrated the expression of
VASH1 in EC cells using immunohistochemistry (IHC) and western
blotting, and it was observed that increased VASH1 expression was
negatively correlated with tumor size, invasion depth and poor
prognosis. Following alteration of VASH1 expression in EC cells
using transfection, it was observed that VASH1 markedly inhibited
the proliferation, clone formation and migration of EC cells. The
results of the present study demonstrated the suppressive effects
of VASH1 in EC and provide evidence of a novel target to inhibit EC
progression.
Materials and methods
Cell lines and culture
EC cell lines EC1 and EC9706 were purchased from the
American Type Culture Collection (Manassas, VA, USA). EC1 and
EC9706 cells were cultured in RPMI-1640 medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in 5% CO2.
IHC
Approved by the Review Board and Ethics Committee of
Yishui Central Hospital of Lingyi (Lingyi, China), 100 primary EC
specimens obtained between January 2006 and January 2009 were
selected from the hospital. No chemotherapy, radiotherapy or
immunotherapy had been performed prior to surgery. Patient
characteristics are presented in Table
I.
 | Table I.Correlation of VASH1 with clinical
parameters of patients with esophageal carcinoma. |
Table I.
Correlation of VASH1 with clinical
parameters of patients with esophageal carcinoma.
|
| VASH1 |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Low | High | r | χ2 | P-value |
|---|
| Sex |
| Male | 28 | 27 | −0.159 | 2.523 | 0.112 |
|
Female | 30 | 15 |
|
|
|
| Age, years |
|
<60 | 35 | 26 | −0.016 | 0.025 | 0.875 |
| ≥60 | 23 | 16 |
|
|
|
| Tumor size, cm |
|
<5 | 18 | 30 | −0.399 | 15.925 | <0.01a |
| ≥5 | 40 | 12 |
|
|
|
| Invasion depth |
| Tis, T1,
T2 | 20 | 28 | −0.318 | 10.109 | <0.01a |
|
T3+T4 | 38 | 14 |
|
|
|
| Metastasis |
|
Absent | 32 | 22 | 0.028 | 0.076 | 0.782 |
|
Present | 26 | 20 |
|
|
|
| Clinical stage |
|
I–IIa | 27 | 18 | 0.037 | 0.134 | 0.714 |
|
IIb-IV | 31 | 24 |
|
|
|
Paraffin-embedded tissue samples were cut into 4-µm
slices. Then, the slides were kept at 60°C for 30 min,
de-paraffinized in xylene at room temperature and rehydrated in
graded ethanol. Following antigen unmasking, the sections were
immersed in 3% H2O2 for 10 min to inhibit the
endogenous peroxidase and in goat serum blocking solution (Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) at room temperature for 15
min to block non-specific antigens. Following incubation at room
temperature for 2 h with primary antibody against VASH1 (1:600;
Abnova, Taipei, Taiwan), the sections were incubated in horseradish
peroxidase goat anti-rabbit/mouse immunoglobulin G polymer (cat.
no. KIT-7710; Fuzhou Maixin Biotech Co., Ltd.) at room temperature
for 30 min. The slides were stained with 3,3′-diaminobenzidine for
5–10 sec and counterstained with hematoxylin for 20 sec at room
temperature. The staining assessment was performed by two
independent pathologists simultaneously at ×400 magnification using
an Olympus BX53 light microscope (Olympus Corporation, Tokyo,
Japan). The proportion score represented the estimated fraction of
positive staining tumor cells (0, ≤25%; 1, 26–50%; 2, 51–75%; 3,
>75%). The intensity score represented the estimated average
staining intensity of positive tumor cells (0, negative; 1, weak;
2, moderate; 3, strong). The expression level of VASH1 was
evaluated using the product of the proportion score and intensity
score at five fields and the mean value was obtained (low
expression, ≤4; high expression, >4). The overall survival (OS)
period was defined as the period of time from surgery to cell death
induced by different factors. Disease-free survival (DFS) was
defined as the period of time from surgery to the recurrence or
progression of disease.
Transfection
Plasmid pEZ-M61/VASH1 (Shanghai Gene Pharma Co.,
Ltd, Shanghai, China) was transfected into EC1 cells to upregulate
VASH1 expression; p-GPU6/VASH1-sh1/2 (Shanghai GenePharma Co., Ltd)
was transfected into EC9706 cells to downregulate VASH1 expression.
All procedures were performed using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol, and empty vectors were used as a control. Cells were
harvested 48 h subsequently and transfection efficiency was
determined using reverse transcription-quantitative polymerase
chain reaction and western blotting. The two shRNA sequences used
for silencing VASH1 were as follows: shRNA1,
5′-AGCGCTACATCAGAGAGCTGCAGTA-3′; shRNA 2,
5′-CCTACTTCTCAGGGAACTACT-3′.
RT-qPCR
Cells were collected and total RNA was extracted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was
synthesized as a template using PrimeScript RT-PCR kit (Takara
Biotechnology Co. Ltd., Dalian, China), according to the
manufacturer's protocol. The qPCR was performed using specific
primers and Universal PCR Master Mix (Thermo Fisher Scientific,
Inc.) with a CFX96 Touch Deep Well™ Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with the following
thermocycling conditions: VASH1; 4°C for 5 min, then 40 cycles of
94°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec, followed by
72°C for 5 min; the same thermocycling conditions were applied for
GAPDH, however, 36 cycles were performed instead of 40. mRNA levels
were normalized to GAPDH. The primers were as follows: VASH1:
forward, 5′-CAAGGACCGGAAGAAGGAUGUUUCU-3′ and reverse,
5′-CAACCAAGGAGAGGAGUAUUGGUCU-3′; and GAPDH: forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′.
The 2−ΔΔCq method was used for quantification (15). The experiment was performed ≥3
times.
Western blot
Protein was extracted from cells using
radioimmunoprecipitation lysis buffer containing 1% PMSF (Beyotime
Institute of Biotechnology, Haimen, China) and the concentration
was detected using a bicinchoninic acid kit (Pierce; Thermo Fisher
Scientific, Inc.). A total of 300 mg/well protein was loaded into a
5% SDS-PAGE gel and then separated by a 10% separating gel and
transferred to polyvinylidene difluoride membrane. Following
blocking in TBS with Tween-20 containing 5% non-fat dried milk for
1 h at room temperature, the membrane was incubated in primary
antibodies of VASH1 (1:1,000) and GAPDH (cat. no. 10494-1-AP;
1:5,000, Proteintech Group, Inc., Wuhan, China) at 4°C overnight.
Following incubation with horseradish peroxidase-conjugated
secondary antibody (cat. no. SA00001-2; 1:5,000, Proteintech Group,
Inc.) at room temperature for 1 h, the protein on the membrane was
detected using a chemiluminescence solution (EMD Millipore,
Billerica, MA, USA) according to the manufacturer's instructions,
and measured using Image-Pro software (version 5.1; Media
Cybernetics, Inc., Rockville, MA, USA).
Viability assay
An MTT assay was used to detect viability. Cells
were counted and plated in 96-well plates in triplicate at a
starting number of 3×103 cells/well. The absorbance of each sample
was measured at 490 nm for 5 days using MTT (Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China) according to the
manufacturer's protocol. The experiment was repeated 3 times.
Clone formation assay
Cells were plated in 6-well plates at 1,000
cells/well. After 2 weeks, cell colonies were stained using giemsa
at room temperature for 15 min (Beijing Solarbio Science and
Technology Co., Ltd.) and counted under a light microscope
(magnification, ×200; >50 cells as one clone; XDS-200; Olympus
Corporation). The experiment was repeated three times.
Migration assay
A total of 1×105 cells were plated in the upper
chambers of Transwell plates in RPMI-1640 without FBS. RPMI 1640
supplemented by 20% FBS was plated in the lower chamber. Following
incubation for 24 h, migrated cells were fixed using 100% methanol
at room temperature for 30 min, stained with 0.1% crystal violet at
room temperature for 25 min and counted in five random fields at
×200 magnification.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean and SPSS software (version 13.0; SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. The differences
between two groups were analyzed using the Student's two-tailed
t-test. The associations between the expression of VASH1 and
clinical parameters were analyzed using Pearson's χ2 test. Survival
curves were drawn using the Kaplan-Meier estimator method and
compared by means of the log-rank test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased VASH1 expression in EC cells
is negatively correlated with tumor size, invasion depth and poor
prognosis
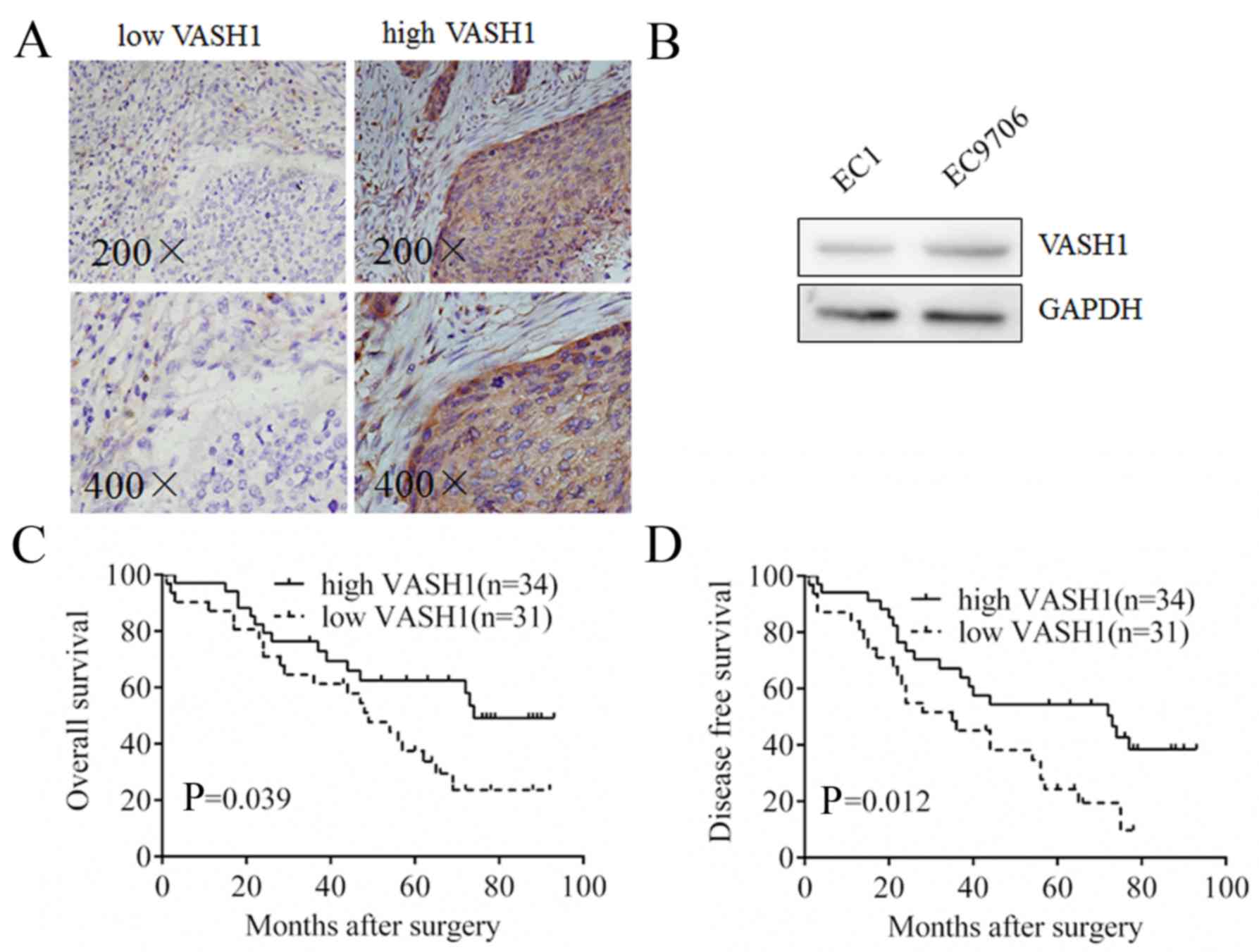
In order to detect VASH1 expression in EC tissues,
IHC was performed and it was observed that VASH1 was expressed in
the cytoplasm of EC cells with different staining intensities;
sporadic staining was observed in the mesenchyme (Fig. 1A). Different expression levels of
VASH1 were observed in EC1 cells and EC9706 cells using western
blotting (Fig. 1B). Due to the
important roles of mesenchymal VASH1 in tumor progression, the
correlation between VASH1 in EC cells and clinical parameters was
analyzed. As presented in Table I,
in the VASH1 high expression group, 12/42 cases exhibited a tumor
size of ≥5 cm; in the VASH1 low expression group, 40/58 cases
exhibited a tumor size of ≥5 cm (P<0.001). In the VASH1 high
expression group, 14/42 cases were at T3/4 stage; in the VASH1 low
expression group, 38/58 cases were at T3/4 stage (P=0.001).
Survival analysis demonstrated that the OS period of VASH1 high
expression group was increased compared with the VASH1 low
expression group (P=0.039; Fig.
1C); additionally, the DFS period of the VASH1 high expression
group was increased compared with the VASH1 low expression group
(P=0.012, Fig. 1D). The results of
the present study confirmed that VASH1 in EC cells exhibited a
negative correlation with progression and poor prognosis for
patients with EC.
VASH1 inhibits the proliferative
ability of EC cells
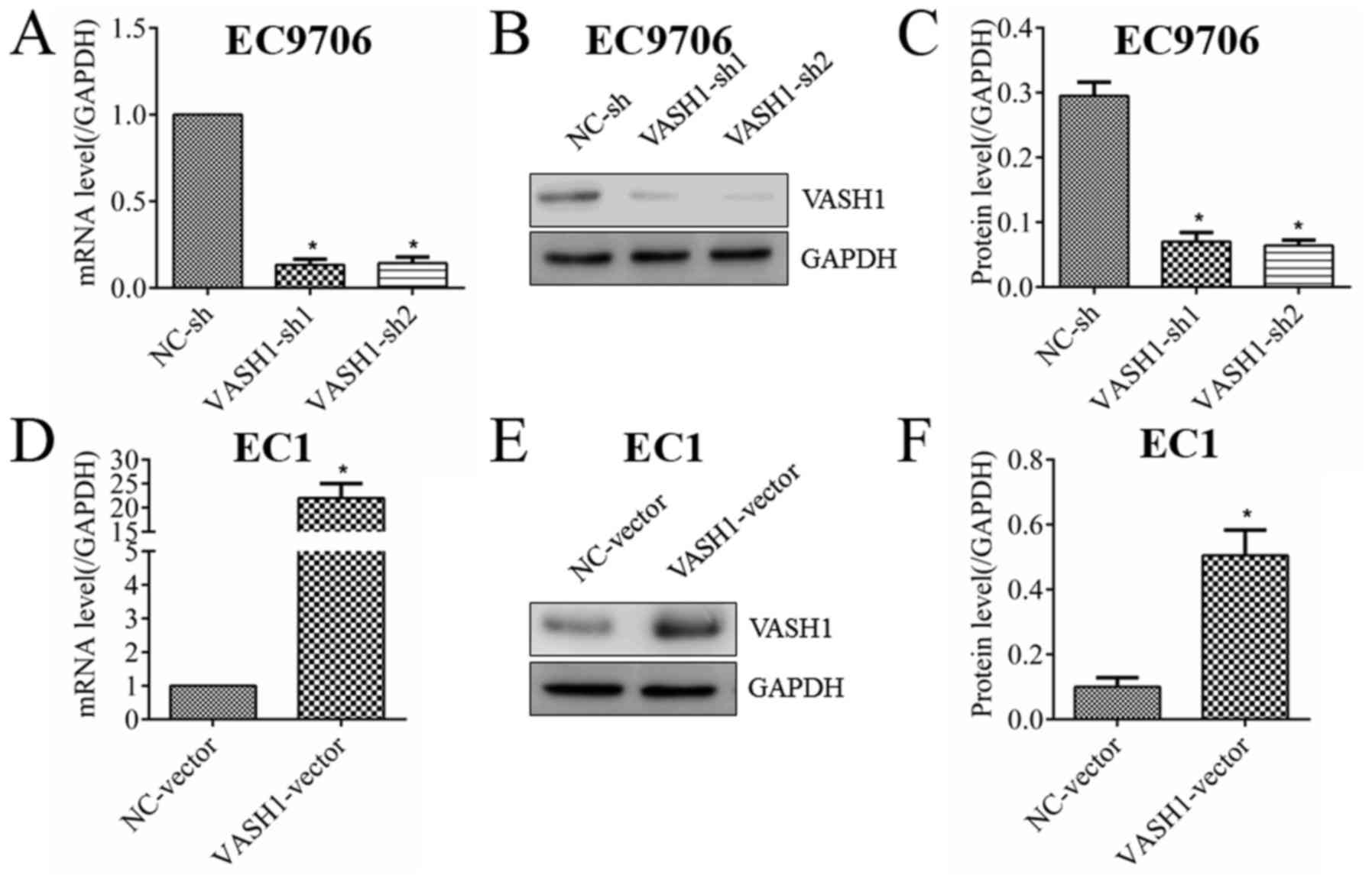
In order to study the direct effect of VASH1 on EC
cells, the expression of VASH1 in EC cells was altered using
transfection. As presented in Fig.
2, VASH1 in EC9706 cells was successfully downregulated at the
RNA (Fig. 2A) and protein
(Fig. 2B and C) levels; VASH1 in
EC1 cells was successfully upregulated at the RNA (Fig. 2D) and protein (Fig. 2E and F) levels. MTT and clone
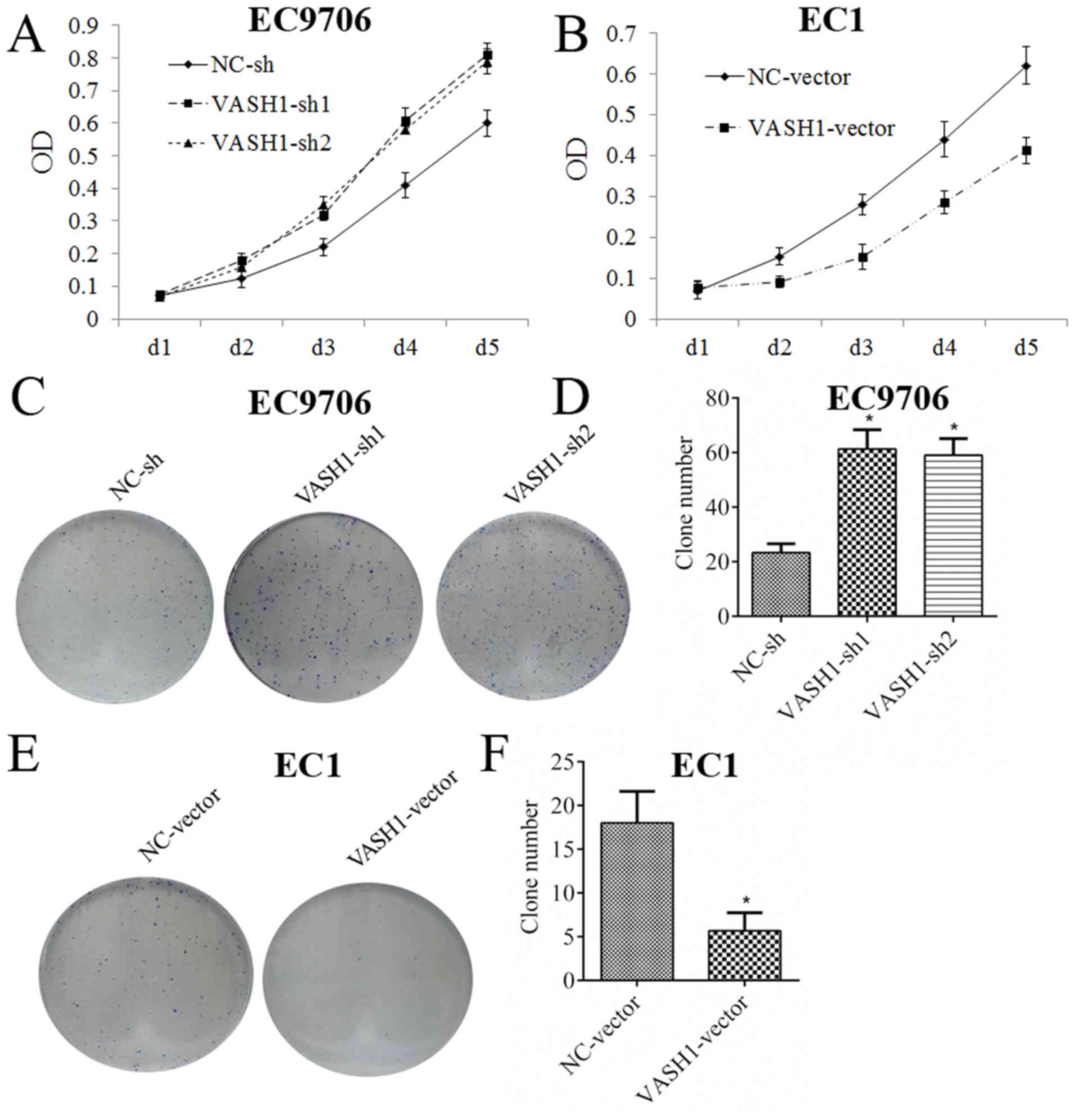
formation assays are presented in Fig.
3. Following downregulation of VASH1 in EC9706 cells, the MTT
assay demonstrated that the growth rate increased markedly
(Fig. 3A); the clone formation
assay demonstrated that clone number increased from 23.33±3.21 to
61.33±7.02 or 59.00±6.08 (P<0.05; Fig. 3C and D). Following upregulation of
VASH1 in EC1 cells, the MTT assay demonstrated that growth rate
decreased markedly (Fig. 3B); the
clone formation assay demonstrated that clone number decreased from
18.00±3.60 to 5.66±2.08 (P<0.05; Fig. 3E and F). The results of the present
study demonstrated that VASH1 was able to directly inhibit the
growth of EC cells.
VASH1 inhibits the migration of EC
cells
Migratory ability is an important biological
behavior of tumor cells and serves important roles in cancer
invasion and metastasis (16,17).
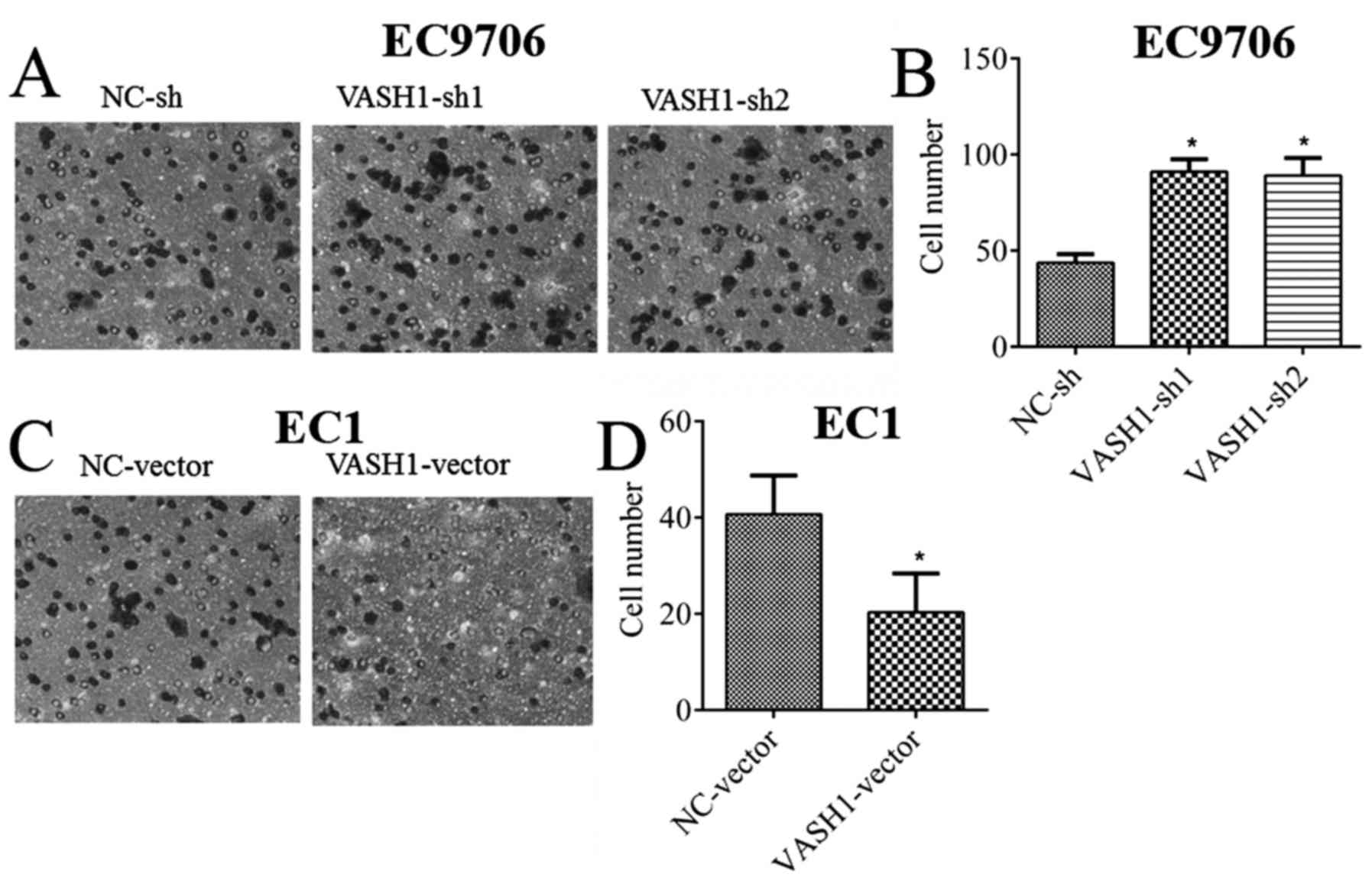
In order to detect the direct effect of VASH1 on the migratory
ability of EC cells, a migration assay was performed; it was
demonstrated that, following downregulation of VASH1 in EC9706
cells, the number of migratory cells increased from 43.66±4.51 to
91.00±6.55 or 89±9.16 (P<0.05; Fig.
4A and B). Following upregulation of VASH1 in EC1 cells, the
number of migratory cells decreased from 40.66±8.08 to 20.33±8.02
(P<0.05; Fig. 4C and D). The
results of the present study demonstrated the negative regulatory
effect of VASH1 on the migratory ability of EC cells.
Discussion
The human VASH1 gene is located on chromosome
14q24.3, including 8 exons and 7 introns. Human VASH1 protein is
composed of 365 amino acids with no glycosylation sites (18,19).
As an acknowledged negative feedback regulator of angiogenesis, the
anti-tumor function of mesenchymal VASH1 has been widely reported
in multiple types of cancer (7,20,21).
Kosaka et al (12) observed
that VASH1 expression was restricted in the mesenchyme and
negatively correlated with advanced clinical stage and poor
recurrence-free survival of prostate cancer patients. Tamaki et
al (9) reported that VASH1 was
detected in human breast cancer endothelial cells and positively
correlated with poor OS and DFS of patients. VASH1 expression has
additionally been detected in parenchymal cells of several types of
tumor. However, the roles of parenchymal VASH1 in cancer remains
controversial. Zhao et al (10) reported that VASH1 was expressed
primarily in the cytoplasm and on the membrane of renal cell
carcinoma cells, and exhibited a negative correlation with tumor
malignancy. In hepatocellular carcinoma, Wang et al
(11) demonstrated that increased
expression of VASH1 in the cytoplasm was positively-correlated with
poor prognosis. In the present study, the expression of VASH1 in EC
cells was observed using IHC and western blotting, and the results
of the present study are consistent with the distribution of VASH1
in renal cell carcinoma and hepatocellular carcinoma reported by
Zhao et al (10) and Wang
et al (11). Further
analysis demonstrated that VASH1 in EC cells was negatively
correlated with tumor size, invasion depth and poor prognosis (OS
and DFS); this is in contrast with the study of Wang et al
and suggests that VASH1 exhibits anti-tumor effects in EC cells.
Only 100 primary EC specimens were used in the present study; due
to this limitation, the results of the present study may not
completely reflect the clinical implications of VASH1 in EC.
Initial studies hypothesized that the anti-tumor
effects of VASH1 were primarily mediated by inhibition of
angiogenesis. The potential direct effects of VASH1 on tumor cells
remain to be elucidated. Increased growth and migratory abilities
are classical biological behaviors of malignant tumors, and are
crucial for tumor occurrence, invasion, metastasis and prognosis
(22–25). Conflicting reports about the direct
effects of VASH1 on biologic behaviors of tumor cells have been
published. Watanabe et al (7) reported that, although overexpression
of VASH1 in lung cancer cells successfully inhibited angiogenesis
in vivo, no effects on the proliferative ability of cancer
cells were observed in vitro. By contrast, Liu et al
(26) demonstrated that
overexpression of VASH1 in colorectal cancer cells was able to
inhibit growth and migration. Due to the results of the IHC in the
present study, demonstrating that VASH1 was negatively correlated
with tumor size and invasion depth, it was hypothesized that VASH1
may affect the growth and migration of EC cells directly. In order
to examine this hypothesis, VASH1 expression in EC cells was
altered using transfection and it was observed that overexpression
of VASH1 inhibited proliferation, clone formation and the migratory
ability of EC1 cells; however, silencing VASH1 enhanced
proliferation, clone formation and the migratory ability of EC9706
cells. The results of the present study demonstrated the direct
inhibitory effects of VASH1 on the growth and migration of EC
cells, consistent with the inhibition of VASH1 on colorectal cancer
cells reported by Liu et al (26). The results of the present study
suggest that the negative correlation of VASH1 with EC progression
and prognosis may be partially attributed to a direct effect on EC
cells.
However, tumor growth is a complex process and may
be regulated by various events, including proliferation, cell cycle
progression, apoptosis and angiogenesis (27,28).
Apart from proliferation and angiogenesis, Liu et al
(26) has also observed that VASH1
was able to effectively promote apoptosis and senescence in CRC
cells. However, apoptosis and senescence of EC cells were not
investigated in the present study. Tumor invasion is a process
which may be affected by the motility of tumor cells and
degradation of the extracellular matrix by proteases produced in
tumor cells (29,30). Although the results of the present
study demonstrated the inhibitory effect of VASH1 on EC cell
migration, this is insufficient to explain the negative correlation
of VASH1 with EC invasion depth. It is possible that VASH1 may
additionally affect the production of certain types of protease,
including matrix metalloproteinase or collagenase, in EC cells;
further research is required to investigate this hypothesis.
In conclusion, VASH1 in EC cells was negatively
correlated with tumor size, invasion depth and poor prognosis.
VASH1 is able to prevent EC progression by anti-angiogenesis, and
additionally through direct inhibition of the growth and migration
of EC cells. The results of the present study increase the
understanding of VASH1 in the context of tumors and contribute to
the search for improved treatments for EC.
References
|
1
|
Zhang H, Chen W, Duan CJ and Zhang CF:
Overexpression of HSPA2 is correlated with poor prognosis in
esophageal squamous cell carcinoma. World J Surg Oncol. 11:1412013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu YH, Yang F, Zhang SS, Zeng TT, Xie X
and Guan XY: High expression of biglycan is associated with poor
prognosis in patients with esophageal squamous cell carcinoma. Int
J Clin Exp Pathol. 6:2497–2505. 2013.PubMed/NCBI
|
|
3
|
Li M, Yang X, Zhang J, Shi H, Hang Q,
Huang X, Liu G, Zhu J, He S and Wang H: Effects of EHD2
interference on migration of esophageal squamous cell carcinoma.
Med Oncol. 30:3962013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hasan MR, Sharma R, Saraya A,
Chattopadhyay TK, DattaGupta S, Walfish PG, Chauhan SS and Ralhan
R: Slug is a predictor of poor prognosis in esophageal squamous
cell carcinoma patients. PLoS One. 8:e828462013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SG: What is the next step after
endoscopic resection of superficial esophageal squamous cell
carcinoma? Gut Liver. 9:693–694. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kern J, Steurer M, Gastl G, Gunsilius E
and Untergasser G: Vasohibin inhibits angiogenic sprouting in vitro
and supports vascular maturation processes in vivo. BMC Cancer.
9:2842009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe K, Hasegawa Y, Yamashita H,
Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, et al:
Vasohibin as an endothelium-derived negative feedback regulator of
angiogenesis. J Clin Invest. 114:898–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato Y: The vasohibin family.
Pharmaceuticals. 3:433–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamaki K, Moriya T, Sato Y, Ishida T,
Maruo Y, Yoshinaga K, Ohuchi N and Sasano H: Vasohibin-1 in human
breast carcinoma: A potential negative feedback regulator of
angiogenesis. Cancer Sci. 100:88–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao G, Yang Y, Tang Y, Han R and Sun Y:
Reduced expression of vasohibin-1 is associated with
clinicopathological features in renal cell carcinoma. Med Oncol.
29:3325–3334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Tian X, Zhang C and Wang Q:
Upregulation of vasohibin-1 expression with angiogenesis and poor
prognosis of hepatocellular carcinoma after curative surgery. Med
Oncol. 29:2727–2736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kosaka T, Miyazaki Y, Miyajima A, Mikami
S, Hayashi Y, Tanaka N, Nagata H, Kikuchi E, Nakagawa K, Okada Y,
et al: The prognostic significance of vasohibin-1 expression in
patients with prostate cancer. Br J Cancer. 108:2123–2129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kimura H, Miyashita H, Suzuki Y, Kobayashi
M, Watanabe K, Sonoda H, Ohta H, Fujiwara T, Shimosegawa T and Sato
Y: Distinctive localization and opposed roles of vasohibin-1 and
vasohibin-2 in the regulation of angiogenesis. Blood.
113:4810–4818. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen Z, Seppänen H, Kauttu T, Vainionpää
S, Ye Y, Wang S, Mustonen H and Puolakkainen P: Vasohibin-1
expression is regulated by transforming growth factor-β/bone
morphogenic protein signaling pathway between tumor-associated
macrophages and pancreatic cancer cells. J Interferon Cytokine Res.
33:428–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang YX, Du ZM, Jiao L, Shao Q, Fu S,
Shao JY, Zhu XF, Ernberg I and Li YH: Inhibition of MiR-155
suppresses cell migration in nasopharyngeal carcinoma through
targeting ZDHHC2. Int J Clin Exp Med. 8:8472–8484. 2015.PubMed/NCBI
|
|
17
|
Zhang Y and Lin Q: MicroRNA-145 inhibits
migration and invasion by down-regulating FSCN1 in lung cancer. Int
J Clin Exp Med. 8:8794–8802. 2015.PubMed/NCBI
|
|
18
|
Zhang T, Yu TT, Zhang DM, Hou XM, Liu XJ,
Zhao D and Shan L: Vasohibin-1 expression detected by
immunohistochemistry correlates with prognosis in non-small cell
lung cancer. Med Oncol. 31:9632014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan Y, Shen Z, Ye Y, Jiang K, Zhang H,
Shen C, Mustonen H, Puolakkainen P and Wang S: A novel molecular
marker of prognosis in colorectal cancer: Vasohibin-1. Med Oncol.
31:8162014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miyashita H, Watanabe T, Hayashi H, Suzuki
Y, Nakamura T, Ito S, Ono M, Hoshikawa Y, Okada Y, Kondo T and Sato
Y: Angiogenesis inhibitor vasohibin-1 enhances stress resistance of
endothelial cells via induction of SOD2 and SIRT1. PLoS One.
7:e464592012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyashita H, Suzuki H, Ohkuchi A and Sato
Y: Mutual balance between vasohibin-1 and soluble VEGFR-1 in
endothelial cells. Pharmaceuticals (Basel). 4:782–793. 2011.
View Article : Google Scholar :
|
|
22
|
Lv ZD, Zhang L, Liu XP, Jin LY, Dong Q, Li
FN, Wang HB and Kong B: NKD1 down-regulation is associated with
poor prognosis in breast invasive ductal carcinoma. Int J Clin Exp
Pathol. 8:4015–4021. 2015.PubMed/NCBI
|
|
23
|
Shen Y, Zhang F, Lan H, Chen K, Zhang Q,
Xie G, Teng L and Jin K: FRZB up-regulation is correlated with
hepatic metastasis and poor prognosis in colon carcinoma patients
with hepatic metastasis. Int J Clin Exp Pathol. 8:4083–4090.
2015.PubMed/NCBI
|
|
24
|
Strong AL, Ohlstein JF, Biagas BA, Rhodes
LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S, et
al: Leptin produced by obese adipose stromal/stem cells enhances
proliferation and metastasis of estrogen receptor positive breast
cancers. Breast Cancer Res. 17:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pathak R, Philizaire M and Mujtaba S:
Dichotomy in the Epigenetic Mark Lysine Acetylation is Critical for
the Proliferation of Prostate Cancer Cells. Cancers (Basel).
7:1622–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Han B, Zhang Q, Dou J, Wang F, Lin
W, Sun Y and Peng G: Vasohibin-1 suppresses colon cancer.
Oncotarget. 6:7880–7898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rusckowski M, Wang Y, Blankenberg FG,
Levashova Z, Backer MV and Backer JM: Targeted scVEGF/(177)Lu
radiopharmaceutical inhibits growth of metastases and can be
effectively combined with chemotherapy. EJNMMI Res. 6:42016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vanajothi R and Srinivasan P: An
anthraquinone derivative from Luffa acutangula induces apoptosis in
human lung cancer cell line NCI-H460 through p53-dependent pathway.
J Recept Signal Transduct Res. 36:292–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiu K, Huang Z, Huang Z, He Z and You S:
miR-22 regulates cell invasion, migration and proliferation in
vitro through inhibiting CD147 expression in tongue squamous cell
carcinoma. Arch Oral Biol. 66:92–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JS, Li HS, Huang JQ, Dong SH, Huang
ZJ, Yi W, Zhan GF, Feng JT, Sun JC and Huang XH: MicroRNA-379-5p
inhibits tumor invasion and metastasis by targeting FAK/AKT
signaling in hepatocellular carcinoma. Cancer Lett. 375:73–83.
2016. View Article : Google Scholar : PubMed/NCBI
|