Introduction
Gastric carcinoma is one of the most common
gastrointestinal malignancies worldwide, and is developed mainly in
gastric endothelial tissue. It is characterized by high incidence
and mortality rates, and it was the second most common type of
cancer in China in 2009, thus posing a serious threat for global
health (1). Cisplatin (DDP) has
significant anticancer effects on gastric carcinoma; however, its
use is limited due to its severe side effects (2). In addition, DDP has been associated
with the development of drug resistance, which may result in
failure of anticancer treatment (3–5).
Therefore, the development of novel therapeutic strategies, as well
as novel drug combinations, characterized by high efficiency and
low toxicity, is of great clinical significance for the treatment
of patients with gastric cancer.
Several polyether ionophores have demonstrated
anticancer activity against the proliferation of various cell
types, such as leukemia, colon carcinoma and prostate cancer cells,
and cancer stem cells, including tumors that exhibit multi-drug
resistance (6). Salinomycin (SAL)
is a carboxyl polyether, first extracted from Streptomyces
albus in 1974 (7). Due to its
cation-neutralizing properties it can potently inhibit the growth
of most Gram-positive bacteria and various Coccidia (8–10).
Gupta et al reported that the inhibitory effects of SAL on
breast cancer stem cell proliferation were ~100 times more potent
than the chemotherapeutic agent paclitaxel (11). Previous studies have demonstrated
that SAL exhibited anticancer effects in various types of cancer
and may have potential as a novel anticancer agent (8,12–18).
It has previously been reported that nuclear factor
(NF)-κB may be implicated in the development of tumor drug
resistance (19), whereas SAL was
demonstrated to effectively inhibit the proliferation of cancer
stem cells with high drug resistance (20–21).
Therefore, it may be hypothesized that SAL can inhibit the
activation of NF-κB, and thus increase the susceptibility of
gastric cancer cells to DDP. In the present study, the anticancer
effects of SAL, DDP and their combination were evaluated in the
SGC-7901 gastric cancer cell line. In addition, the mechanisms
underlying their actions in the induction of cancer cell apoptosis
were investigated.
Materials and methods
Reagents
SAL was purchased from Shunbo Biological Technology
and Engineering Co., Ltd. (Shanghai, China). DDP was obtained from
Jiangsu Hansoh Pharmaceutical Group Co., Ltd. (Jiangsu, China). MTT
solution was purchased from Merck KGaA (Darmstadt, Germany).
RPMI-1640 medium was purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Fetal bovine serum (FBS) was purchased from
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.
(Hangzhou, China). Propidium iodide (PI) was purchased from Merck
KGaA. Acridine orange (AO) was obtained from Amresco, LLC (Solon,
OH, USA). Annexin V-fluorescein isothiocyanate (FITC)/PI apoptosis
detection kit was purchased from BD Biosciences (Franklin Lakes,
NJ, USA). The rabbit anti-human NF-κB p65 polyclonal antibody (cat.
no. A00224) was obtained from GenScript (Nanjing) Co., Ltd.
(Nanjing, China). The 3,3′-diaminobenzidine (DAB) color developing
kit, rabbit anti-human Fas protein ligand (L) polyclonal antibody
(cat. no. BA0049) and biotin-labeled secondary antibody (cat. no.
BA1003) were purchased from Wuhan Boster Biological Technology,
Ltd. (Wuhan, China).
Cell culture
The SGC-7901 human gastric cancer cell line was
purchased from Digestion Research Center of Xi'an Jiaotong
University Health Science Center (Xi'an, China). Cells were
cultured in RPMI-1640 medium, supplemented with 10% FBS, 100 U/ml
penicillin and 100 U/ml streptomycin and maintained in a humidified
5% CO2 atmosphere at 37°C.
MTT assay
SGC-7901 cells at the logarithmic growth phase were
seeded in 96-well plates at a density of 1×104 cells/well, and
incubated at 37°C overnight to form a monolayer. Cells were divided
into the following four groups: SAL group, where cells were treated
with 4, 8 and 16 µmol/l SAL (dissolved in ethanol); DDP group,
where cells were treated with 6 µmol/l DDP (dissolved in normal
saline); combination group, where cells were treated with SAL (4, 8
and 16 µmol/l) and DDP (6 µmol/l); and control group, where cells
did not receive drug treatment. Experiments were repeated 5 times
for each group. Cells were incubated in a 95% humidified atmosphere
at 37°C and 5% CO2 for 24, 48 and 72 h. Following
incubation, MTT solution (20 µl) was added and cells were incubated
at 37°C for an additional 4 h. Subsequently, the supernatants were
removed and the crystals were dissolved with 150 µl DMSO. The
absorbance at 490 nm was measured to assess cellular proliferation.
Results were averaged from three independent measurements.
Cellular morphology
SGC-7901 cells were seeded in a 6-well plate at a
density of 2×105 cells/well. Following incubation for 24 h, drugs
were added. The cells were divided into four groups: SAL group (8
µmol/l), DDP group (6 µmol/l), combination group [SAL (8 µmol/l)
and DDP (6 µmol/l)] and control group. Following incubation for 48
h, cellular morphology was observed under an inverted
phase-contrast microscope (Nikon Corporation, Tokyo, Japan). All
experiments were repeated three times.
In another set of experiments, following drug
treatment for 48 h as aforementioned, cells were washed twice with
PBS and fixed with 75% ice cold ethanol for 2 h at −20°C.
Subsequently, PI (50 µg/l) was added and incubated at 4°C for 30
min in the dark; after which, AO (50 µg/ml) was added and incubated
at 4°C for 10 min in the dark. Cellular morphology was observed
under a fluorescence microscope (Nikon Corporation). All
experiments were repeated three times.
Flow cytometry
SGC-7901 cells were seeded in a 6-well plate at a
density of 2×105 cells/well. Following incubation for 24 h, fresh
medium containing 0.5% FBS was added and cells were cultured for an
additional 24 h to synchronize. Cells received treatment with
different drugs as aforementioned and were incubated for 48 h.
Subsequently, cells were digested with trypsin, collected and fixed
with 75% ice-cold ethanol for 2 h at −20°C. The cell suspension was
centrifuged at 350 × g for 5 min at room temperature and the
supernatant was discarded. Following washing with PBS, cells were
incubated with RNase (20 µg/l) at 37°C for 30 min. For cell cycle
analysis, cells were fixed with 75% ice-cold ethanol for 2 h at
−20°C and were then stained with PI (50 µg/l) for 30 min at 4°C.
Cell cycle analysis was performed using a Sysmex CyFlow® Cube 8
flow cytometer (Sysmex Europe GmbH, Norderstedt, Germany) and data
were analyzed using FCS Express Application V3 software (De Novo
Software, Glendale, CA, US).
To evaluate cellular apoptosis, SGC-7901 cells were
prepared as aforementioned in the Cellular morphology subsection.
Following drug treatment for 48 h, cells were collected, washed
twice with PBS, transferred to Eppendorf tubes and stained with
Annexin V-FITC and PI for 10 min at 4°C, according to the
manufacturer's protocol. The apoptotic rate was assessed in all
experimental groups using a Sysmex CyFlow® Cube 8 flow cytometer
(Sysmex Europe GmbH) and data were analyzed using FCS Express
Application V3 software (De Novo Software).
Immunocytochemistry
SGC-7901 cells were prepared as aforementioned in
the Cellular morphology subsection. Following incubation for 48 h,
cells were washed with PBS three times and fixed with 4%
paraformaldehyde for 20 min at room temperature. After air-drying,
cells were fixed to glass slides using neutral gum and
permeabilized with 0.3% Triton X-100 for 15 min at room
temperature. Following a wash with PBS, cells were incubated with
trypsin at 37°C for 30 min followed by 3%
H2O2 for 20 min at 37°C, the cells were then
blocked with goat serum (Wuhan Boster Biological Technology, Ltd.)
for 30 min at 37°C. Subsequently, cells were incubated with
anti-NF-κB p65 (dilution 1:100) and anti-FasL primary antibodies
(dilution 1:100) at 4°C overnight. Following a wash with PBS, cells
were incubated with biotin-labeled secondary antibodies (dilution
1:500) (cat. no. BA1003; Wuhan Boster Biological Technology, Ltd.)
for 2 h at room temperature, and then stained with DAB in the dark
for 5 min. The staining was monitored under a microscope and the
slides were washed with water to terminate the reaction.
Hematoxylin was used for counter-staining at room temperature for 5
min. Hydrochloric acid alcohol (0.1%) was used to differentiate the
staining, and sections were dehydrated with alcohol, cleared with
xylene and sealed with neutral gum. Photomicrographs were captured
with a Nikon E600 microscope (Nikon Corporation, Tokyo, Japan).
Statistical analysis
The statistical significance of the difference
between groups was assessed by one-way analysis of variance,
followed by a post hoc least significant difference test. All
experiments were repeated three times. Data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference. The analysis was performed
using IBM SPSS software version 22.0 (IBM SPSS, Armonk, NY,
USA).
Results
Effects of SAL and DDP on SGC-7901
proliferation
To assess the inhibitory effects of SAL and DDP
alone, as well as their combination, on cellular proliferation, the
MTT assay was performed. Results revealed that SAL and DDP
inhibited the proliferation of SGC-7901 cells, and their inhibitory
rate increased with the concentration used and the incubation time
(Table I). The combination of SAL
(4, 8 and 16 µmol/l) and DDP (6 µmol/l) exerted a significant
inhibitory effect on cellular proliferation following 24, 48 and 72
h of incubation, compared with the control, DDP alone and SAL alone
groups (P<0.05). These results suggested that SAL may be able to
enhance the susceptibility of SGC-7901 cells to DDP.
 | Table I.Inhibitory rate of SAL and DDP on
SGC-7901 cellular proliferation. |
Table I.
Inhibitory rate of SAL and DDP on
SGC-7901 cellular proliferation.
|
| Inhibitory rate
(%) |
|---|
|
|
|
|---|
| Group | 24 h | 48 h | 72 h |
|---|
| Control | 0 | 0 | 0 |
| DDP (6 µmol/l) |
15.52±0.75a |
27.38±0.63a |
40.99±1.11a |
| SAL (4 µmol/l) |
17.63±1.48a |
31.56±1.34a |
42.48±1.24a |
| SAL (8 µmol/l) |
26.47±1.30a |
46.64±1.03a |
55.18±0.80a |
| SAL (16
µmol/l) |
33.75±1.46a |
58.23±1.09a |
70.91±1.82a |
| SAL (4 µmol/l) +
DDP (6 µmol/l) |
25.63±1.03a,b |
41.73±1.35a,b |
51.74±1.02a,b |
| SAL (8 µmol/l) +
DDP (6 µmol/l) |
38.54±1.14a,b |
57.36±1.17a,b |
69.98±1.08a,b |
| SAL (16 µmol/l) +
DDP (6 µmol/l) |
57.44±0.73a,b |
72.35±0.86a,b |
89.76±1.25a,b |
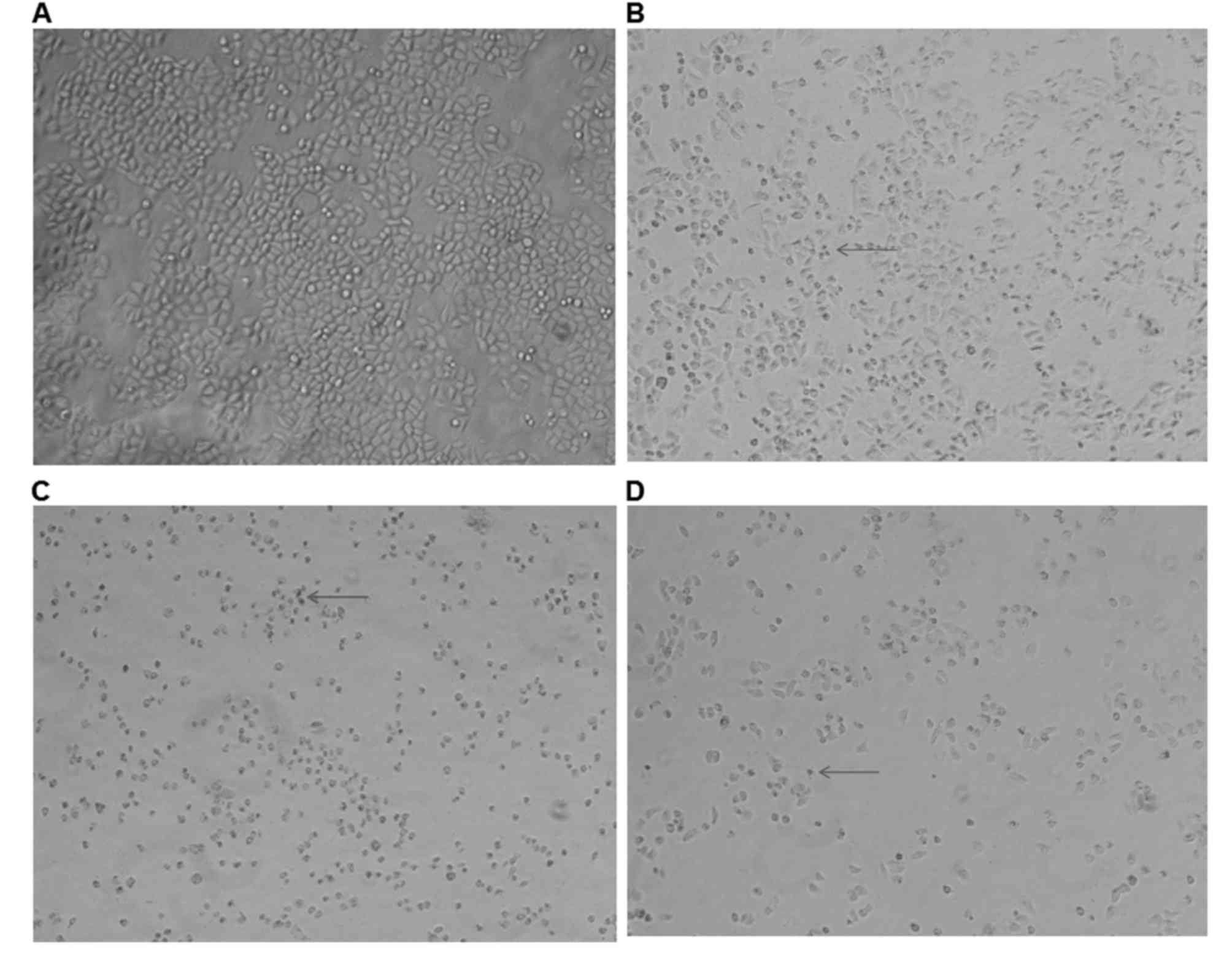
Alterations in cellular morphology
following treatment with SAL and DDP
To assess the effects of SAL and DDP alone, as well
as their combination, on cellular morphology, the shapes of
SGC-7901 cells were observed following treatment. In the control
group, SGC-7901 cells grew adherently, connected closely and
tightly, and were characterized by a full cytoplasm and a polygonal
or spindle shape (Fig. 1A).
Following treatment with SAL or DDP for 48 h, cells appeared to
shrink, gaps between cells became larger, cell-cell connections
disappeared, nuclear membranes started to shrink and cellular
volume decreased (Fig. 1B and C).
When the combination of SAL and DDP was applied, cellular volumes
appeared to be further reduced compared with the DDP alone and SAL
alone groups, and cell numbers also decreased (Fig. 1D).
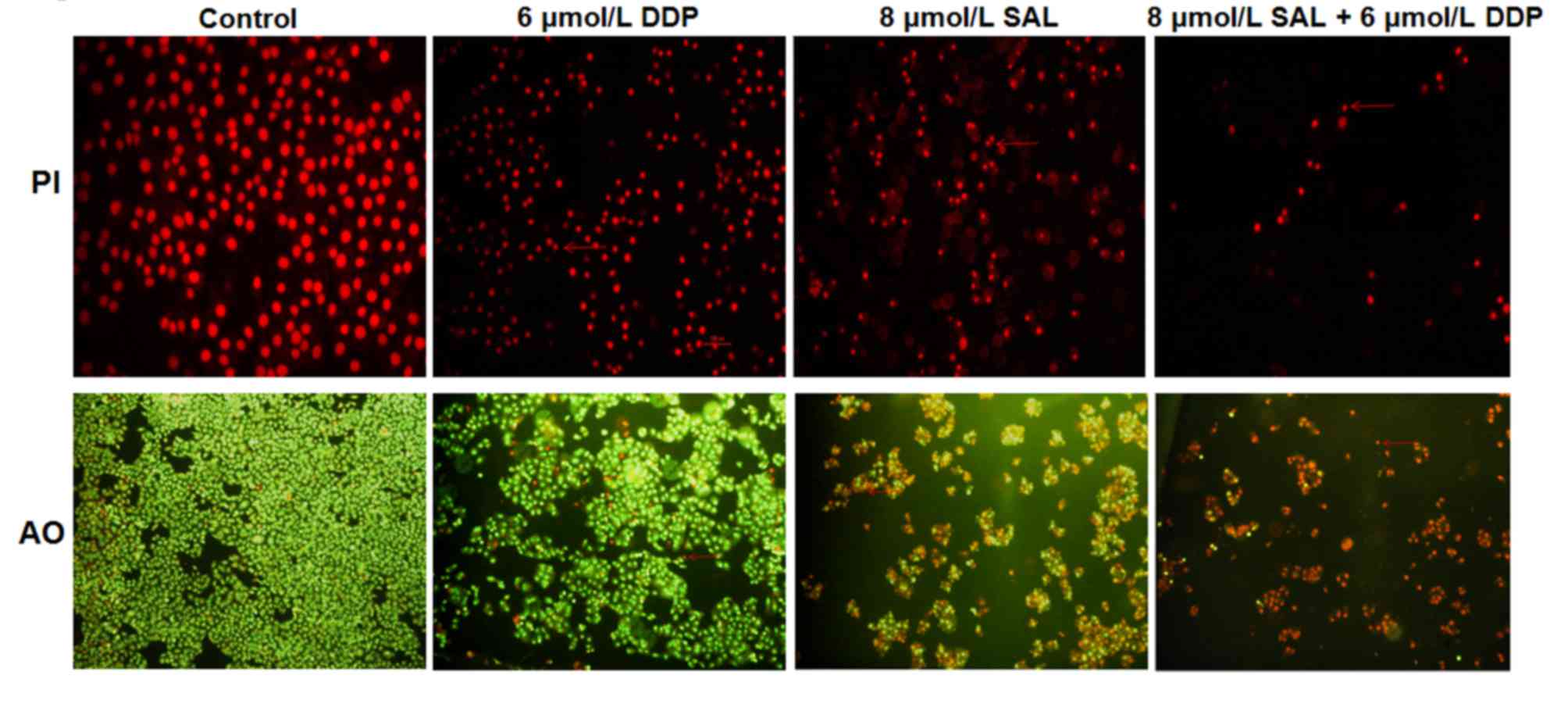
To determine the effects of SAL or DDP alone, as
well as their combination, on SGC-7901 apoptosis, the apoptotic
morphology of cells was observed under a fluorescence microscope.
SGC-7901 cells in the control group grew well and exhibited
homogeneous sizes, regular nuclei, smooth nuclear membranes and
evenly-distributed chromatin (Fig.
2). Following treatment with DDP or SAL, cellular morphology
appeared altered. Cell numbers were reduced, and DNA appeared
condensed and near the nuclear membrane. Apoptotic bodies were
formed, and the cellular nuclei became more condensed. The
combination of DDP and SAL produced more pronounced morphological
alterations on SGC-7901 cells compared with the DDP alone and SAL
alone groups (Fig. 2).
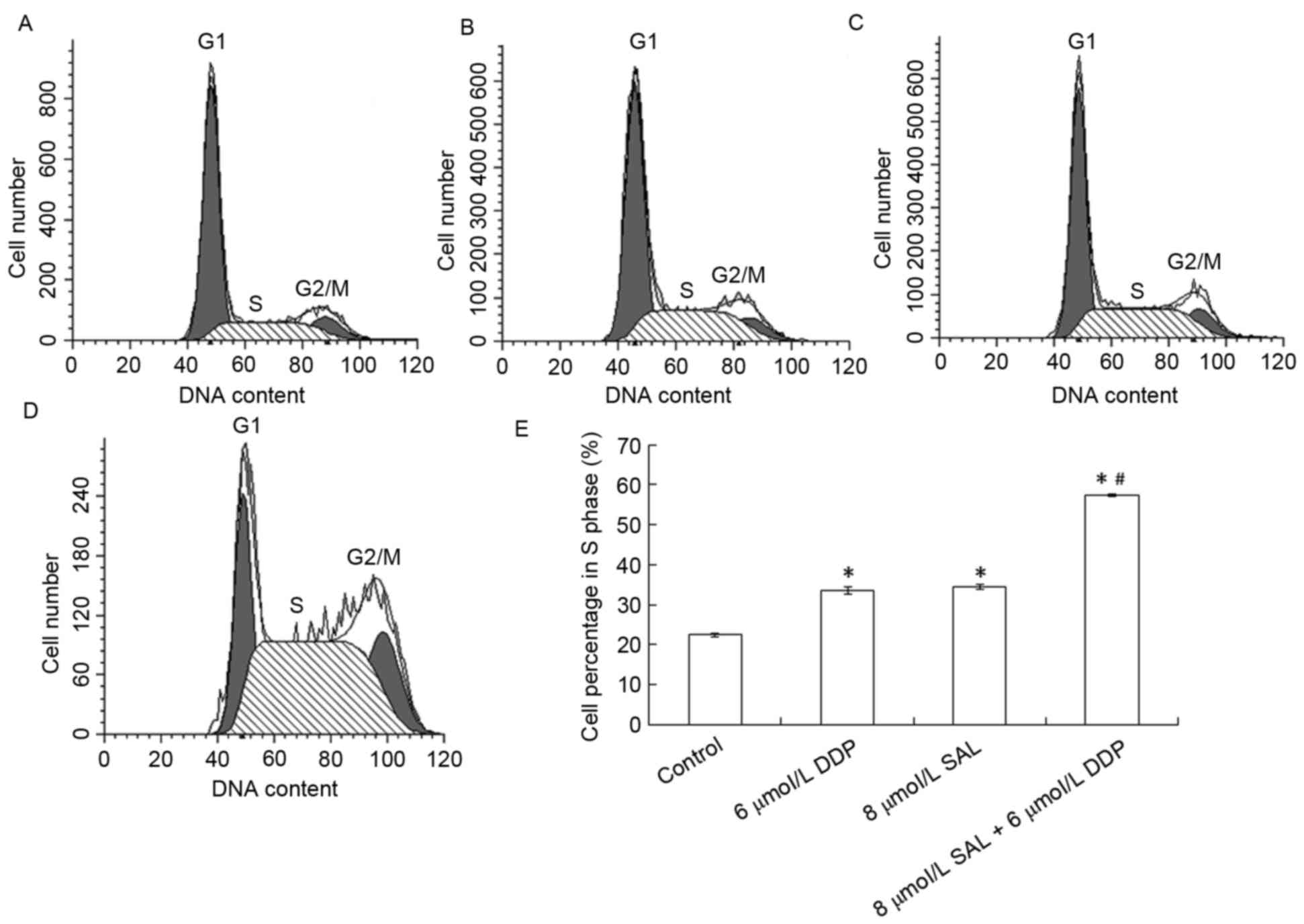
Effects of SAL and DDP on SGC-701 cell
cycle progression
To determine the effects of SAL or DDP alone, as
well as their combination, on cell cycle distribution, flow
cytometry was used. Following treatment with DDP or SAL for 48 h,
the number of SGC-7901 cells in G0/G1 phase
decreased, whereas the number of cells in S phase increased
(Fig. 3A-D). SGC-7901 cells in S
phase accounted for 33.52 and 34.57% of total cells in the DDP- and
SAL-treated groups, respectively, which were significantly
different compared with the control group (22.43%; P<0.05).
These results suggested that SAL and DDP caused S phase arrest in
SGC-7901 cells. The cells in S phase in the SAL and DDP combined
treatment group accounted for 57.45% of the population, which was
significantly higher compared with the control, DDP alone and SAL
alone groups (P<0.05).
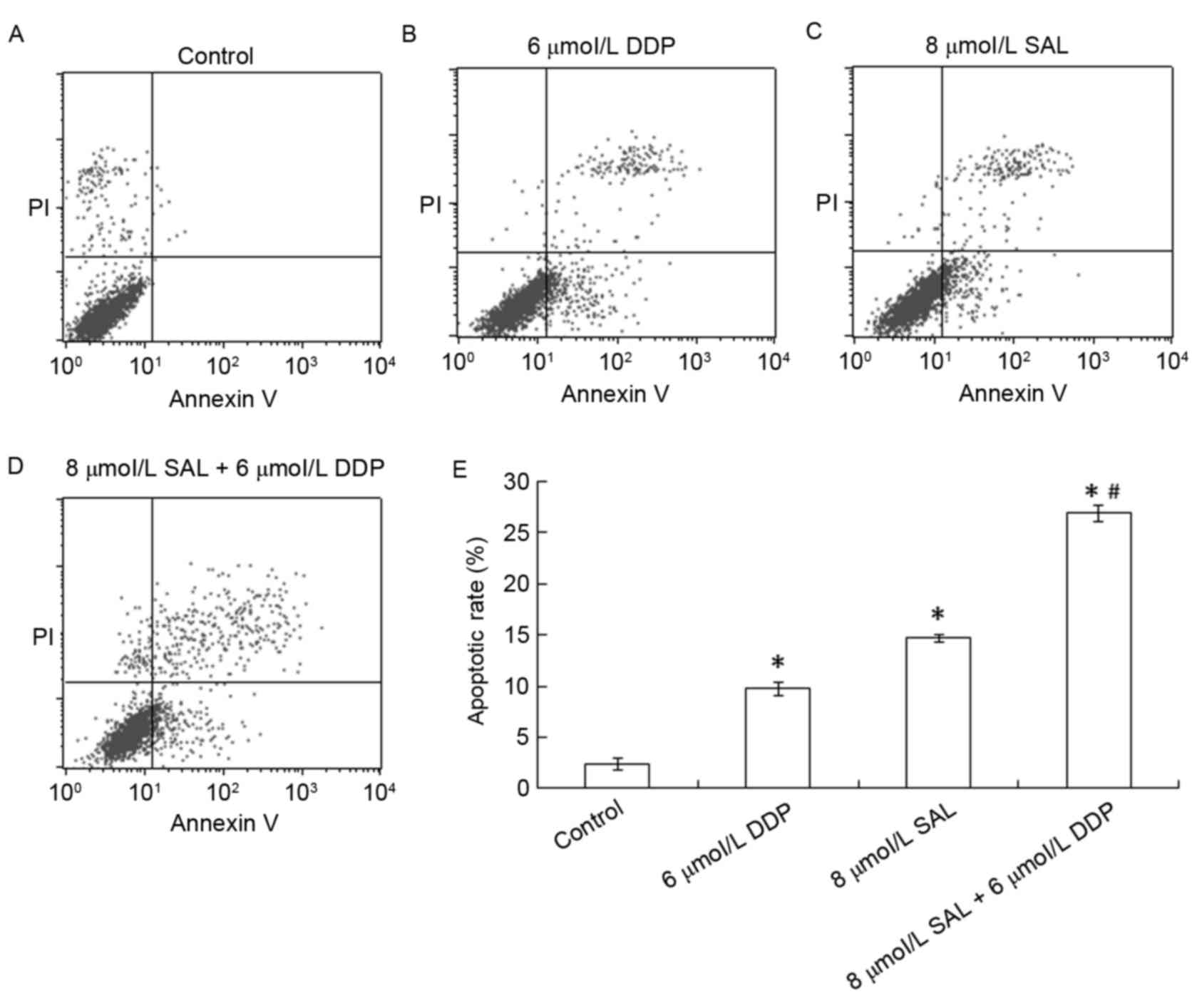
SAL and DDP induce cellular
apoptosis
To determine the effects of SAL or DDP alone, as
well as their combination, on SGC-7901 cell apoptosis, apoptotic
cells were detected using flow cytometry. Apoptotic rates of
SGC-7901 cells were 9.76 and 14.69% following treatment with DDP
and SAL respectively (Fig. 4), and
were significantly higher compared with the control group (2.37%;
P<0.05). The apoptotic rate of SGC-7901 cells was 27.79%
following treatment with the combination of DDP and SAL, which was
significantly higher compared with the control, DDP alone and SAL
alone groups (P<0.05). These results suggested that SAL and DDP
alone, as well as their combination, were able to induce SGC-7901
cell apoptosis, and the combined treatment produced more potent
proapoptotic effects.
Effects of SAL and DDP on NF-κB p65
and FasL expression
To investigate the mechanism underlying the
proapoptotic effects of SAL and DDP, as well as their combination,
immunohistochemistry was performed to assess the expression of
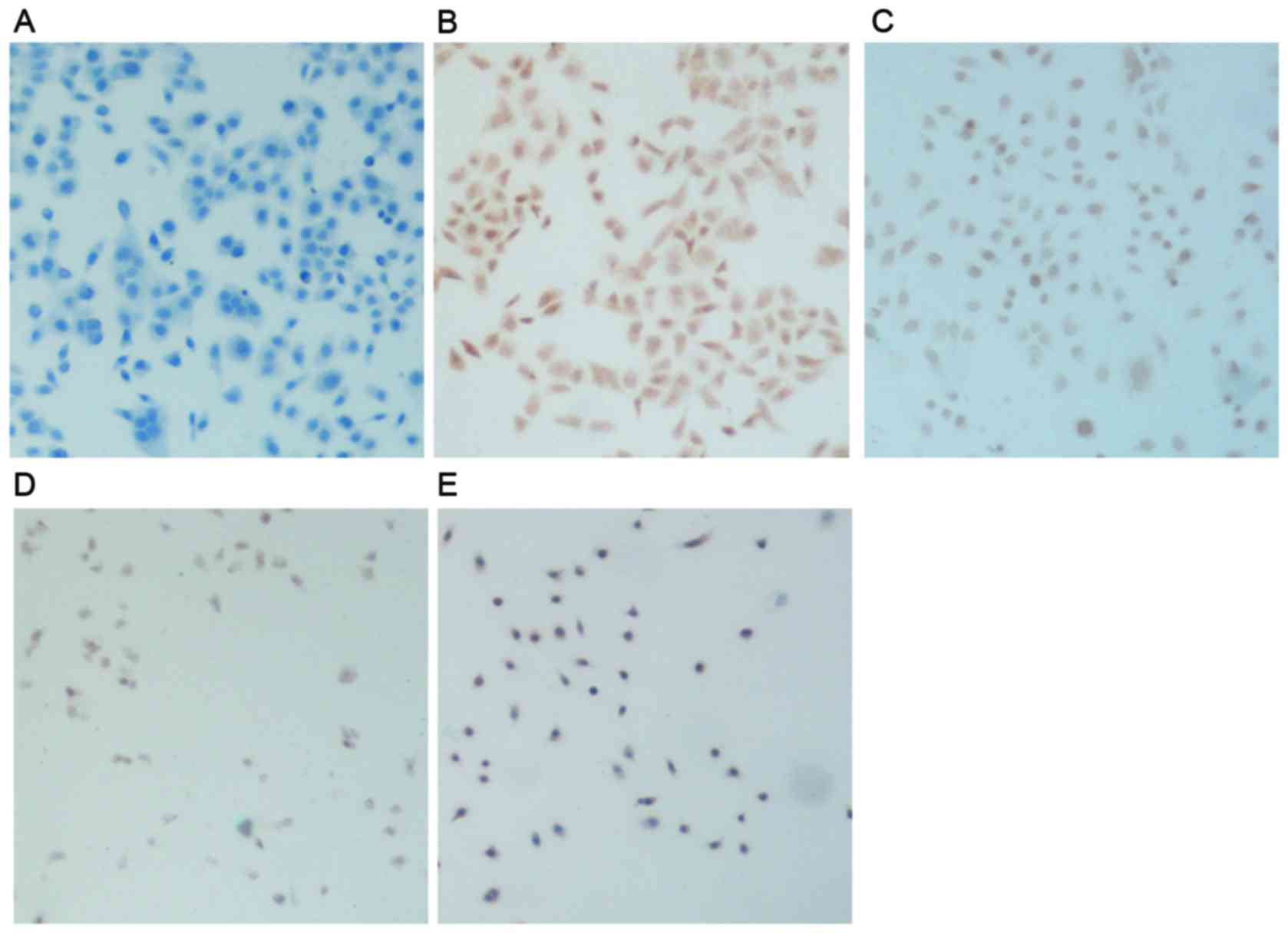
NF-κB p65 and FasL following treatment. The present results
demonstrated that NF-κB p65 was expressed in control cells, as
nuclei were stained dark brown (Fig.
5B). Following treatment with DDP or SAL, NF-κB p65 expression
was decreased, as the intensity of nuclear and cytosolic staining
appeared reduced (Fig. 5C and D).
The combination treatment group had much lower NF-κB p65 expression
than those in the control group and the DDP and SAL separate
treatment groups. The cell nuclei exhibited decreased brown color
and presented some blue color, and part of the cytosol showed light
brown color (Fig. 5E).
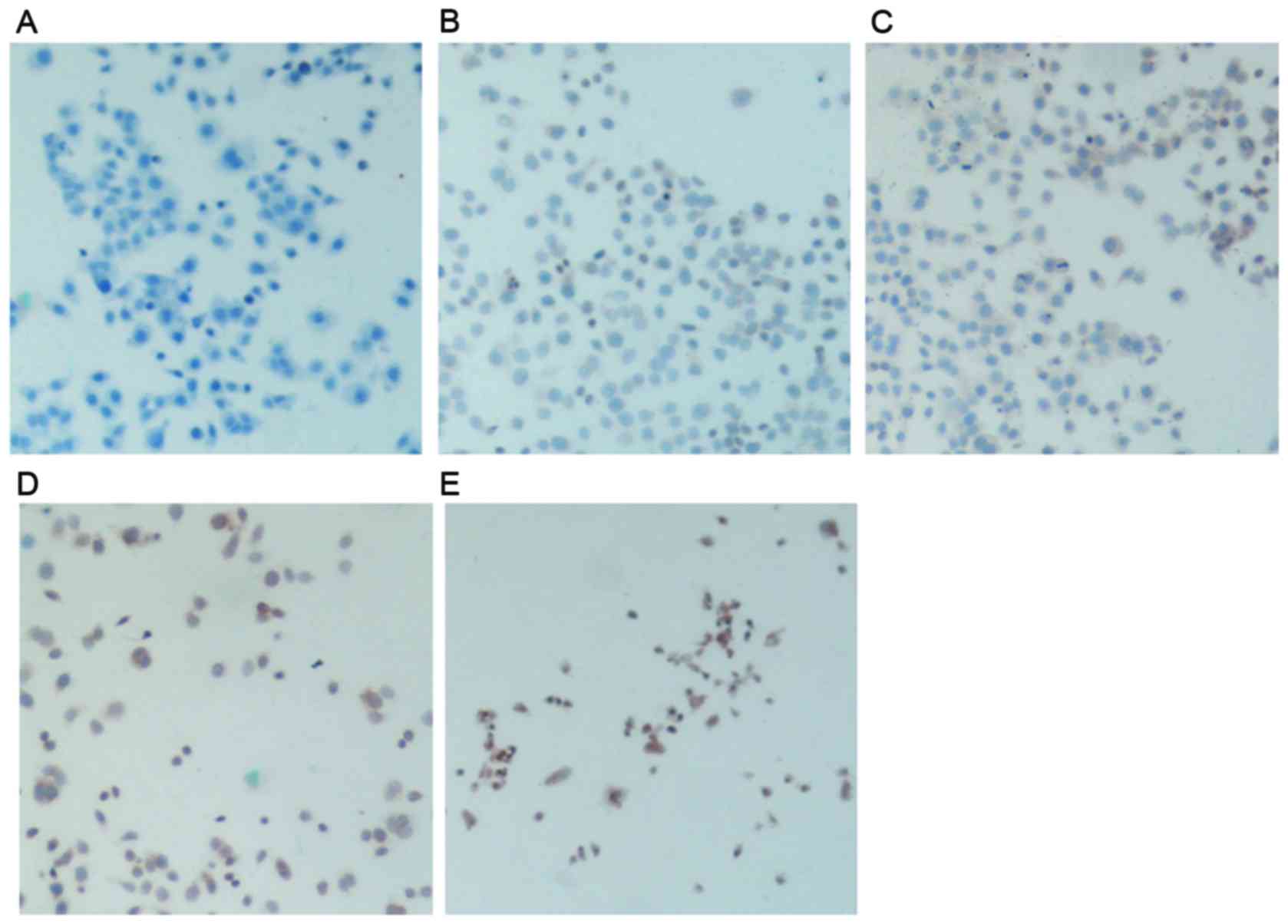
The expression of FasL in control SGC-7901 cells
appeared low, as revealed by the low intensity of brown staining
present in the cytosol and cell membrane (Fig. 6B). Following treatment with DDP or
SAL, the expression of FasL in the cytosol appeared to be
increased, as the intensity of staining increased. SAL-treated
gastric cancer cells exhibited higher FasL expression compared with
DDP-treated cells, as suggested by the higher intensity of nuclear
and cytosolic staining present in SAL-treated cells (Fig. 6C and D). Cells treated with a
combination of DDP and SAL exhibited increased FasL staining
compared with the control, DDP alone and SAL alone groups, as the
intensity of dark brown nuclear and cytosolic staining appeared
markedly increased (Fig. 6E).
Discussion
Gastric cancer is one of the most common malignant
tumors in the clinical setting. As an ionophore antibiotic, SAL may
contribute to the inhibition of tumor cell proliferation. However,
it has previously been reported that SAL may exhibit strong
neurotoxic effects (22). DDP is a
conventional chemotherapy drug used in clinical practice that
exhibits significant antitumor effects. However, it has been
reported to be prone to induce drug resistance during the treatment
of gastric cancer, which may eventually lead to treatment failure
(4,5). The results of the present study
suggested that SAL may enhance the susceptibility of SCG-7901
gastric cancer cells to DDP, and the mechanism underlying its
effects may involve NF-κB p65 downregulation and FasL
upregulation.
NF-κB is a nuclear transcription factor that has
been implicated in various physiological and pathophysiological
processes, such as embryonic development, tissue injury and repair,
inflammation, viral infection, tumor development and progression,
and in the regulation of apoptosis-related gene expression
(23–25). Under physiological conditions,
NF-κB remains in the cytosol in the form of inactive
p65/p50/inhibitor of κB (IκB-α) complexes, and thus its
transcriptional actions are suppressed (26). Following stimulation by cytokines,
physical and chemical factors, such as X-rays and chemotherapy
drugs, and other activators, IκB-α is phosphorylated and
ubiquitinated. As a result, the conformation of the p65/p50/IκB-α
complex is altered, allowing NF-κB to translocate into the nucleus
and activate the transcription of target genes (27,28).
Previous research has suggested that the suppression of NF-κB may
enhance apoptosis mediated by the Fas/FasL signaling pathway
(29–31). Fas and FasL are a pair of molecules
located on cell membranes, which promote apoptosis via the death
receptor pathway. Binding of FasL to Fas can initiate death
signaling cascades, leading to cancer cell apoptosis (32–34).
Chen et al reported that the expression of Fas/FasL genes
may be associated with NF-κB (29). Travert et al indicated that
NF-κB activation may indirectly inhibit Fas, tumor necrosis factor
(TNF) receptor 1 and TNF-related apoptosis-inducing ligand, to
ultimately promote cellular apoptosis (30). Therefore, since NF-κB and FasL may
serve a role in the promotion of tumor cell apoptosis (35–39),
the present study investigated the implication of NF-κB and FasL in
the mechanisms underlying the proapoptotic actions of SAL.
SAL has previously been reported to inhibit the
proliferation of breast cancer stem-like cells via an
apoptosis-independent pathway (40). Zhi et al demonstrated that
SAL selectively inhibited the proliferation of gastric cancer cells
characterized by high aldehyde dehydrogenase activity, which are
resistant to 5-fluorouracil and DDP (21). In the present study, SAL was
demonstrated to inhibit the proliferation of gastric cancer cells
in a dose- and time-dependent manner. These results are in
accordance with the study by Zhi et al (21), as they demonstrated the cytotoxic
effects of SAL on gastric cancer cells. Furthermore, the present
study suggested that the combination of SAL and DDP may possess
more potent cytotoxic potential compared with treatment with SAL or
DDP alone. The addition of SAL appeared to enhance the
susceptibility of gastric cancer cells to DDP. Treatment with SAL
or DDP alone, as well as their combination, was revealed to alter
cellular morphology, and combined treatment exhibited stronger
effects compared with separate treatments. PI and AO staining, and
flow cytometry demonstrated that the apoptotic rate of cells
treated with the combination of SAL and DDP was markedly higher
compared with the control, DDP alone and SAL alone groups. These
results suggested that SAL and DDP may be able to synergistically
induce gastric cancer cell apoptosis. These results are consistent
with the study by Liu et al, which reported that SAL alone
and in combination with vincristine induced apoptosis of Jurkat
cancer cells (41).
The present study demonstrated that SAL alone and in
combination with DDP could alter cell cycle distribution and
prolong the S phase, and combined treatment exhibited stronger
effects compared with treatment with SAL or DDP alone. These
results suggested that the mechanisms underlying the inhibitory
effects of SAL and DDP may involve interference in DNA synthesis
and replication in cancer cells. Immunocytochemistry was used to
further investigate the mechanisms underlying the proapoptotic
actions of SAL and DDP. The present results revealed that SAL and
DDP inhibited the translocation of NF-κB p65 into the nuclei of
cancer cells. The combination of SAL and DDP markedly downregulated
the expression of NF-κB p65 and upregulated the expression of FasL.
These results suggested that SAL and DDP may induce cancer cell
apoptosis via inhibiting the activation of NF-κB p65 and promoting
the activation of Fas/FasL pathways, consistent with previous
findings (29–31). Combination of the drugs enhanced
the proapoptotic actions of DDP on SGC-7901 cells. In accordance
with the present results, Parajuli et al reported that SAL
inhibited the nuclear translocation of NF-κB (42).
In conclusion, the present study demonstrated that
the novel anticancer agent SAL inhibited gastric cancer cell
proliferation, alone or in combination with DDP, and induced
cellular apoptosis. The present results suggested that the
mechanisms underlying its actions may involve upregulation of FasL
and downregulation of NF-κB p65 expression.
Acknowledgements
The present study was supported by the National
Science Foundation (grant no. 81470140) and the Health Science and
research project of Shaanxi Province (grant no. 2014D31).
References
|
1
|
Chen W, Zhang R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
2
|
Lenz HJ, Lee FC, Haller DG, Singh D,
Benson AB III, Strumberg D, Yanagihara R, Yao JC, Phan AT and Ajani
JA: Extended safety and efficacy data on S-1 plus cisplatin in
patients with untreated, advanced gastric carcinoma in a
multicenter phase II study. Cancer. 109:33–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li G, Yang F, Gu S, Li Z and Xue M:
MicroRNA-101 induces apoptosis in cisplatin-resistant gastric
cancer cells by targeting VEGF-C. Mol Med Rep. 13:572–578.
2016.PubMed/NCBI
|
|
4
|
Chen DD, Feng LC, Ye R, He YQ and Wang YD:
miR-29b reduces cisplatin resistance of gastric cancer cell by
targeting PI3K/Akt pathway. Zhongguo Yi Xue Ke Xue Yuan Xue Bao.
37:514–519. 2015.(In Chinese). PubMed/NCBI
|
|
5
|
Zhou X, Jin W, Jia H, Yan J and Zhang G:
MiR-223 promotes the cisplatin resistance of human gastric cancer
cells via regulating cell cycle by targeting FBXW7. J Exp Clin
Cancer Res. 34:282015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huczyński A: Polyether
ionophores-promising bioactive molecules for cancer therapy. Bioorg
Med Chem Lett. 22:7002–7010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyazaki Y, Shibuya M, Sugawara H,
Kawaguchi O and Hirsoe C: Salinomycin, a new polyether antibiotic.
J Antibiot (Tokyo). 27:814–821. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daugschies A, Gässlein U and Rommel M:
Comparative efficacy of anticoccidials under the conditions of
commercial broiler production and in battery trials. Vet Parasitol.
76:163–171. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Danforth HD, Ruff MD, Reid WM and Miller
RL: Anticoccidial activity of salinomycin in battery raised broiler
chickens. Poult Sci. 56:926–932. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mahmoudi N, de Julián-Ortiz JV, Ciceron L,
Gálvez J, Mazier D, Danis M, Derouin F and García-Domenech R:
Identification of new antimalarial drugs by linear discriminant
analysis and topological virtual screening. J Antimicrob Chemother.
57:489–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naujokat C, Fuchs D and Opelz G:
Salinomycin in cancer: A new mission for an old agent. Mol Med Rep.
3:555–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kopp F, Hermawan A, Oak PS, Ulaganathan
VK, Herrmann A, Elnikhely N, Thakur C, Xiao Z, Knyazev P, Ataseven
B, et al: Sequential salinomycin treatment results in resistance
formation through clonal selection of epithelial-like tumor cells.
Transl Oncol. 7:702–711. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Chae M, Kim WK, Kim YJ, Kang HS,
Kim HS and Yoon S: Salinomycin sensitizes cancer cells to the
effects of doxorubicin and etoposide treatment by increasing DNA
damage and reducing p21 protein. Br J Pharmacol. 162:773–784. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao Z, Sperl B, Ullrich A and Knyazev P:
Metformin and salinomycin as the best combination for the
eradication of NSCLC monolayer cellsand their alveospheres (cancer
stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status.
Oncotarget. 5:12877–12890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Antoszczak M, Popiel K, Stefańska J,
Wietrzyk J, Maj E, Janczak J, Michalska G, Brzezinski B and
Huczyński A: Synthesis, cytotoxicity and antibacterial activity of
new esters of polyether antibiotic - salinomycin. Eur J Med Chem.
76:435–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kopp F, Hermawan A, Oak PS, Herrmann A,
Wagner E and Roidl A: Salinomycin treatment reduces metastatic
tumor burden by hampering cancer cell migration. Mol Cancer.
13:162014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huczynski A: Salinomycin: A new cancer
drug candidate. Chem Biol Drug Des. 79:235–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moretti M, Bennett J, Tornatore L,
Thotakura AK and Franzoso G: Cancer: NF-κB regulates energy
metabolism. Int J Biochem Cell Biol. 44:2238–2243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni M, Xiong M, Zhang X, Cai G, Chen H,
Zeng Q and Yu Z: Poly (lactic-co-glycolic acid) nanoparticles
conjugated with CD133 aptamers for targeted salinomycin delivery to
CD133+ osteosarcoma cancer stem cells. Int J Nanomedicine.
10:2537–2554. 2015.PubMed/NCBI
|
|
21
|
Zhi QM, Chen XH, Ji J, Zhang JN, Li JF,
Cai Q, Liu BY, Gu QL, Zhu ZG and Yu YY: Salinomycin can effectively
kill ALDH (high) stem-like cells on gastric cancer. Biomed
Pharmacother. 65:509–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song SP, Zhang XG, Wang M, et al: Change
and significance of serum creatine kinase and its isoenzyme in
salinomycin poisoning patients. Zhong Guo Wei Sheng Jian Yan Za
Zhi. 21:28–29. 2011.
|
|
23
|
Cai Z, Tchou-wong KM and Rom WN: NF-kappaB
in lung tumorigenesis. Cancers (Basel). 3:4258–4268. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varfolomeev E, Goncharov T, Maecker H,
Zobel K, Kömüves LG, Deshayes K and Vucic D: Cellular inhibitors of
apoptosis are global regulators of NF-κB and MAPK activation by
members of the TNF family of receptors. Sic Signal. 5:ra222012.
|
|
26
|
Majdalawieh A and Ro HS: Regulation of
IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel
proinflammatory mediator in macrophages. Mediators Inflamm.
2010:8238212010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee HJ, Seo HS, Kim GJ, Jeon CY, Park JH,
Jang BH, Park SJ, Shin YC and Ko SG: Houttuynia cordata Thunb
inhibits the production of pro-inflammatory cytokines through
inhibition of the NFκB signaling pathway in HMC-1 human mast cells.
Mol Med Rep. 8:731–736. 2013.PubMed/NCBI
|
|
28
|
Ferreiro DU and Komives EA: Molecular
mechanisms of system control of NF-kappaB signaling by
IkappaBalpha. Biochemistry. 49:1560–1567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, Dong Y, Xu C, Jiang L, Chen Y,
Jiang C, Hou W and Li W: Involvement of a chromatin modifier in
response to mono-(2-ethylhexyl) phthalate (MEHP)-induced Sertoli
cell injury: Probably an indirect action via the regulation of
NF-κB/FasL circuitry. Biochem Biophys Res Commun. 440:749–755.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Travert M, Ame-Thomas P, Pangault C,
Morizot A, Micheau O, Semana G, Lamy T, Fest T, Tarte K and
Guillaudeux T: CD40 ligand protects from TRAIL-induced apoptosis in
follicular lymphomas through NF-kappaB activation and up-regulation
of c-FLIP and Bcl-xL. J Immunol. 181:1001–1111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Zhao S, Wang HX and Zou P:
Inhibition of NF-kappa B can enhance Fas-mediated apoptosis in
leukemia cell line HL-60. Front Med China. 4:323–328. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strasser A, Jost PJ and Nagata S: The many
roles of FAS receptor sig-naling in the immune system. Immunity.
30:180–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mahmood Z and Shukla Y: Death receptors:
Targets for cancer therapy. Exp Cell Res. 316:887–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen L, Park SM, Tumanov AV, Hau A, Sawada
K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E and Peter ME:
CD95 promotes tumour growth. Nature. 465:492–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kong FC, Zhang JQ, Zeng C, Chen WL, Ren
WX, Yan GX, Wang HX, Li QB and Chen ZC: Inhibitory effects of
parthenolide on the activity of NF-κB in multiple myeloma via
targeting TRAF6. J Huazhong Univ Sci Technolog Med Sci. 35:343–349.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Diab S, Fidanzi C, Léger DY, Ghezali L,
Millot M, Martin F, Azar R, Esseily F, Saab A, Sol V, et al:
Berberis libanotica extract targets NF-κB/COX-2, PI3K/Akt and
mitochondrial/caspase signalling to induce human erythroleukemia
cell apoptosis. Int J Oncol. 47:220–230. 2015.PubMed/NCBI
|
|
37
|
Gmeiner WH, Jennings-Gee J, Stuart CH and
Pardee TS: Thymineless death in F10-treated AML cells occurs via
lipid raft depletion andFas/FasL co-localization in the plasma
membrane with activation of the extrinsic apoptotic pathway. Leuk
Res. 39:229–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu W, Lin YT, Yan XL, Ding YL, Wu YL,
Chen WN and Lin X: Hepatitis B virus core protein inhibits
Fas-mediated apoptosis of hepatoma cells via regulation of
mFas/FasL and sFas expression. FASEB J. 29:1113–1123. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li L, Yao YC, Fang SH, Ma CQ, Cen Y, Xu
ZM, Dai ZY, Li C, Li S, Zhang T, et al: Pigment epithelial-derived
factor (PEDF)-triggered lung cancer cell apoptosis relies on p53
protein-driven Fas ligand (Fas-L) up-regulation and Fas protein
cell surface translocation. J Biol Chem. 289:30785–30799. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
An H, Kim JY, Lee N, Cho Y, Oh E and Seo
JH: Salinomycin possesses anti-tumor activity and inhibits breast
cancer stem-like cells via an apoptosis-independent pathway.
Biochem Biophys Res Commun. 466:696–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu PP, Zhu JC, Liu GX, Shui CX and Li XM:
Salinomycin enhances the apoptosis of T-cell acute lymphoblastic
leukemia cell line jurkat cells induced by vincristine. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 23:653–657. 2015.(In Chinese).
PubMed/NCBI
|
|
42
|
Parajuli B, Shin SJ, Kwon SH, Cha SD,
Chung R, Park WJ, Lee HG and Cho CH: Salinomycin induces apoptosis
via death receptor-5 up-regulation in cisplatin-resistant ovarian
cancer cells. Anticancer Res. 33:1457–1462. 2013.PubMed/NCBI
|