Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory
disease, with activated T cells serving important roles in the
pathological progress (1,2). Pristane-induced arthritis (PIA) in
rats is one of the animal models commonly used for the study of RA,
and it exhibits many clinical features similar to human RA
(3,4). Pristane is a small alkane molecule,
that induces arthritogenic T cells and cannot form a stable complex
with the major histocompatibility complex class II molecule.
Therefore, the PIA rat model is suitable to study the pathological
role of T cells or cytokines in arthritis progression (5,6).
T helper (Th) 17 cells were discovered in 2005, and
were demonstrated to secrete interleukin (IL)-17A, IL-17F, IL-21
and IL-22, to express the key transcription factors RAR-related
orphan receptor (ROR) γt and RORα, and to be important in local
inflammation (7,8). Another subtype of T helper cells was
discovered in 2009, Th22 cells, and were demonstrated to secrete
IL-22, to exhibit the phenotype
CCR4+CCR6+CCR10+ and to express
the key transcription factor aryl hydrocarbon receptor (9). The receptor complex that binds IL-22
is IL-10 receptor (R) 2/IL-22R1. Since IL-10R2 is expressed
ubiquitously, expression of IL-22R1 indicates the location of
functional IL-22 signaling (10).
In vivo, IL-22 binding protein (BP), a soluble form of the
IL-22R1 subunit which binds IL-22 without activating downstream
signaling, is a natural antagonist of IL-22 signaling (11). IL-17A and IL-22 are associated with
RA or RA animal models. IL-17A mRNA expression levels are increased
in RA patients and in the synovial tissues of collagen-induced
arthritis (CIA) mice (12). IL-17A
is also increased in the synovial fluid of patients with RA
(13). Ikeuchi et al
(14) reported that IL-22 mRNA
expression levels are increased in the synovial tissues of patients
with RA and that IL-22 is a pro-inflammatory cytokine, while Sarkar
et al (15) reported that
IL-22 reduces the severity of CIA and is protective against the
disease in mice, suggesting that these two studies reported
contradictory results. Further studies that will lead to a more
comprehensive understanding of the expression pattern of IL-22 in
an animal model of RA are thus needed.
In the present report, different time points were
examined in a PIA rat model, in order to simulate the initial
phase, onset, acute and chronic arthritis phases at 6, 12, 26 and
70 days following pristane injection, respectively. The expression
of various Th17 and Th22 cell-related cytokines, cytokine receptors
and transcription factors were measured in the different disease
phases in the PIA rats. IL-17F and IFN-γ were significantly
increased in the synovium of acute PIA rats, while IL-22 expression
was increased predominantly in the chronic phase of PIA rats.
Materials and methods
Animals and the PIA model
Dark Agouti rats (originating from Zentralinstitut
Fur Versuchstierzucht, Hannover, Germany) were bred in the animal
house under specific pathogen-free conditions and with 12 h
light/dark cycles. The rats were housed in polystyrene cages at 4
rats/cage with standard rodent chow and water ad libitum. A total
of 42 rats (21 female and 21 male; age, 8 to 12 weeks; weight,
174.5±34.2 g), were randomly divided into two groups matched for
sex and age. In the PIA group, rats were subcutaneously injected
with 150 µl pure pristane (Acros Organics; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at the base of the tail.
Control rats were subcutaneously injected with 150 µl PBS and
sacrificed at 26 days following the injection. The PIA group was
then divided into four subgroups (8–10 rats per group), which were
sacrificed at different days post-injection: Day (D) 6 was
considered as the initial phase, D12 as the onset, D26 as acute
arthritis and D70 as chronic arthritis (3). Rats were anesthetized by
intraperitoneal administration of 2% pentobarbital sodium (0.15
ml/100 g body weight). Spleens and synovium from the right
posterior ankles were harvested from the rats immediately following
sacrifice, and stored at −80°C. The left posterior paws of the rats
were removed and fixed with 4% paraformaldehyde at 20°C for 1 week,
then decalcified in 12.5% EDTA solution at 20°C for 4 weeks, during
which the solution was changed every two days. The decalcified
samples were subsequently embedded in paraffin and cut into 6 µm
tissue sections. The experiments were approved by the Institutional
Animal Ethics Committee of the Xi'an Jiaotong University (Xi'an,
China).
RNA isolation and reverse
transcription (RT)
Total RNA was isolated from spleens and synovium
using the TRIzol method (Invitrogen; Thermo Fisher Scientific,
Inc.). Quantification of RNA samples was performed using a
GeneQuest CE 2301 instrument (Cecil Instruments, Inc., Cambridge,
UK), and samples with optical density (OD) 260/OD280 ratios of
1.8–2.0 were selected for further analysis. The quality of the
extracted RNA was also examined using an automated gel imaging
analysis system (Syngene, Frederick, MA, USA). cDNA was prepared by
RT using the RevertAid First Strand cDNA synthesis kit, according
to manufacturer's protocol (Fermentas; Thermo Fisher Scientific,
Inc.).
Quantitative polymerase chain reaction
(qPCR)
The cDNA product was diluted 8 times with pure
water. PCR reactions were prepared with 4 µl diluted cDNA, 5 µl 2X
SYBR® Premix Ex Taq™ II (Takara Biotechnology Co., Ltd., Dalian,
China), 0.5 µl sense primers and 0.5 µl antisense primers into 200
µl PCR tubes, and then run on a iQ5 Real-Time PCR detection
instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
reaction conditions were as follows: 95°C for 3 min, then 40 cycles
of 95°C for 10 sec, annealing temperature for 20 sec (as
illustrated in Table I for each
primer) and 72°C for 20 sec. Primer sequences and annealing
temperatures are listed in Table
I, and β-actin was used as the internal control. Relative gene
expression was calculated by the ΔΔCq method (16).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer | Sequence (5′-3′) | Product (bp) | Annealing temperature
(°C) |
|---|
| rIL-17A | Sense |
CTACCTCAACCGTTCCACTT | 191 | 65 |
|
| Antisense |
ACTTCTCAGGCTCCCTCTTC |
|
|
| rIL-17F | Sense |
CTCTGCTGCTGTTGATGT | 228 | 54 |
|
| Antisense |
GGTCTCGGGTGATATTGT |
|
|
| rIL-22 | Sense |
TTCTCCTCCCAGTTATCAGTTGT | 205 | 54 |
|
| Antisense |
GGTGCGGTTGACGATGTAT |
|
|
| rIFN-γ | Sense |
CCCTCTCTGGCTGTTACTGC | 149 | 65 |
|
| Antisense |
TTTCGTGTTACCGTCCTTTTG |
|
|
| rIL-22R1 | Sense |
TGAGGAGGAGGCACAGAGACC | 141 | 60 |
|
| Antisense |
ACAGAGGACAGGAGGGACAGC |
|
|
| rIL-22BP | Sense |
CGTATGGACAGGGACAATGGAAAG | 102 | 61 |
|
| Antisense |
AGTATGGCTCGTATGGGTCTAAGG |
|
|
| rRORα | Sense |
TGAGAACTACCAGAACAAGCAGAG | 192 | 61 |
|
| Antisense |
GTCAAAGGCACGGCACATCC |
|
|
| rβ-actin | Sense |
CTATCGGCAATGAGCGGTTCC | 146 | 61 |
|
| Antisense |
TGTGTTGGCATAGAGGTCTTTACG |
|
|
Immunohistochemistry
Immunohistochemistry was used to detect the location
of IL-17A, IL-21, IL-22 and IL-22R1 expression in ankle tissues
from D26 PIA and control rats. Samples were deparaffinized,
retrieved for antigens by incubating compound enzymes at 37°C for
20 mins, washed with PBS buffer 3 times, and then they were
incubated with 5% BSA (cat. no. ZLI-9027; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd, Beijing, China) in PBS buffer at
37°C for 20 mins. Samples were then incubated overnight at 4°C with
primary antibodies, and normal rabbit serum (cat. no. ZLI-9025;
Zhongshan Golden Bridge Biotechnology Co., Ltd) or goat serum (cat.
no. ZLI-9056; Zhongshan Golden Bridge Biotechnology Co., Ltd) was
used instead of primary antibody as a negative control. Primary
antibodies were: Rabbit anti-IL-17A (cat. no. sc-7927; 1:100
dilution), goat anti-IL-21 (cat. no. sc-17649; 1:200 dilution) and
goat anti-IL-22 (cat. no. sc-14436; 1:200 dilution) from Santa Cruz
Biotechnology, Inc. (Dallas, TX USA) and goat anti-IL-22R1 (cat.
no. AF2770; 1:200 dilution) from R&D Systems, Inc.
(Minneapolis, MN, USA). Biotin conjugated secondary antibodies
[goat anti-rabbit immunoglobulin (Ig)G (cat. no. ZB-2010) or rabbit
anti-goat IgG (cat. no. ZB-2050), purchased from Zhongshan Golden
Bridge Biotechnology Co., Ltd] with 1:1,000 dilutions, were
incubated at 37°C for 20 min. Finally, the reaction was developed
by adding the substrate reagent 3,30-diaminobenzidine
tetrahydrochloride (DAB; Zhongshan Golden Bridge Biotechnology Co.,
Ltd) and the sections were counterstained with hematoxylin. A total
of 18 slides per group (n=6 rats) were evaluated under a light
microscope.
Statistical analysis
Results were analyzed by one-way analysis of
variance followed by a Dunnett test using SPSS 15.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of cytokines in the spleen
of PIA rats
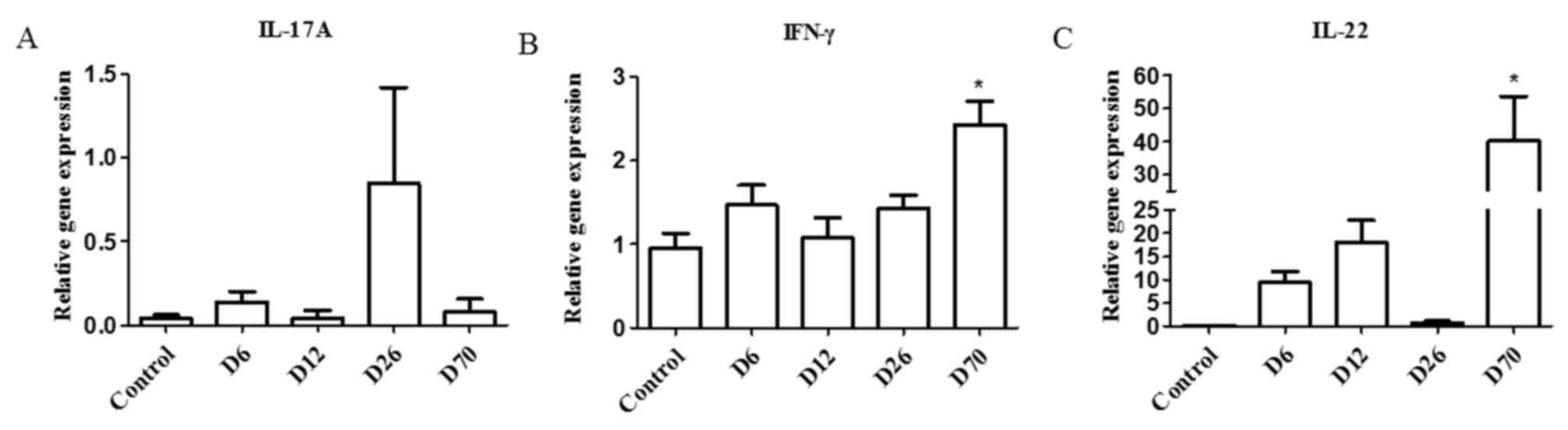
The mRNA expression levels of IL-17A, IFN-γ and
IL-22 were examined in the spleens of D6, D12, D26 and D70 PIA rats
by RT-qPCR. The results demonstrated that, in the spleen, IL-17A
exhibited an increasing trend in the D26 group but this was not
statistically significant (Fig.
1A). IFN-γ mRNA expression levels increased significantly in
the D70 group compared with the control group (Fig. 1B). IL-22 exhibited a trend towards
increased expression in the D6 and D12 groups, and increased
significantly in the D70 group compared with control (Fig. 1C).
Expression of cytokine receptors and
transcription factors in the spleen of PIA rats
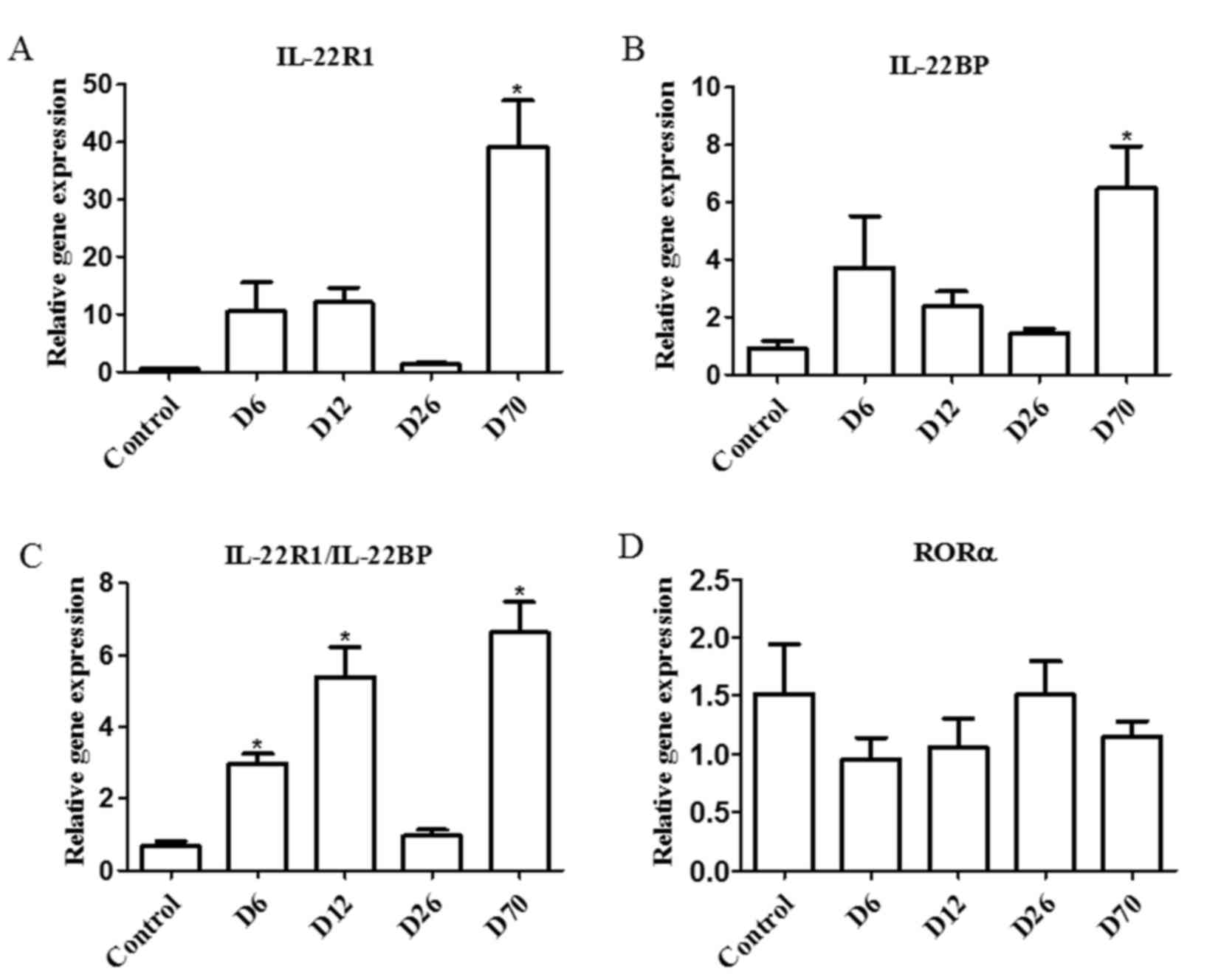
The mRNA expression levels of two types of IL-22
receptor, IL-22R1 and IL-22BP, and of the transcription factor RORα
(which is predominantly expressed by Th17 cells), were examined in
the spleens of D6, D12, D26 and D70 PIA rats by RT-qPCR. IL-22R1
expression levels were significantly increased in the D70 group
compared with the control group (Fig.
2A). IL-22BP expression levels were increased in the D70 group
as well (Fig. 2B). Since IL-22R1
is a functional receptor for IL-22, but IL-22BP is an antagonist of
IL-22, the ratio of IL-22R1/IL-22BP expression was also calculated
as a measure of IL-22 activity. The results demonstrated that the
IL-22R1/IL-22BP ratio was significantly increased in the D6, D12
and D70 groups compared with control, a pattern similar with the
expression pattern of IL-22 (Fig.
2C). Finally, the mRNA expression levels of the RORα
transcription factor were not altered during the progression of the
disease (Fig. 2D).
Expression of cytokines in the
synovium of PIA rats
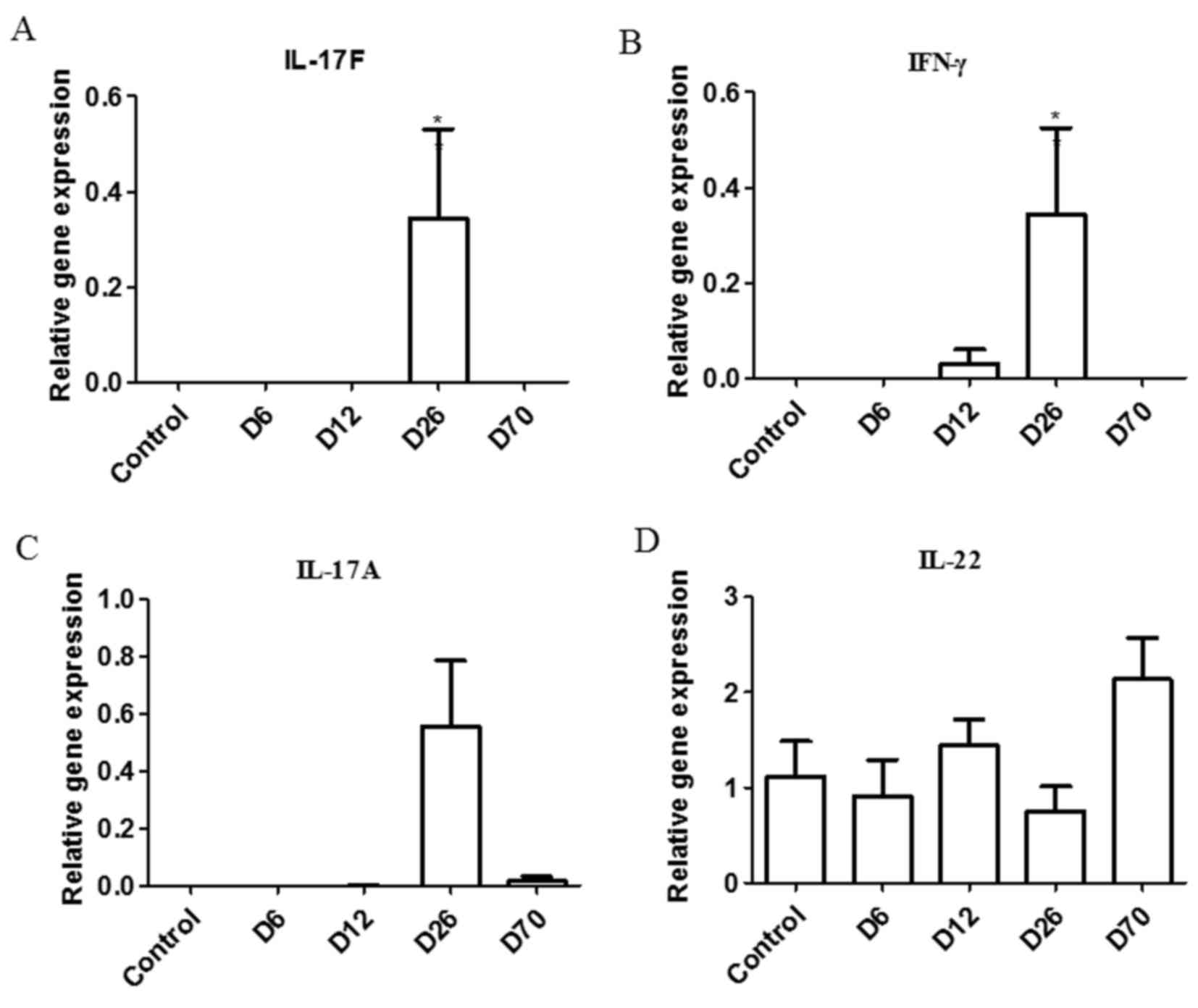
Ankle joints are the most affected joints in the PIA
rat model. The pathological changes in the synovial tissue reflect
the local disease condition (3).
In the ankle synovium, the mRNA expression levels of IL-17F and
IFN-γ were significantly increased in the D26 group compared with
the control group (Fig. 3A and B).
IL-17A expression appeared increased in the D26 group (Fig. 3C) and IL-22 expression appeared
increased in the D70 group (Fig.
3D) compared with control, but none of these differences were
significant.
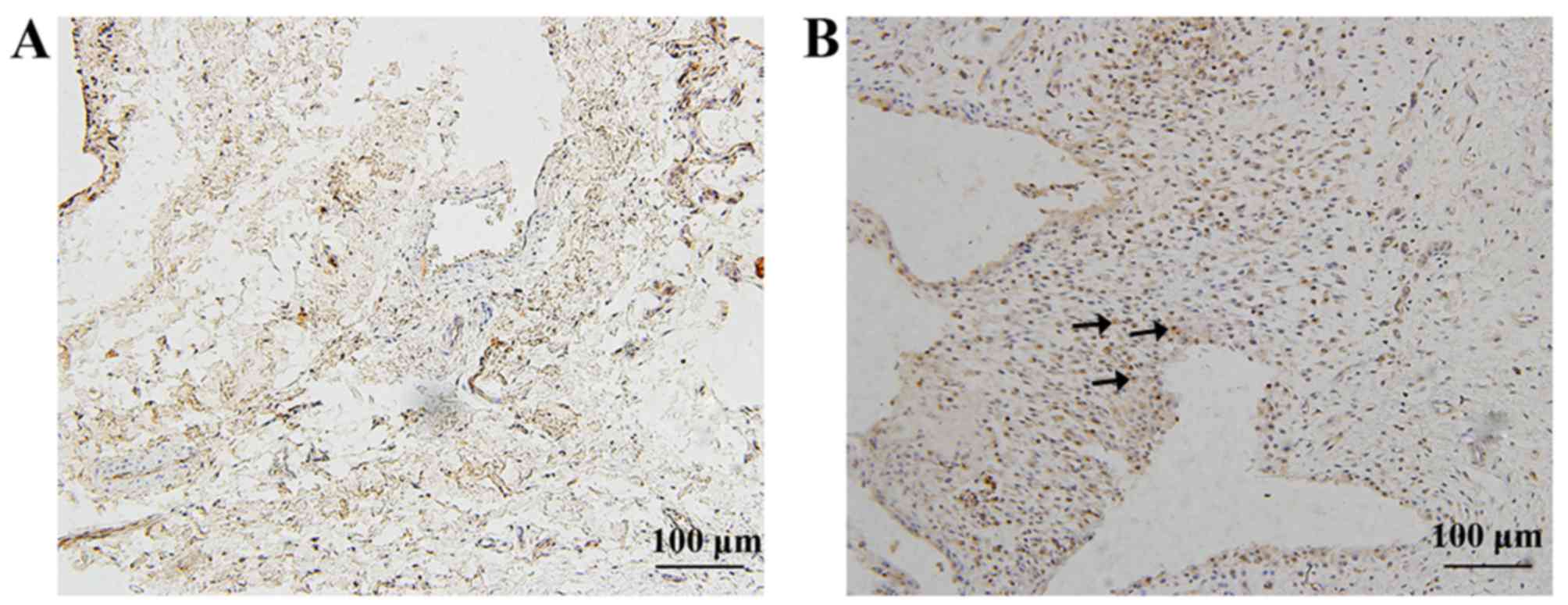
Location of cytokines and cytokine
receptor in the ankles of PIA rats
Immunohistochemistry analysis was used to detect the
location of IL-17A, IL-21, IL-22 and IL-22R1 expression in the
ankle joints of D26 PIA and control rats. The results demonstrated
that IL-17A was mainly expressed in infiltrated inflammatory cells
in PIA rats, while in the control group, few synovial cells were
observed, the synovium was not proliferated and no IL-17A positive
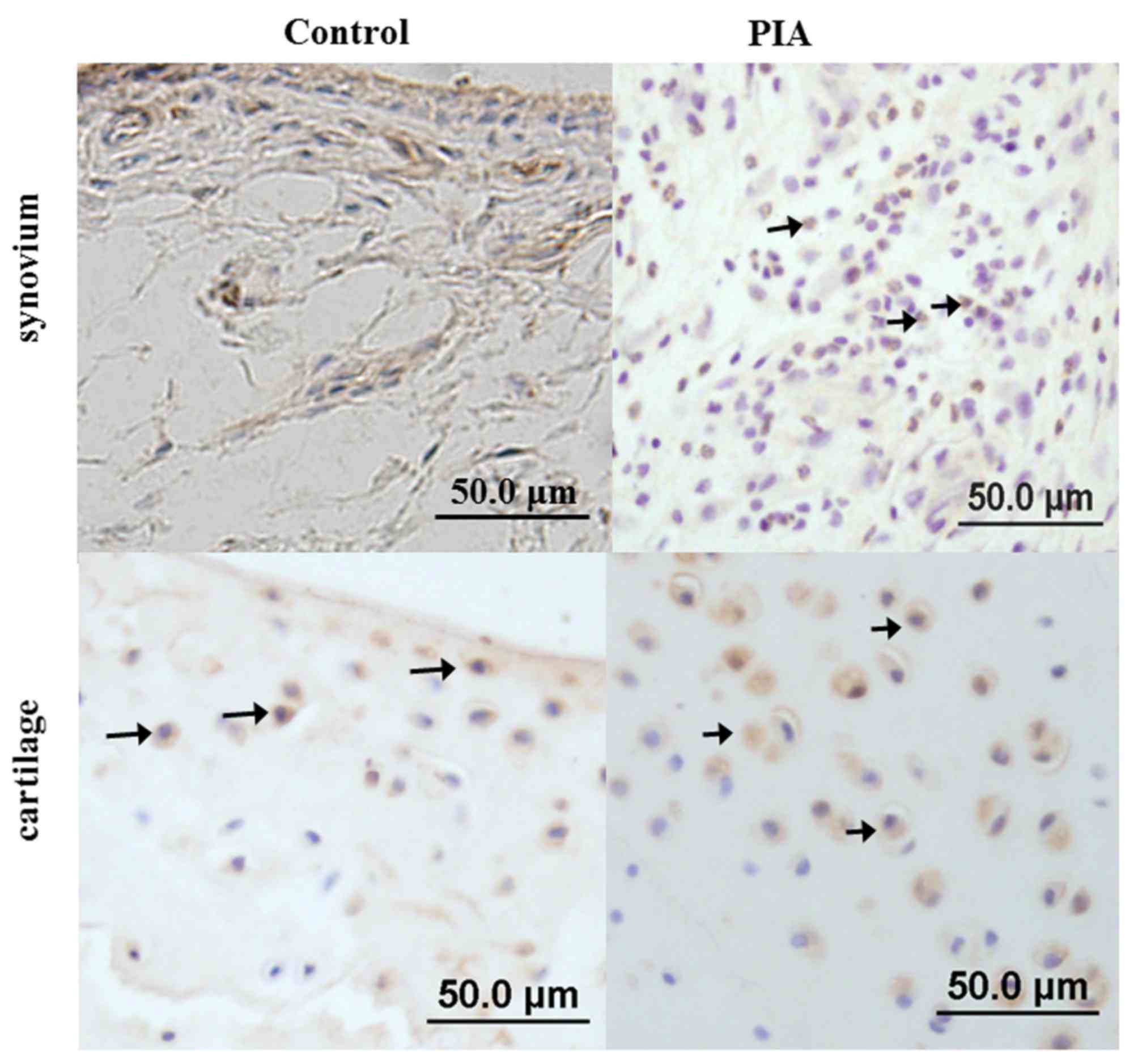
cells were detected (Fig. 4). In
the PIA group, IL-21 was expressed in the infiltrated inflammatory
cells, as well as in the proliferating layer of the articular
cartilage (Fig. 5). A small number
of proliferating chondrocytes in the normal control group were also
positive for IL-21 expression (Fig.
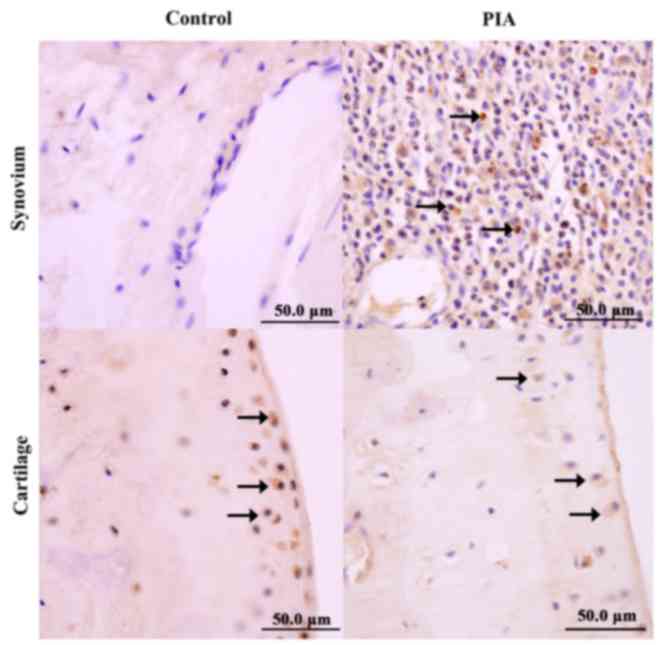
5). Concerning IL-22 expression, in the PIA group, IL-22 was
predominantly expressed in the infiltrated inflammatory cells
located at invasion sites, and in a small number of cells in the
proliferating layer of articular cartilage (Fig. 6). In the control group, however,
IL-22 was expressed in the proliferating layer of the articular
cartilage (Fig. 6). Finally,
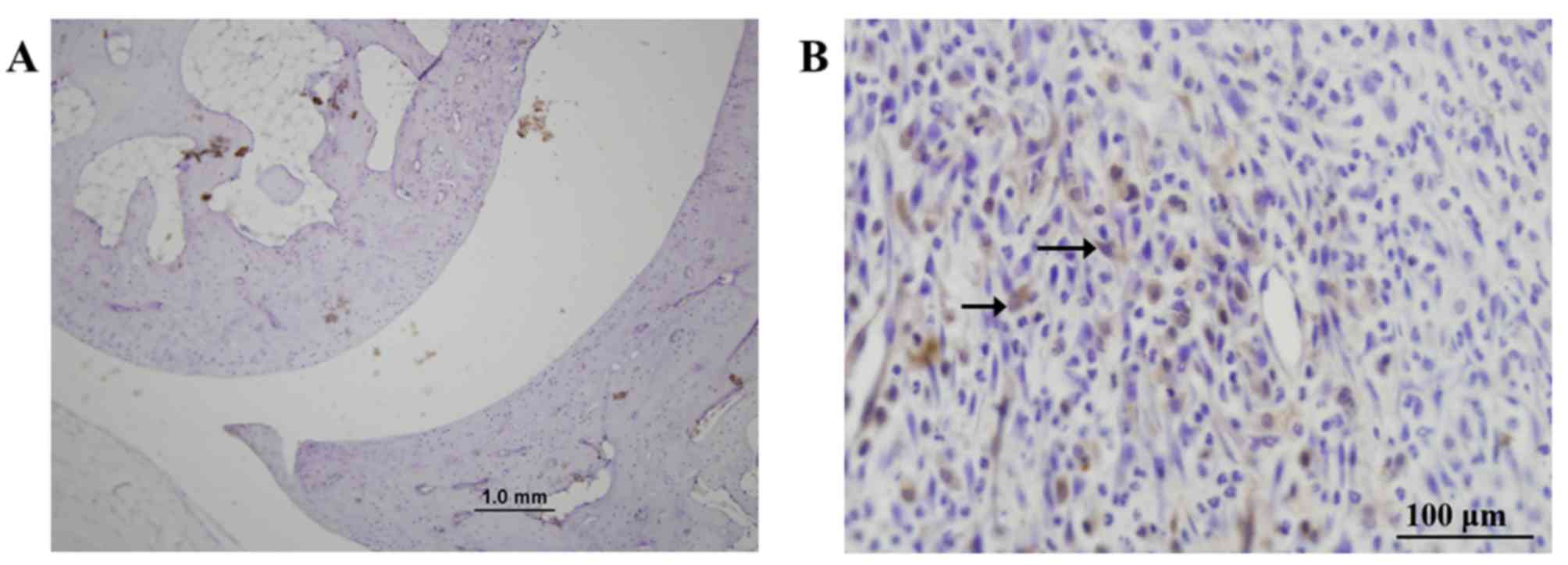
IL-22R1 was expressed in proliferated synovial cells in the PIA
group (Fig. 7).
Discussion
The PIA rat model shares similar clinical features
with RA, and its dominant features of different phases are two
disease peaks, one at the acute phase and one at the chronic phase
(17). As previously demonstrated,
the onset arthritis phase in the PIA model is 12 days
post-immunization, and the acute phase lasts from D12 to D32, then
progresses to the chronic phase with a fluctuating incidence and
level of disease (3).
Control rats were subcutaneously injected with PBS
as it is pathogen free. A previous study revealed that, following
an injection with PBS, there were no significant changes in the
levels of cytokines in rats from day 0 to day 50 (4). Thus, in the present study, control
group rats were sacrificed at 26 days following the injection,
particularly as the PIA group rats exhibit the most serious
clinical and pathological symptoms of the disease (4).
In the present study, the expression pattern of
IL-22 was screened for the first time in the PIA rat model and
IL-22 mRNA expression level was demonstrated to be varied in the
different phases of PIA.
In the initial phase of arthritis, D6 group in the
PIA rat model, which is prior to the onset phase, the deleterious
factors have started to produce and migrate to the immune organs.
Expression of IL-22 and IL-22R1 exhibited a trend to increased
expression and the ratio of IL-22R1/IL-22BP in the spleen was
increased compared with control. There was no significant change in
the expression of the detected cytokines in the synovium. Geboes
et al (18) used
IL-22−/− mice to induce a CIA model, and demonstrated
that CIA incidence was decreased and the severity of the disease
was palliative compared with wild-type mice. Similar results were
reported by Pinto et al (19) using an antigen-induced arthritis
mouse model. These findings suggest that IL-22 may serve an
important role in the initial phase of arthritis, earlier than
other cytokines, in a systemic manner.
The next phase of arthritis progression is the onset
phase, in which the immune reaction and pro-inflammatory factors
transfer from the immune organs to target joints, thus inducing
arthritis (3,17). In the present results, IL-22
expression had an increasing tendency and the ratio of
IL-22R1/IL-22BP increased significantly in the spleens of PIA rats
compared with control. In the synovium, IFN-γ, a pro-inflammatory
factor, was positively detected. IL-22 has been hypothesized to
play a protective role against arthritis in this phase. Sarkar
et al (15) observed that
administration of recombinant IL-22 during the effector phase in a
CIA mouse model, delays the progression and severity of arthritis,
and reduces the scores for inflammation, synovitis, and cartilage
and bone damage in paws, and these effects are mediated through
IL-10 production.
During the acute phase of arthritis, most
pro-inflammatory factors are secreted and aggregated into joints
(4). In the present study, IL-17F
and IFN-γ were significantly increased in the synovium of PIA rats
compared with control, while no significant changes were observed
in IL-22 expression. IL-17A is the predominant cytokine secreted by
Th17 cells, and it is related with many autoimmune diseases,
including rheumatoid arthritis. IL-17A may be one of the key
pro-inflammatory factors and may mediate the pathological progress
of synovitis during the acute phase of arthritis. IL-17A expression
levels are increased both in the synovium and synovial fluid of
patients with RA and in the inflammatory synovial tissues of CIA
mice (13). Intra-articular
injection of IL-17A in wild-type mice can induce symptoms similar
to RA, while injection with a neutralizing anti-murine IL-17A
antibody after the onset of CIA reduces joint inflammation,
cartilage destruction, and bone erosion, and decreases the serum
level of IL-6 (20–22). In IL-17A−/− mice, the
progression of CIA is suppressed and the production of antigen
specific T cells and collagen specific IgG2a is also influenced
(23). IL-17A promotes the
production of granulocyte macrophage colony-stimulating factor and
prostaglandin E2 in synovial cells, and promotes macrophages to
produce IL-1 and tumor necrosis factor-α (20,22,24).
IL-17A promotes the differentiation of osteoclasts by inducing
expression of receptor activator of nuclear factor kB ligand in
osteoblasts (25). IL-17A induces
synovial cells and chondrocytes to produce matrix
metalloproteinases, promotes the degradation of extracellular
matrix and inhibits the synthesis of proteoglycans and collagens,
which are the main components of matrix repair (26–28).
Ikeuchi et al (14)
reported that IL-22 induces synovial fibroblast proliferation and
monocyte chemotactic protein-1 production in vitro. In the
present results, the role of IL-22 may be covered by other
pro-inflammatory factors.
Progression to the chronic phase is one of the key
features of RA, with disease fluctuation and lasting progression
(29). In the present study, IL-22
expression in the spleen of PIA rats was demonstrated to be
increased predominantly in the chronic phase, an expression pattern
that was similar to the IL-22R1/IL-22BP expression ratio in PIA
rats. Of note, IL-22 expression in the synovium was stable during
the disease progression, except in the chronic phase, which
exhibited a trend towards increased expression. This indicated that
IL-22 may be important systemically and locally. Previously, it has
been reported that the predominant pathological features of PIA in
the chronic phase are the destruction and repair of joints
(3). IL-22 may serve important
roles in the joint repair process. Marijnissen et al
(30) observed that IL-22
neutralization does not influence the number of osteoclasts, but
induces a significant reduction in bone erosion. Geboes et
al (18) reported that IL-22R1
is expressed in osteoclast precursors, a CD11b+ fraction
of splenocytes, and that IL-22 stimulates osteoclast
differentiation and activity. In IL-22−/− mice, synovial
tissue hyperplasia and pannus formation was reduced compared with
wild-type mice (18).
In conclusion, the present results demonstrated
that, in PIA rats, IL-17F and IFN-γ expression increased in the
synovium during the acute phase of arthritis. In addition, IL-22
increased predominantly in the spleen during the chronic phase of
arthritis, with an increasing trend observed in the initial and
onset phases in the spleen and in the chronic phase in the
synovium. Further studies will be necessary in order to establish
the role of IL-22 in the various cytokine milieus and in the
different phases of arthritis progression.
Acknowledgements
The authors wish to thank Mr. Fujun Zhang for the
excellent work at histological and pathological research. The
present study was supported by grants from the National Natural
Science Foundation of China (grant no. 81201373) and Postdoctoral
Foundation of Shaanxi Province (grant no. 2016BSHEDZZ93).
References
|
1
|
Huber LC, Distler O, Tarner I, Gay RE, Gay
S and Pap T: Synovial fibroblasts: Key players in rheumatoid
arthritis. Rheumatology (Oxford). 45:669–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Mahony R, Richards A, Deighton C and
Scott D: Withdrawal of disease-modifying antirheumatic drugs in
patients with rheumatoid arthritis: A systematic review and
meta-analysis. Ann Rheum Dis. 69:1823–1826. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hou W, Meng L, Tian L, Zhu W, Jiang C and
Lu S: A systematic comparison between collagen-induced arthritis
and pristane-induced arthritis in Dark Agouti rats. Clin Exp
Rheumatol. 28:532–538. 2010.PubMed/NCBI
|
|
4
|
Vingsbo C, Sahlstrand P, Brun JG, Jonsson
R, Saxne T and Holmdahl R: Pristane-induced arthritis in rats: A
new model for rheumatoid arthritis with a chronic disease course
influenced by both major histocompatibility complex and non-major
histocompatibility. complex genes. 149:1675–1683. 1996.
|
|
5
|
Lu S, Nordquist N, Holmberg J, Olofsson P,
Pettersson U and Holmdahl R: Both common and unique susceptibility
genes in different rat strains with pristane-induced arthritis. Eur
J Hum Genet. 10:475–483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holmberg J, Tuncel J, Yamada H, Lu S,
Olofsson P and Holmdahl R: Pristane, a non-antigenic adjuvant,
induces MHC class II-restricted, arthritogenic T cells in the rat.
J Immunol. 176:1172–1179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage
distinct from the T helper type 1 and 2 lineages. Nat Immunol.
6:1123–1132. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solt LA, Kumar N, Nuhant P, Wang Y, Lauer
JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D, et al:
Suppression of TH17 differentiation and autoimmunity by a synthetic
ROR ligand. Nature. 472:491–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duhen T, Geiger R, Jarrossay D,
Lanzavecchia A and Sallusto F: Production of interleukin 22 but not
interleukin 17 by a subset of human skin-homing memory T cells. Nat
Immunol. 10:857–863. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolk K, Witte E, Witte K, Warszawska K and
Sabat R: Biology of interleukin-22. Semin Immunopathol. 32:17–31.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dumoutier L, Lejeune D, Colau D and
Renauld JC: Cloning and characterization of IL-22 binding protein,
a natural antagonist of IL-10-related T cell-derived inducible
factor/IL-22. J Immunol. 166:7090–7095. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarkar S, Justa S, Brucks M, Endres J, Fox
DA, Zhou X, Alnaimat F, Whitaker B, Wheeler JC, Jones BH and
Bommireddy SR: Interleukin (IL)-17A, F and AF in inflammation: A
study in collagen-induced arthritis and rheumatoid arthritis. Clin
Exp Immunol. 177:652–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ziolkowska M, Koc A, Luszczykiewicz G,
Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H and
Maslinski W: High levels of IL-17 in rheumatoid arthritis patients:
IL-15 triggers in vitro IL-17 production via cyclosporin
A-sensitive mechanism. J Immunol. 164:2832–2838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko
Y, Hiromura K, Ueki K and Nojima Y: Expression of interleukin-22 in
rheumatoid arthritis: Potential role as a proinflammatory cytokine.
Arthritis Rheum. 52:1037–1046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarkar S, Zhou X, Justa S and Bommireddy
SR: Interleukin-22 reduces the severity of collagen-induced
arthritis in association with increased levels of interleukin-10.
Arthritis Rheum. 65:960–971. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olofsson P and Holmdahl R:
Pristane-induced arthritis in the rat. Methods Mol Med.
136:255–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geboes L, Dumoutier L, Kelchtermans H,
Schurgers E, Mitera T, Renauld JC and Matthys P: Proinflammatory
role of the Th17 cytokine interleukin-22 in collagen-induced
arthritis in C57BL/6 mice. Arthritis Rheum. 60:390–395. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pinto LG, Talbot J, Peres RS, Franca RF,
Ferreira SH, Ryffel B, Aves-Filho JC, Figueiredo F, Cunha TM and
Cunha FQ: Joint production of IL-22 participates in the initial
phase of antigen-induced arthritis through IL-1β production.
Arthritis Res Ther. 17:2352015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fossiez F, Djossou O, Chomarat P,
Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E,
Saeland S, et al: T cell interleukin-17 induces stromal cells to
produce proinflammatory and hematopoietic cytokines. J Exp Med.
183:2593–2603. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jovanovic DV, Di Battista JA,
Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F and
Pelletier JP: IL-17 stimulates the production and expression of
proinflammatory cytokines, IL-beta and TNF-alpha, by human
macrophages. J Immunol. 160:3513–3521. 1998.PubMed/NCBI
|
|
22
|
Katz Y, Nadiv O and Beer Y: Interleukin-17
enhances tumor necrosis factor alpha-induced synthesis of
interleukins 1,6 and 8 in skin and synovial fibroblasts: A possible
role as a ‘fine-tuning cytokine’ in inflammation processes.
Arthritis Rheum. 44:2176–2184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kotake S, Udagawa N, Takahashi N,
Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N,
Gillespie MT, et al: IL-17 in synovial fluids from patients with
rheumatoid arthritis is a potent stimulator of osteoclastogenesis.
J Clin Invest. 103:1345–1352. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jovanovic DV, Di Battista JA,
Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F and
Pelletier JP: IL-17 stimulates the production and expression of
proinflammatory cytokines, IL-beta and TNF-alpha, by human
macrophages. J Immunol. 160:3513–3521. 1998.PubMed/NCBI
|
|
25
|
Kotake S, Udagawa N, Takahashi N,
Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N,
Gillespie MT, et al: IL-17 in synovial fluids from patients with
rheumatoid arthritis is a potent stimulator of osteoclastogenesis.
J Clin Invest. 103:1345–1352. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chabaud M, Garnero P, Dayer JM, Guerne PA,
Fossiez F and Miossec P: Contribution of interleukin 17 to synovium
matrix destruction in rheumatoid arthritis. Cytokine. 12:1092–1099.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benderdour M, Tardif G, Pelletier JP, Di
Battista JA, Reboul P, Ranger P and Martel-Pelletier J: Interleukin
17 (IL-17) induces collagenase-3 production in human osteoarthritic
chondrocytes via AP-1 dependent activation: Differential activation
of AP-1 members by IL-17 and IL-1beta. J Rheumatol. 29:1262–1272.
2002.PubMed/NCBI
|
|
28
|
Koshy PJ, Henderson N, Logan C, Life PF,
Cawston TE and Rowan AD: Interleukin 17 induces cartilage collagen
breakdown: Novel synergistic effects in combination with
proinflammatory cytokines. Ann Rheum Dis. 61:704–713. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grassi W, de Angelis R, Lamanna G and
Cervini C: The clinical features of rheumatoid arthritis. Eur J
Radiol. 27 Suppl 1:S18–S24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marijnissen RJ, Koenders MI, Smeets RL,
Stappers MH, Nickerson-Nutter C, Joosten LA, Boots AM and van den
Berg WB: Increased expression of interleukin-22 by synovial Th17
cells during late stages of murine experimental arthritis is
controlled by interleukin-1 and enhances bone degradation.
Arthritis Rheum. 63:2939–2948. 2011. View Article : Google Scholar : PubMed/NCBI
|