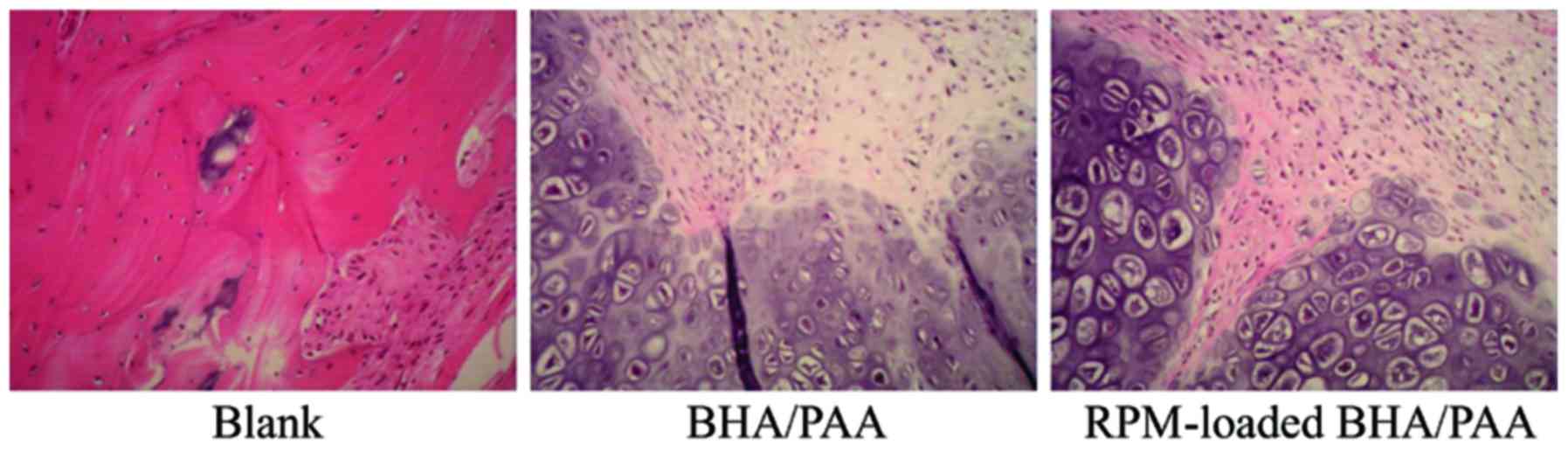
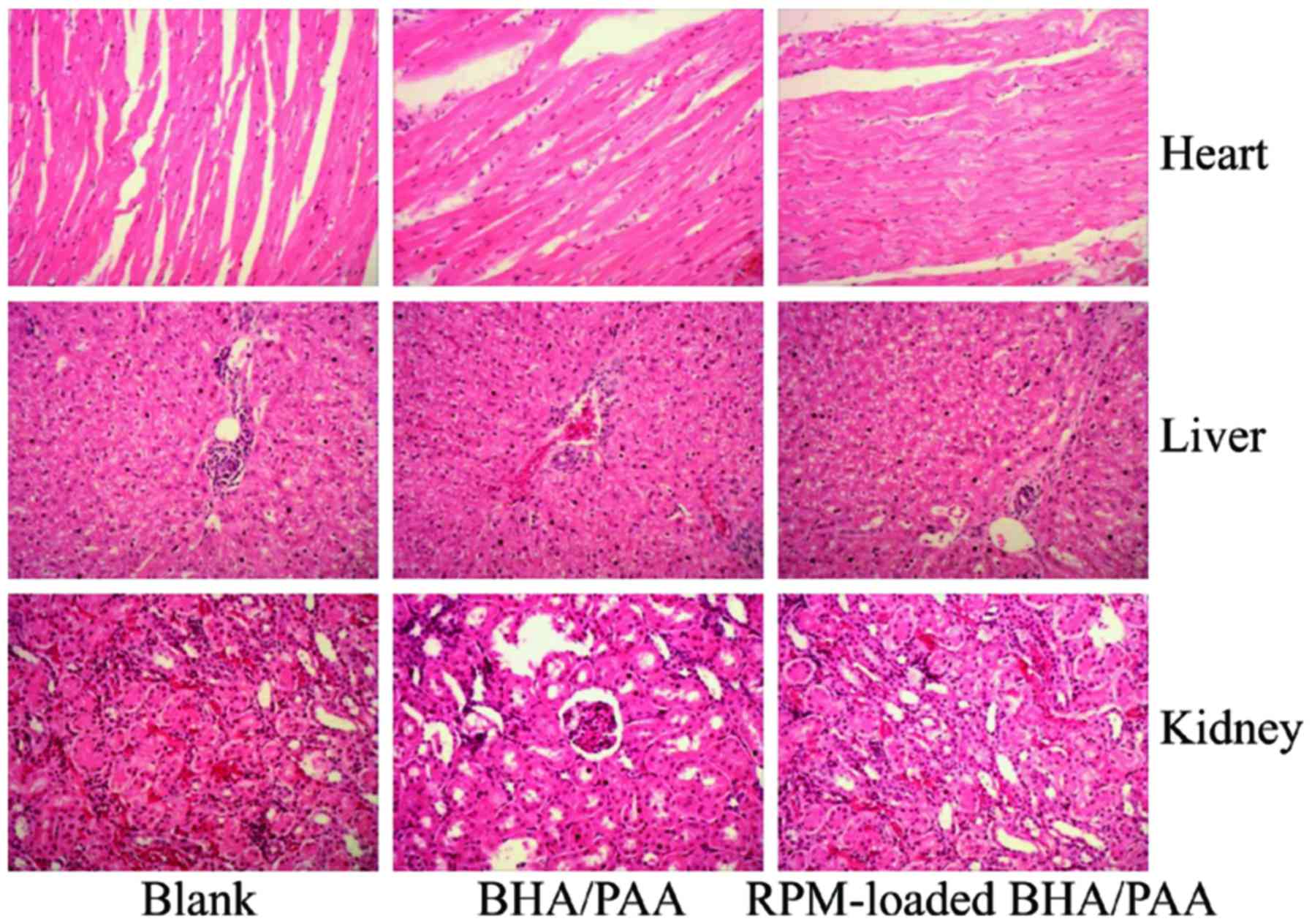
|
1
|
Dye C and Williams BG: The population
dynamics and control of tuberculosis. Science. 328:856–861. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen M, Gan H and Remold HG: A mechanism
of virulence: Virulent Mycobacterium tuberculosis strain H37Rv, but
not attenuated H37Ra, causes significant mitochondrial inner
membrane disruption in macrophages leading to necrosis. J Immunol.
176:3707–3716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barry CE III, Boshoff HI, Dartois V, Dick
T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ and Young D: The
spectrum of latent tuberculosis: Rethinking the biology and
intervention strategies. Nat Rev Microbiol. 7:845–855.
2009.PubMed/NCBI
|
|
4
|
Nagashima H, Yamane K, Nishi T, Nanjo Y
and Teshima R: Recent trends in spinal infections: Retrospective
analysis of patients treated during the past 50 years. Int Orthop.
34:395–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
WHO global tuberculosis control report
2010. Summary. Cent Eur J Public Health. 18:2372010.PubMed/NCBI
|
|
6
|
Sequeira W, Co H and Block JA:
Osteoarticular tuberculosis: Current diagnosis and treatment. Am J
Ther. 7:393–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge Z, Wang Z and Wei M: Measurement of the
concentration of three antituberculosis drugs in the focus of
spinal tuberculosis. Eur Spine J. 17:1482–1487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saifullah B, Hussein MZ and Al Ali SH
Hussein: Controlled-release approaches towards the chemotherapy of
tuberculosis. Int J Nanomedicine. 7:5451–5463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moore WR, Graves SE and Bain GI: Synthetic
bone graft substitutes. ANZ J Surg. 71:354–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freitas S, Merkle HP and Gander B:
Microencapsulation by solvent extraction/evaporation: Reviewing the
state of the art of microsphere preparation process technology. J
Control Release. 102:313–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aristoff PA, Garcia GA, Kirchhoff PD and
Showalter HD: Rifamycins-obstacles and opportunities. Tuberculosis
(Edinb). 90:94–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keung A, Eller M, McKenzie K and Weir S:
Single and multiple dose pharmacokinetics of rifapentine in man:
Part II. Int J Tuberc Lung Dis. 3:437–444. 1999.PubMed/NCBI
|
|
13
|
Bemer-Melchior P, Bryskier A and Drugeon
HB: Comparison of the in vitro activities of rifapentine and
rifampicin against Mycobacterium tuberculosis complex. J Antimicrob
Chemother. 46:571–576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Assandri A, Ratti B and Cristina T:
Pharmacokinetics of rifapentine, a new long lasting rifamycin, in
the rat, the mouse and the rabbit. J Antibiot (Tokyo).
37:1066–1075. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan JG, Bai X and Traini D: An update on
the use of rifapentine for tuberculosis therapy. Expert Opin Drug
Deliv. 11:421–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Zuo Y, Hu Y, Wang J, Li J, Qiao B
and Jiang D: Development and in vitro characterization of drug
delivery system of rifapentine for osteoarticular tuberculosis.
Drug Des Devel Ther. 9:1359–1366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gentile P, Chiono V, Boccafoschi F, Baino
F, Vitale-Brovarone C, Vernè E, Barbani N and Ciardelli G:
Composite films of gelatin and hydroxyapatite/bioactive glass for
tissue-engineering applications. J Biomater Sci Polym Ed.
21:1207–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roohani-Esfahani SI, Nouri-Khorasani S, Lu
Z, Appleyard R and Zreiqat H: The influence hydroxyapatite
nanoparticle shape and size on the properties of biphasic calcium
phosphate scaffolds coated with hydroxyapatite-PCL composites.
Biomaterials. 31:5498–5509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y and Bose S: Nanocrystalline
hydroxyapatite: Micelle templated synthesis and characterization.
Langmuir. 21:3232–3234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mastrogiacomo M, Scaglione S, Martinetti
R, Dolcini L, Beltrame F, Cancedda R and Quarto R: Role of scaffold
internal structure on in vivo bone formation in macroporous calcium
phosphate bioceramics. Biomaterials. 27:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turco G, Marsich E, Bellomo F, Semeraro S,
Donati I, Brun F, Grandolfo M, Accardo A and Paoletti S:
Alginate/Hydroxyapatite biocomposite for bone ingrowth: A
trabecular structure with high and isotropic connectivity.
Biomacromolecules. 10:1575–1583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan L and Jiang DM: Study of bone-like
hydroxyapatite/polyamino acid composite materials for their
biological properties and effects on the reconstruction of long
bone defects. Drug Des Devel Ther. 9:6497–6508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan L, Jiang DM, Cao ZD, Wu J, Wang X,
Wang ZL, Li YJ and Yi YF: Treatment of Staphylococcus
aureus-induced chronic osteomyelitis with bone-like
hydroxyapatite/poly amino acid loaded with rifapentine
microspheres. Drug Des Devel Ther. 9:3665–3676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng XL, Li YB, Wang XJ, Yan YG, Wei J and
Zhang L: Study on the soaking behaviors of
nano-hydroxyapatite/polyamide 66 biomedical composite in vitro.
Functional Mater. 2:253–255. 2004.
|
|
25
|
Rastogi N, Goh KS, Berchel M and Bryskier
A: Activity of rifapentine and its metabolite
25-O-desacetylrifapentine compared with rifampicin and rifabutin
against Mycobacterium tuberculosis, Mycobacterium africanum,
Mycobacterium bovis and M. bovis BCG. J Antimicrob Chemother.
46:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bos R, van der Mei HC and Busscher HJ:
Physico-chemistry of initial microbial adhesive interactions-its
mechanisms and methods for study. FEMS Microbiol Rev. 23:179–230.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei G and Ma PX: Structure and properties
of Nano-hydroxyapatite/polymer composite scaffolds for bone tissue
engineering. Biomaterials. 25:4749–4757. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Owen GR, Jackson J, Chehroudi B, Burt H
and Brunette DM: A PLGA membrane controlling cell behaviour for
promoting tissue regeneration. Biomaterials. 26:7447–7456. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang J, Zhao D, Dangaria SJ, Luan X,
Diekwisch TG, Jiang G, Saiz E, Liu G and Tomsia AP: Combinatorial
design of hydrolytically degradable, bone-like biocomposites based
on PHEMA and hydroxyapatite. Polymer (Guildf). 54:909–919. 2013.
View Article : Google Scholar : PubMed/NCBI
|