Introduction
Detrusor underactivity (DU) is a common bladder
dysfunction in clinical practice. It is characterized by reduced
bladder detrusor contractility or decreased contractility duration,
leading to delayed or incomplete bladder voiding (1). Typical clinical manifestations of DU
include urinary hesitancy and dysuria. In addition, those with DU
can experience increased nocturia, increased urinary frequency and
urgency, and urinary incontinence. Therefore, DU can seriously
affect patient quality of life. The pathogenesis of DU involves
neurogenic, muscle-genic, age-related and iatrogenic factors.
However, the pathophysiologic mechanisms of DU remains to be fully
elucidated (2).
The interstitial cells of Cajal (ICCs) are a
specific type of interstitial cell in the gastrointestinal tract;
the activation of c-kit receptors on the surface of ICCs is
intimately associated with their proliferation, differentiation and
function (3). The activation of
c-kit relies on its ligand, stem cell factor (SCF) (3). SCF is of neural and smooth muscle
origin (4,5), and is involved in the regulation of
cell proliferation, differentiation and migration (6). Therefore, SCF is important in
regulating the proliferation, differentiation and function of ICCs
(7,8).
ICCs are present in multiple smooth muscle organs
and are closely associated with organ dysfunction (9–11).
It has been suggested that ICCs have a pacemaker-like function in
the gastrointestinal tract (12).
Studies have shown that a change in SCF leads to altered ICC
numbers, which contributes to gastrointestinal dysmotility
(9,10,13).
In the urinary tract, ICCs serving as a pacemaker are found in the
tissues of the renal pelvis and ureter (14–16).
ICCs are also present in the bladder (17,18).
The association between ICCs and bladder dysfunction has been
examined previously. An overactive bladder, a common bladder
dysfunction, is associated with an increased number of ICCs
(19,20). However, there have been few
investigations evaluating the association between an underactive
bladder and ICCs, and the pathophysiological mechanisms of an
underactive bladder remain to be elucidated. The present study
hypothesized that DU may be associated with the SCF-mediated
alterations in ICCs. Therefore, the present study aimed to examine
changes in ICCs in the context of DU, and evaluate the effect of
SCF on ICCs in addition to the changes in detrusor
contractility.
Materials and methods
Animals
A total of 90 female Sprague-Dawley (SD) rats
(weight, 190–220 g; age, 3 months) were used in the present study.
Rats were housed at ~24°C with a 12 h light/dark cycle (on 7 AM/
off 7 PM) and 35–40% humidity. Rats were given free access to food
and water throughout the study. All experiments were performed at
the Central Laboratory of the Third Military Medical University
Affiliated Southwest Hospital (Chongqing, China). All animal
experiments were approved by the animal ethics committee of the
Third Military Medical University. The bladder outlet obstruction
(BOO) rat model was established using the bladder outlet
obstruction method (21). Briefly,
anesthesia was achieved by intraperitoneal injection of 3%
pentobarbital sodium (30 mg/kg; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). Subsequently, a median abdominal incision was
performed to expose the bladder and proximal urinary tract. A 1.1
mm diameter polyethylene pipe was inserted into the bladder
transurethrally, with 1–0 thread used for urethral ligation and the
degree of ligation defined by appropriate movement with the
urethral catheter. The incision was sutured following catheter
removal. In the sham group, the urethra was exposed but not
ligated. After 8 weeks, bladder filling pressure measurements were
performed using a micro perfusion pump (AVI 270; 3M Company, Saint
Paul, MN, USA) and a urodynamic instrument (Nidoc 970A+; Wearnes
UEST New Tech Co., Ltd., Chengdu, China), and rats with DU were
observed. Animal condition was monitored by measuring animal body
temperature at 8.00 a.m., 4.00 p.m., and 12.00 p.m. daily using a
biological remote sensing system (TA10TA-F40 Data Sciences
International, St. Paul, MN, USA). In addition, mental health and
overall activity were assessed twice every day; animal weights were
measured weekly. Following surgery, the rats were housed in
separate cages; at 3 and 6 days post-surgery, they were housed with
3 and 5 per cage, respectively. The current protocols required that
seriously ill or moribund rats (no activity and/or eating) be
administered with injectable anesthetic euthanasia.
The animals were divided into control (n=18),
control+SCF (n=18), DU (n=18) and DU+SCF (n=18) groups. SCF was
dissolved in PBS and administered at 0.2 µg/kg/d by intraperitoneal
injection. The control and DU group animals were intraperitoneally
injected with equal volumes of PBS. The rats were sacrificed 2
weeks later by intraperitoneal injection of 3% pentobarbital sodium
(100 mg/kg), and bladder specimens were collected for the
determination of ICC number, expression levels of c-kit and SCF in
the detrusor, and alterations of detrusor contractility using an
in vitro muscle strip experiment.
No animals became ill or died in the sham-operated
group. When suspected with illness, the rats were housed in single
cages, which were cleaned daily. In the BOO group, one rat died on
day 3, two rats died on day 42, and one rat died on day 48
post-surgery. In addition, one animal in the DU+SCF group died 5
days post-injection. The possible causes of death are described
below. In the BOO group, the rat which died on day 3 post-surgery
had surgical trauma-induced stress. Postoperative infection
(increased body temperature) was the cause of death of one of the
rats on day 42, whereas the other died of unknown causes.
Postoperative kidney disease with retention, which can lead to
hydronephrosis or renal failure, led to the death of the animal on
day 48. In the DU+SCF group, the rat died on day 5 as a result of
bladder stones.
Assessment of ICC numbers in the
detrusor using immunohistochemistry
The rats were sacrificed, and the bladder and
proximal urinary tract were exposed. Proximal urinary obstruction
was removed, and a polyethylene catheter was inserted. The
bilateral ureters were ligated using 1–0 silk, following which the
bladder was inflated by injection of 4% paraformaldehyde through
the urethral catheter, which was subsequently removed prior to
rapid ligation of the proximal urethra. The proximal urethra and
bilateral ureter were incised, followed by bladder removal and
preservation in 4% paraformaldehyde at 21°C for 30 min. The samples
were then transferred to 0.1 M PBS and incubated at 21°C overnight.
The bladder was longitudinally incised the following day, and
lateral walls measuring 4×4 mm were harvested. The mucosal and
serosal layers were carefully dissected and separated under a
microscope (XTS-4A; Zhenjiang Zhongtian Optical Instrument Co.,
Ltd., Zhenjiang, China). The specimens were blocked in 1% bovine
serum albumin (Sigma-Aldrich; Merck Millipore) at room temperature
for 1 h and subsequently incubated with anti-c-kit primary antibody
(1:100; cat.no. sc-1494; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 24 h at 4°C. Finally, the samples were incubated with
secondary antibody (1:100; cat.no. sc-2356; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h and then rinsed in
0.01 M PBS for 2 h prior to examination under a confocal microscope
(LSCM, Leica TCS-NT, Germany) for determination of the expression
of c-kit in the cells. The rat small intestines were used as
positive controls. Five high power fields from each sample were
randomly selected, and samples were collected from different groups
in a blinded manner by different examiners. Cell counts of the
c-kit positive cells were recorded.
Expression of SCF in plasma determined
using an enzyme-linked immunosorbent assay (ELISA)
Blood samples (2 ml) were collected from the rats
prior to sacrifice. The concentrations of SCF in the plasma was
determined using an ELISA kit (Abcam, Cambridge, UK) according to
manufacturer's protocol.
Protein levels of c-kit and SCF in
detrusor using western blot analysis
The detrusor (~25 mg) was harvested and homogenized.
The homogenate was added to lysate buffer for 30 min at 4°C, and
subjected to centrifugation at 12,000 g for 8 min at 4°C. The
supernatants were collected, and protein concentrations determined
using a NanoDrop ND-1000 (NanoDrop; Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). The total protein samples (50 µg) were
resolved by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and electrotransferred onto nitrocellulose
membranes. Following blocking with 5% skimmed milk for 2 h, the
membranes were incubated with primary antibodies raised against SCF
(1:200; cat.no sc-9132; Santa Cruz Biotechnology, Inc.), c-kit
(1:100; cat.no. sc-1494; Santa Cruz Biotechnology, Inc.) and
glyceraldehyde 3 phosphate dehydrogenase (GAPDH; 1:500; cat.no.
A00191; GenScript, Piscataway, NJ, USA). The membranes were then
washed with PBST three times prior to incubation with appropriate
HRP-conjugated secondary antibodies (1:500; cat.nos. sc-2357 and
sc-2354; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C; detection
was performed with Millipore Immobilon Western Chemiluminescent HRP
substrate (EMD Millipore, Billerica, MD USA). Band intensities were
determined using image process software (Bio-Rad Gel Doc 2000;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Determination of gene expression
levels of c-kit and SCF in detrusor samples using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The bladder tissue specimens (~30 mg) were
homogenized and lysed using Tripure lysate (Roche Diagnostics,
Basel, Switzerland) for RNA preparation, according to the
manufacturer's protocol. Total RNA was extracted using TRIzol
reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. RNA quantity and
integrity were assessed using spectrophotometry and gel
electrophoresis, respectively. RNA (1 µg) was used as a template
for cDNA synthesis using a GeneAmp RNA PCR kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. qPCR analysis was performed using Power SYBR® Green
PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
on an Applied Biosystems 7900HT Fast Real-Time qPCR system. qPCR
was performed in a 20 µl reaction volume, containing 2 µl cDNA, 0.6
µl primers, 10 µl 2X SYBR green and 7.4 µl RNA free H2O.
Thermocycling conditions involved an initial denaturation step of
96°C for 5 min, followed by 40 cycles of a three-step program of
96°C for 30s, 57°C for 30s and 72°C for 30s, followed by a final
extension step at 72°C for 10 min. qPCR was conducted three times
for each gene of interest. The primers used were as follows: c-kit,
forward 5′-TGCCGGTCGATTCCAAGTTT-3′ and reverse
5′-GCCGACGGAATTGACCCTC-3′ (target fragment 269 bp); SCF, forward
5′-CCTGCAATATGAAGCCCCAAGAC-3′ and reverse
5′-GGTGCCCTCCTGCTACTTTTAC-3′ (target fragment 166 bp); GAPDH,
forward 5′-CCGCCCCTTCCGCTGATG-3′ and reverse
5′-CCGCCTGCTTCACCACCTTCTT-3′ (target fragment 432 bp). The
2−ΔΔCq method was used to analyze the PCR data (22), expressed as the fold change
relative to the expression of GAPDH.
Detection of detrusor contractility
using in vitro assays of muscle strips
The rats underwent anesthesia, and the bladder and
proximal urethra were exposed as described above. The muscle strips
(7×2 mm) were prepared and stored in 5 ml Krebs's solution (pH 7.4)
at 37°C in an atmosphere containing 5% CO2. The ends of
the longitudinal muscle strips were placed on metal hooks with a
tension sensor (Chengdu Instrument Company, Chengdu, China)
connected to a biological laboratory data system (RM6240; Chengdu
Instrument Company). The muscle strips were placed in an organ bath
for 30 min prior to recording contractility, with an initial
tension of 0.3 g. The amplitude of the contraction wave was defined
as the wave height, between the base and peak. The frequency of
contraction was defined as the mean contraction frequency within 5
min.
Statistical analysis
All experiments were performed three times in total.
Values are presented as the mean ± standard deviation. Differences
among or between groups were assessed using one-way analysis of
variance or Student's t-test, using SPSS 19.0 software (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
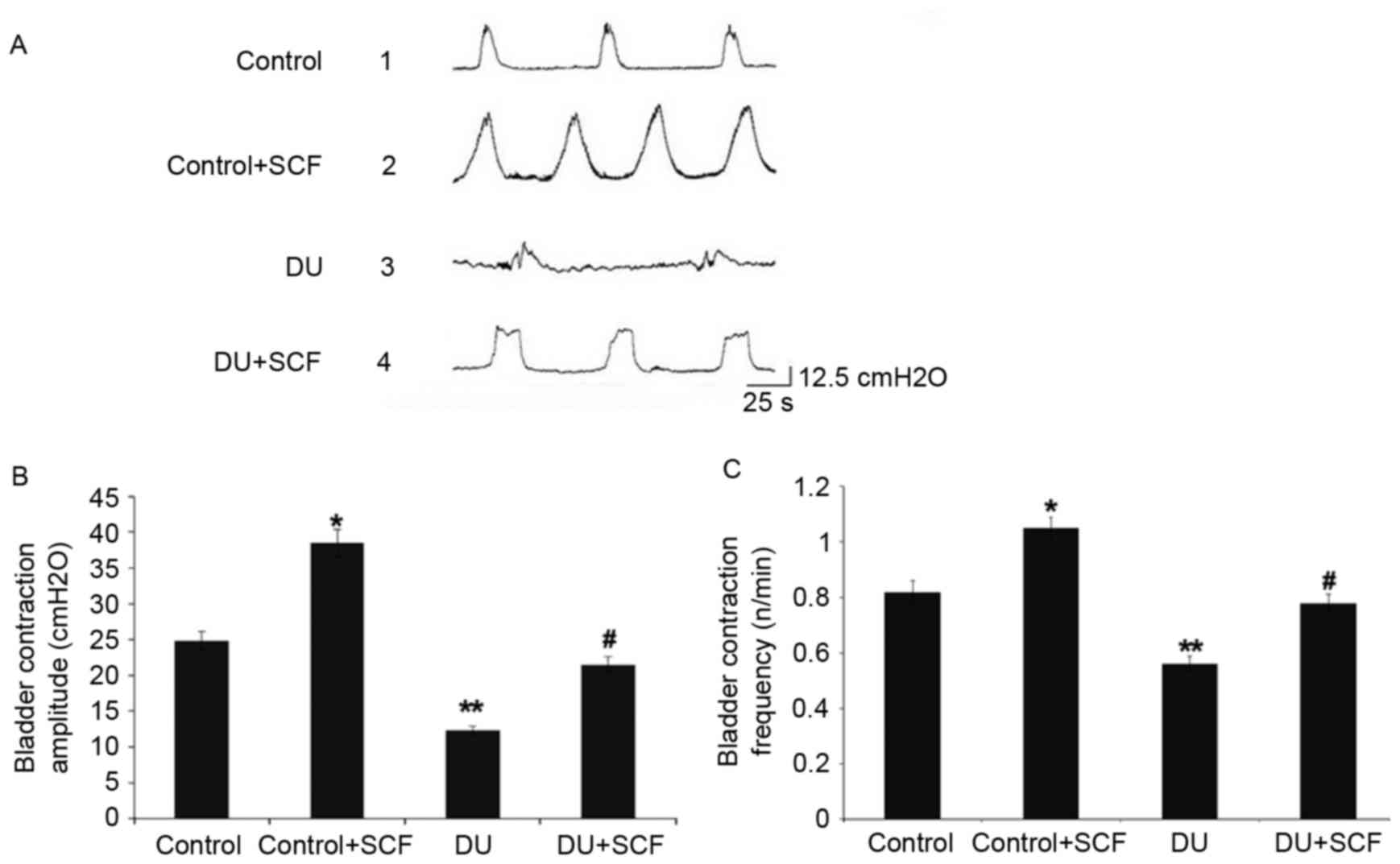
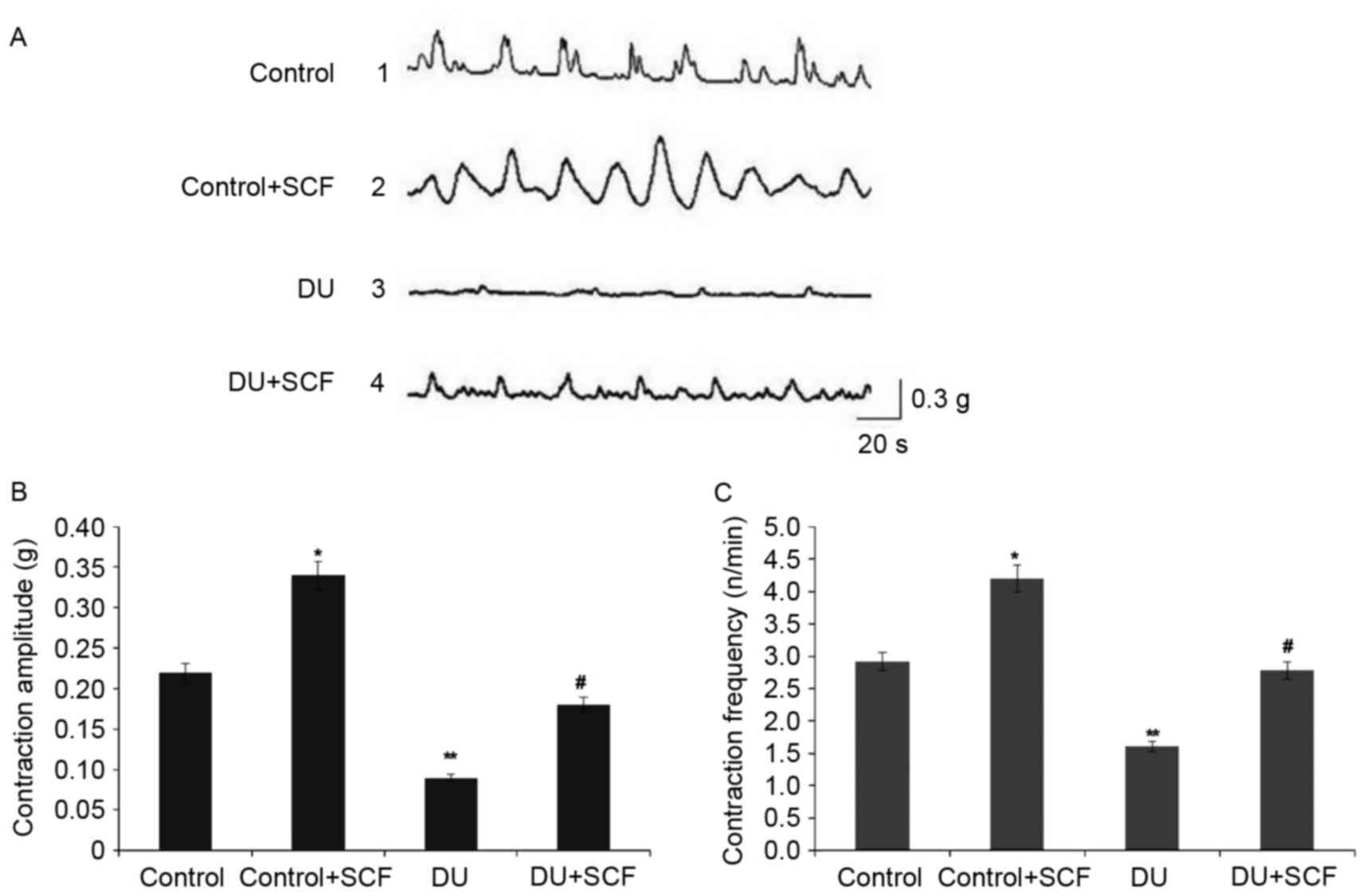
SCF improves bladder micturition
contraction in rats with DU
The bladder pressure was measured in vivo by
the micturition contraction wave during gradual bladder filling,
the results of which are shown in Fig.
1A. Compared with the control group, the amplitude and
frequency of bladder micturition contraction were significantly
decreased in the DU group, as shown in Fig. 1B and C, respectively. The
control+SCF group showed significantly higher amplitude and
frequency of bladder micturition contraction, compared with the
control group. Similarly, the amplitude and frequency of bladder
micturition contraction were higher in the DU+SCF group, compared
with the DU group.
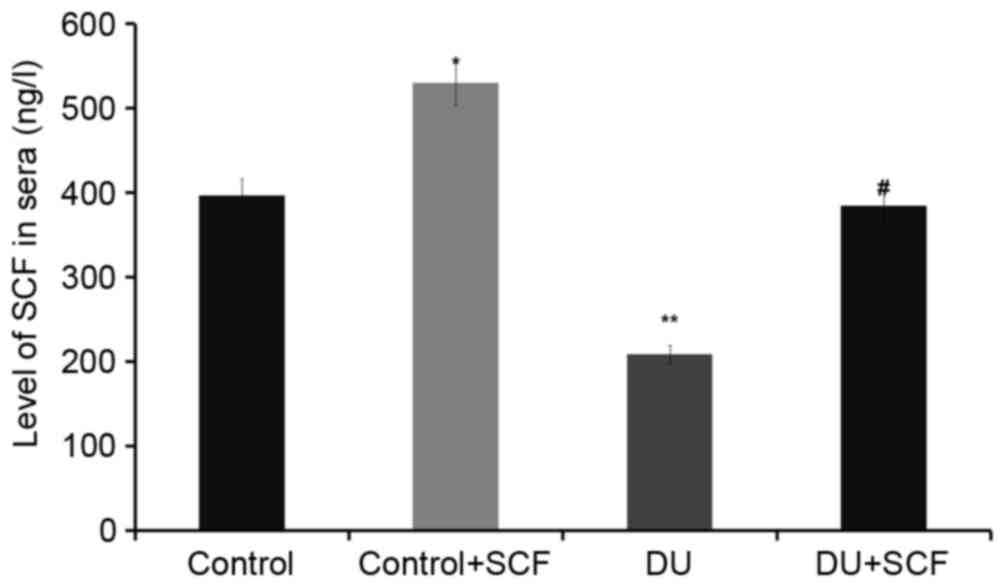
Exogenous administration of SCF
results in increased plasma levels of SCF
The levels of SCF in plasma, as assessed using
ELISA, are shown in Fig. 2. The
plasma levels of SCF in the control+SCF group animals (529.6±28.3
ng/l) were higher, compared with the values obtained in the control
group animals (396.1±20.2 ng/l; P<0.05). Compared with the
control group, the plasma levels of SCF in rats of the DU group
(208.5±13.9 ng/l) were significantly decreased. However, following
administration of exogenous (DU+SCF group), the plasma levels of
SCF were significantly increased to 384.5±18.7 ng/l (P<0.05),
suggesting that exogenous SCF markedly improved the levels of SCF
in animals with DU.
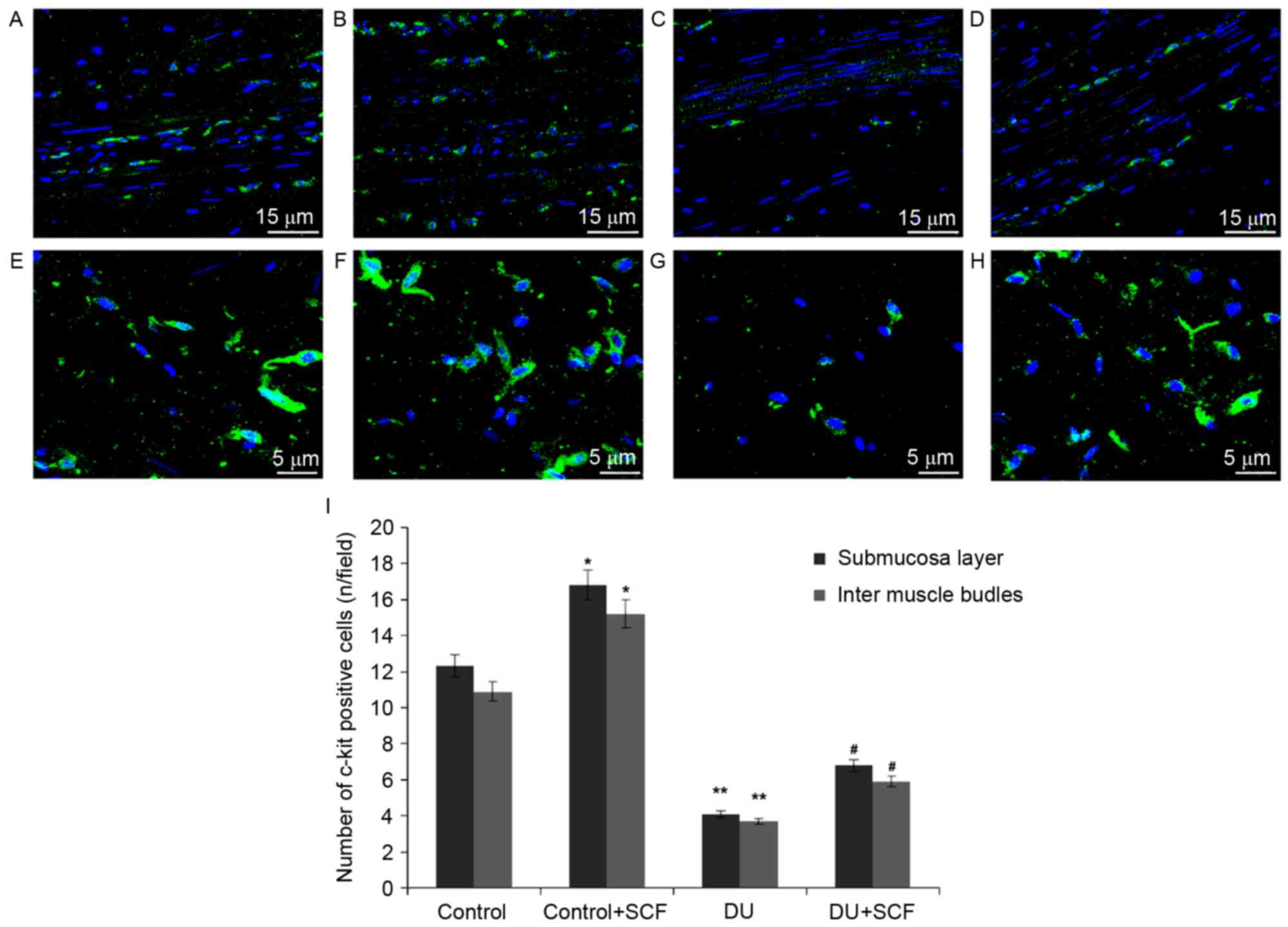
SCF increases ICC counts in rats with
DU
The ICC counts in rat detrusor specimens were
determined using immunofluorescence and shown in Fig. 3A-H. Classical spindle-like ICCs
were widely distributed in the submucosal and muscular layers.
Compared with the control rats, significantly fewer ICCs were found
in the submucosal and intermuscle bundles layers of the DU group,
(Fig. 3I; P<0.05). Upon
administration of exogenous SCF (DU+SCF group), ICC counts in the
rat detrusor specimens were significantly increased (Fig. 3I), suggesting that SCF markedly
improved ICC count in the underactive detrusor. This was also
reflected by increased values in the control+SCF group, compared
with the control group (Fig.
3I).
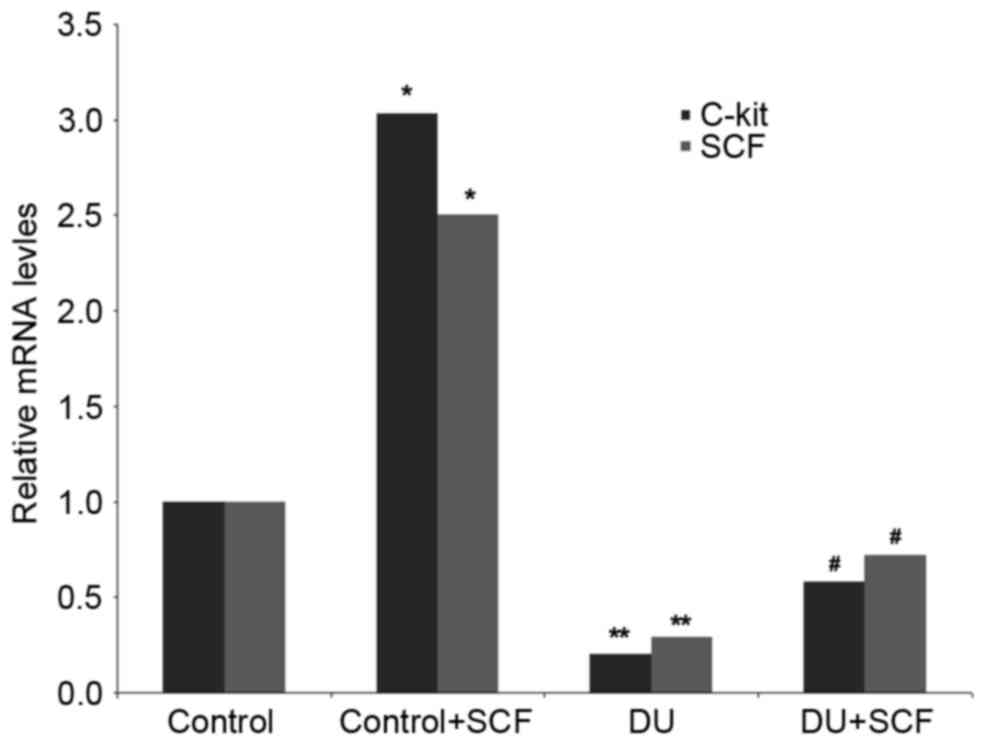
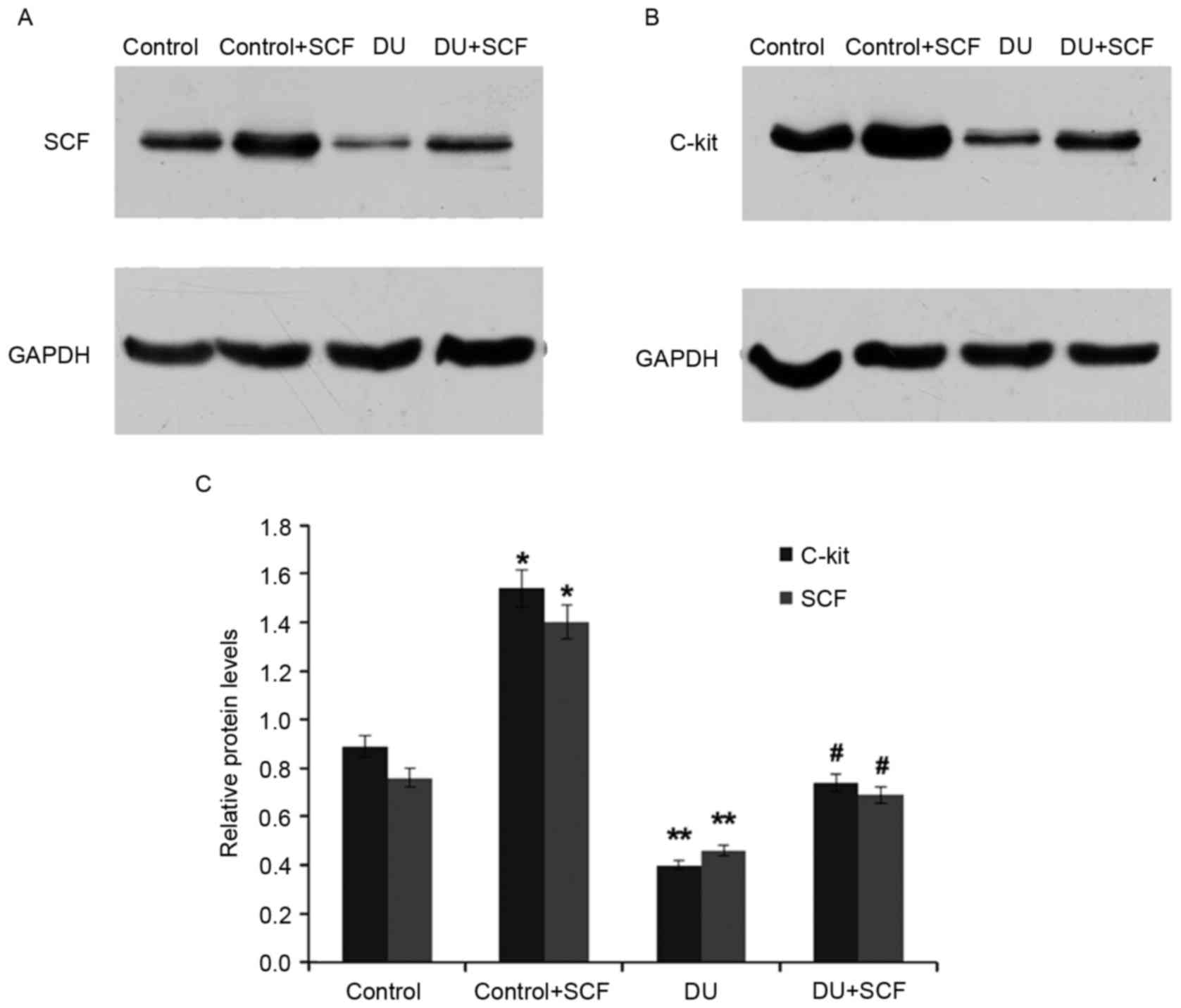
SCF administration results in induced
expression of SCF and c-kit, at the gene and protein levels
The gene expression levels of SCF and c-kit in rat
detrusor samples were detected using RT-qPCR analysis. Compared
with the control group, the mRNA levels of SCF and c-kit in the DU
group were significantly decreased (P<0.05). However,
administration of exogenous SCF (DU+SCF group) significantly
increased the mRNA levels of SCF and c-kit (Fig. 4), indicating that SCF induced the
gene expression of SCF and c-kit in DU. In agreement, the
control+SCF group showed significantly higher mRNA levels of SCF
and c-kit, compared with the control group (Fig. 4).
As shown in Fig. 5,
the protein levels of SCF and c-kit in the DU group were also
significantly reduced, compared with those in the control group
(P<0.05), an effect alleviated by the exogenous administration
of SCF, as observed in the DU+SCF group. Similarly, the protein
levels of SCF and c-kit were significantly increased in the
control+SCF group, compared with the control group. These findings
indicated that exogenous SCF increased the protein expression
levels of SCF and c-kit in DU.
SCF restores detrusor contractility in
rats with DU
The results of the in vitro muscle
contraction experiment are shown in Fig. 6. As shown in Fig. 6A, and quantified in Fig. 6B and C, the detrusor contraction
amplitude and frequency were markedly decreased in the DU group,
compared with those in the control group (P<0.05). Of note, SCF
administration significantly increased the amplitude and frequency
of muscle contraction (P<0.05 for DU+SCF group, vs. DU group and
control+SCF, vs. control group), suggesting that exogenous SCF
restored contractility in DU.
Discussion
The present study revealed that partial obstruction
of bladder outlet contributed to a significant change of bladder
function. Obstruction for 8 weeks led to significant decreases in
contraction amplitude and frequency, suggesting successful
establishment of the DU model.
Several studies investigating bladder dysfunction
have focused on the overactive bladder (19,20),
whereas few have investigated the underactive bladder. The
pathophysiological mechanisms of underactive detrusor remain to be
elucidated and have attracted increasing attention.
ICCs are interstitial cells distributed in smooth
muscles in several organs; they are closely associated with
multiple organ dysfunction (8–11).
Following 6 weeks of obstruction, the bladder detrusor shows
compensatory hypertrophy with markedly increased function and a
significant increase in ICC cell counts (23). These findings correspond with
previous studies (24,25) demonstrating that ICCs are closely
associated with detrusor function.
In the present study, a BOO rat model was
established, and animals with DU were assessed. As described above,
ICC counts in the detrusor from rats with underactive bladders were
significantly decreased, compared with those in the control rats;
however, the underlying mechanisms remain to be elucidated.
Previous studies have shown that decreased ICC counts are caused by
ICC phenotype redifferentiation instead of death (7), which may be closely associated with
SCF. A study by Lin et al (13) found a significant decrease in ICC
counts in the colon of diabetic mice, which was associated with
decreased levels of SCF. Theresults of the present study showed
that the levels of SCF were decreased in underactive detrusor
tissues and plasma samples, and the levels of c-kit in the detrusor
tissues were significantly reduced, resulting in markedly reduced
ICC counts and dysfunctional contraction. These findings
corroborate with other reports demonstrating that SCF is involved
in regulating the proliferation and differentiation of ICC, and is
thus involved in regulating organ motility. Nakahara et al
(26) showed that ICCs have a
dose-dependent and time-limited proliferation response to SCF. In
addition, Tong et al (27)
revealed that exogenous SCF improved ICC number and function via
the SCF/c-kit pathway, with a high SCF concentration having
increased potency. In agreement, the results of the present study
demonstrated that exogenous SCF upregulated the expression of c-kit
in damaged detrusor tissues, contributing to increased ICC number
and restoring contractility of the underactive detrusor.
The results of the present study indicated that
exogenous SCF and c-kit were involved in regulating ICC count and
function. Exogenous SCF improved the organ dysfunction caused by a
reduction in ICC number, providing a novel approach for repairing
the dysfunction of organs, including DU.
Acknowledgements
This study was supported by a grant from the
Chongqing Natural Science Foundation (grant no.
cstc2012jjA1566).
References
|
1
|
Abrams P, Cardozo L, Fall M, Griffiths D,
Rosier P, Ulmsten U, van Kerrebroeck P, Victor A and Wein A:
Standardisation Sub-committee of the International Continence
Society: The standardisation of terminology of lower urinary tract
function: Report from the Standardisation Sub-committee of the
International Continence Society. Neurourol Urodyn. 21:167–178.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor JA III and Kuchel GA: Detrusor
underactivity: Clinical features and pathogenesis of an
underdiagnosed geriatric condition. J Am Geriatr Soc. 54:1920–1932.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lorincz A, Redelman D, Horvath VJ,
Bardsley MR, Chen H and Ordög T: Progenitors of interstitial cells
of cajal in the postnatal murine stomach. Gastroenterology.
134:1083–1093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horvath VJ, Vittal H, Lörincz A, Chen H,
Almeida-Porada G, Redelman D and Ordög T: Reduced stem cell factor
links smooth myopathy and loss of interstitial cells of cajal in
murine diabetic gastroparesis. Gastroenterology. 130:759–770. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nishimura M, Koda K, Oda K, Seike K,
Shimizu K and Miyazaki M: Mesenteric transection decreases
expression of interstitial cells of Cajal in an experimental model.
Br J Surg. 94:483–490. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galli SJ, Tsai M and Wershil BK: The c-kit
receptor, stem cell factor, and mast cells. What each is teaching
us about the others. Am J Pathol. 142:965–974. 1993.PubMed/NCBI
|
|
7
|
Torihashi S, Nishi K, Tokutomi Y, Nishi T,
Ward S and Sanders KM: Blockade of kit signaling induces
transdifferentiation of interstitial cells of cajal to a smooth
muscle phenotype. Gastroenterology. 117:140–148. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu JJ, Rothman TP and Gershon MD:
Development of the interstitial cell of Cajal: Origin, kit
dependence and neuronal and nonneuronal sources of kit ligand. J
Neurosci Res. 59:384–401. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forrest A, Huizinga JD, Wang XY, Liu LW
and Parsons M: Increase in stretch-induced rhythmic motor activity
in the diabetic rat colon is associated with loss of ICC of the
submuscular plexus. Am J Physiol Gastrointest Liver Physiol.
294:G315–G326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto T, Watabe K, Nakahara M, Ogiyama
H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y and Hayashi N:
Disturbed gastrointestinal motility and decreased interstitial
cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol.
23:660–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubota M, Kanda E, Ida K, Sakakihara Y and
Hayashi M: Severe gastrointestinal dysmotility in a patient with
congenital myopathy: Causal relationship to decrease of
interstitial cells of Cajal. Brain Dev. 27:447–450. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomsen L, Robinson TL, Lee JC, Farraway
LA, Hughes MJ, Andrews DW and Huizinga JD: Interstitial cells of
Cajal generate a rhythmic pacemaker current. Nat Med. 4:848–851.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin L, Xu LM, Zhang W, Ge YB, Tang YR,
Zhang HJ, Li XL and Chen JD: Roles of stem cell factor on the
depletion of interstitial cells of Cajal in the colon of diabetic
mice. Am J Physiol Gastrointest Liver Physiol. 298:G241–G247. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lang RJ, Tonta MA, Zoltkowski BZ, Meeker
WF, Wendt I and Parkington HC: Pyeloureteric peristalsis: Role of
atypical smooth muscle cells and interstitial cells of Cajal-like
cells as pacemakers. J Physiol. 576:695–705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pezzone MA, Watkins SC, Alber SM, King WE,
de Groat WC, Chancellor MB and Fraser MO: Identification of
c-kit-positive cells in the mouse ureter: The interstitial cells of
Cajal of the urinary tract. Am J Physiol Renal Physiol.
284:F925–F929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Zhang Y and Hu J: The expression
of Cajal cells at the obstruction site of congenital pelviureteric
junction obstruction and quantitative image analysis. J Pediatr
Surg. 44:2339–2342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCloskey KD and Gurney AM: Kit positive
cells in the guinea pig bladder. J Urol. 168:832–836. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davidson RA and McCloskey KD: Morphology
and localization of interstitial cells in the guinea pig bladder:
Structural relationships with smooth muscle and neurons. J Urol.
173:1385–1390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SO, Song SH, Ahn KY and Kwon DD:
Distribution of interstitial cells of cajal in menopausal rat
urinary bladder showing detrusor overactivity. Int Neurourol J.
14:48–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kubota Y, Kojima Y, Shibata Y, Imura M,
Sasaki S and Kohri K: Role of KIT-positive interstitial cells of
Cajal in the urinary bladder and possible therapeutic target for
overactive bladder. Adv Urol. 2011:8163422011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sutherland RS, Baskin LS, Kogan BA and
Cunha G: Neuroanatomical changes in the rat bladder after bladder
outlet obstruction. Br J Urol. 82:895–901. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Fang Q, Lu Y, Song B, Li W and Li
L: Effects of mechanical stretch on interstitial cells of Cajal in
guinea pig bladder. J Surg Res. 164:e213–e219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SO, Oh BS, Chang IY, Song SH, Ahn K,
Hwang EC, Oh KJ, Kwon D and Park K: Distribution of interstitial
cells of Cajal and expression of nitric oxide synthase after
experimental bladder outlet obstruction in a rat model of bladder
overactivity. Neurourol Urodyn. 30:1639–1645. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kubota Y, Hashitani H, Shirasawa N, Kojima
Y, Sasaki S, Mabuchi Y, Soji T, Suzuki H and Kohri K: Altered
distribution of interstitial cells in the guinea pig bladder
following bladder outlet obstruction. Neurourol Urodyn. 27:330–340.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakahara M, Isozaki K, Vanderwinden JM,
Shinomura Y, Kitamura Y, Hirota S and Matsuzawa Y: Dose-dependent
and time-limited proliferation of cultured murine interstitial
cells of Cajal in response to stem cell factor. Life Sci.
70:2367–2376. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tong W, Jia H, Zhang L, Li C, Ridolfi TJ
and Liu B: Exogenous stem cell factor improves interstitial cells
of Cajal restoration after blockade of c-kit signaling pathway.
Scand J Gastroenterol. 45:844–851. 2010. View Article : Google Scholar : PubMed/NCBI
|