Introduction
Diabetic nephropathy (DN) is an important endocrine
metabolic dysfunctional disease and the leading cause of end-stage
renal disease worldwide (1).
Increasing evidence indicates that injury, detachment, apoptosis
and loss of podocytes are observed in humans with DN and in DN
animal models (2–4). Podocytes are key in maintaining the
integrity of the glomerular filtration barrier, together with
mesangial cells, and have been reported to be important for the
progression of diabetic kidney disease (5). In patients with type I and II
diabetes mellitus, the density of podocytes is significantly
reduced in those who have had the diabetes for a short duration
prior to the onset of microalbuminuria (6). A correlation between the rate of
albumin excretion and the reduction in podocyte number has been
demonstrated in rats with streptozotocin-induced diabetes mellitus
(7). In addition, high glucose
(HG) provokes adhesion capacity and phenotypic alterations in
cultured podocytes (8). Taken
together, these data indicate that podocyte injury is closely
associated with hyperglycemia. Although there is considerable
evidence suggesting that chronic hyperglycemia is the primary cause
of podocyte injury, the underlying molecular mechanisms of
hyperglycemia-induced podocyte injury remain to be elucidated.
Endoplasmic reticulum (ER) is a central organelle
engaged in lipid synthesis, protein folding and maturation
(9). A variety of toxic insults,
including hypoxia (10),
glucocorticoids (11) and HG
(12), can disturb ER function,
and result in ER stress. There is increasing evidence that ER
stress is crucial in the regulation of apoptosis (13), with a previous study reporting that
ER stress is triggered in angiotensin II-treated podocytes
(14). In addition, palmitate
induces ER calcium depletion and apoptosis in mouse podocytes
following mitochondrial oxidative stress (9), and HG induces the apoptosis of
podocytes through ER stress in vivo and in vitro
(15,16). These results suggest that ER stress
is involved in the pathogenesis of podocyte dysfunction and is
being recognized as an emerging target for DN therapy.
Huaiqihuang (HQH) is predominantly composed of
Trametes robiniophila Murr, Fructus Lycii and
Polygonatum sibiricum, and has been widely used for the
treatment of primary nephrotic syndrome (17). In renal tissues of rats with
adriamycin-induced nephrosis, HQH can maintain the integrity of the
slit diaphragm in podocytes, alleviate lesions of the glomerular
filtration membrane, and decrease proteinuria by upregulating the
expressions of nephrin and podocin (18). However, the protective effect of
HQH in hyperglycemia-induced MPC5 podocyte dysfunction remains to
be fully elucidated. To the best of our knowledge, the present
study is the first to attempt to determine the protective effect of
HQH in hyperglycemia-induced MPC5 podocytes. The data provided
evidence that HQH attenuated hyperglycemia-induced reactive oxygen
species (ROS) generation and ER stress in MPC5 podocytes.
Materials and methods
Cell culture
MPC5 podocytes were obtained from the Cell Resource
Center, Shanghai Institutes for Biological Sciences (Shanghai,
China), and maintained in RPMI-1640 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified incubator (Thermo Fisher Scientific, Inc.), 5%
CO2, 95% air atmosphere. RPMI-1640 medium containing
high glucose (HG; 30 mM D-glucose) or normal glucose (5 mM
D-glucose) was used.
Cell viability detection using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT)
The proliferation of MPC5 podocytes (1×105) was
monitored using an MTT Cell Proliferation/Viability Assay kit
(R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol.
Caspase-3 activity
Activity of caspase-3 was determined using the
caspase-3 activity assay kit (cat. no. C1116; Beyotime Institute of
Biotechnology, Haimen, China), according to the manufacturer's
protocol. Briefly, ~1×106 cells were incubated for 30 min at 0°C in
2 ml of lysis buffer, containing 25 mM Hepes, pH 7.5
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 5 mM EDTA
(Sigma-Aldrich; Merck KGaA), 1 mM EGTA (Sigma-Aldrich; Merck KGaA),
5 mM MgCl2 (Thermo Fisher Scientific, Inc.), 10 mM Sucrose (Thermo
Fisher Scientific, Inc.), 5 mM dithiothreitol (DTT; Sigma-Aldrich;
Merck KGaA), 1% 3-[-(3-chloramidopropyl)
dimethylammonio]-1-propanesulfonic acid (CHAPS; Sigma-Aldrich;
Merck KGaA), protease inhibitor cocktail (10 µl/ml; Sigma-Aldrich;
Merck KGaA), and 1 mM PMSF (Sigma-Aldrich; Merck KGaA). Cell
lysates were freeze/thawed three times and centrifuged at 12,000 ×
g for 60 min at 4°C. The supernatants were collected and incubated
with caspase-3 substrate in PBS for 2 h at 37°C. The release of
p-nitroaniline was measured at 405 nm using an ELISA reader (MD
SpectraMax M5; Molecular Devices LLC, Sunnyvale, CA, USA) according
to the manufacturer's protocol. The results indicated the
percentage change in activity compared with the untreated
control.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick end labeling
(TUNEL) assay
Quantitative assessment of the apoptotic cells was
performed using the TUNEL method, which examines DNA-strand breaks
during apoptosis, using a BD ApoAlert™ DNA Fragmentation
Assay kit (BD Biosciences, Franklin Lakes, NJ, USA). The cells were
trypsinized, fixed with 4% paraformaldehyde for 30 min at room
temperature and permeabilized with 0.1% Triton-X-100 in 0.1% sodium
citrate for 5 min at room temperature. Following washing with PBS
three times, the cells (1×105) were incubated with the reaction
mixture for 60 min at 37°C. The cells were immediately analyzed
using FACScan flow cytometry and the CellQuest™ software
version 5.1 (BD Biosciences).
Measurement of ROS
The generation of ROS in cells was evaluated with a
fluorometric assay using intracellular oxidation of
dichlorodihydrofluorescein diacetate (DCFH-DA). The cells (2×106)
were incubated in a 6-well plate for 24 h at 37°C for
stabilization, and were then detected and analyzed using flow
cytometry (BD Biosciences.).
Measurement of
H2O2, malondialdehyde (MDA) and permea
bility
An Amplex Red assay (Thermo Fisher Scientific, Inc.)
was used to measure H2O2 levels, which were
measured at an excitation wavelength of 560 nm and emission
detection wavelength of 590 nm using an ELISA reader (MD SpectraMax
M5; Molecular Devices LLC) according to the manufacturer's
protocol. A Biochemical Analysis kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) was used for the
measurement of MDA, according to manufacturer's protocol. The
permeability of the podocytes was measured, as described previously
(19).
Detection of Ca2+
concentrations
The MPC5 podocytes were plated and treated in
12-well plates, and were incubated to detect changes in
Ca2+ levels. The cells were harvested and washed with
PBS twice, and resuspended in Indo 1/AM (3 µg/ml) at 37°C for 30
min, followed by analysis using flow cytometry (BD
Biosciences).
Determination of mitochondrial
membrane potential
The mitochondrial membrane potential was assessed
using a fluorometric probe (DiOC6; Molecular Probes;
Thermo Fisher Scientific, Inc.). Briefly, cells (2×106) were plated
in 6-well culture dishes. On reaching confluence, the cells were
treated with HG (30 mM) or HQH (0, 0.2, 1 or 2 mg/ml) for 24 h at
37°C. Following incubation, the cells were stained with
DiOC6 (40 nM) for 15 min at 37°C. The cells were then
collected, washed twice in PBS and analyzed using FACScan flow
cytometry (BD Biosciences).
Small interfering RNA (siRNA)
transfection
The siRNAs against glucose-related protein 78
(GRP78) and scrambled siRNA were obtained from GE Dharmacon
(Lafayette, CO, USA). The cells (1×105) were transfected with the
siRNAs (at a final concentration of 100 nM) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific. Inc.) according to the
manufacturer's protocol. Sequences of the siRNAs used were as
follows: si-GRP78, sense 5′-AAGGUUACCCAUGCAGUUGTT-3′, antisense
5′-CAACUGCAUGGGUAACCUUTT-3′; and scrambled siRNA, sense
5′-UUCUGCGAUGCUGUCACGUAT-3′ and antisense
5′-ACCUGACUCGAUCGCAGAAAT-3′.
Comet assay
Briefly, fully frosted slides were precoated at each
end with 100 ml of 0.8% agarose in PBS (pH 7.4), covered with a
22×22 mm glass coverslip and left at room temperature for 20 min.
Subsequently, 30 ml of the cell culture was mixed with 70 ml of 1%
low-melting point agarose in PBS and maintained at 42°C on a
dry-bath incubator. The mixture was immediately spread onto each
end of a precoated slide and covered with a fresh glass coverslip.
Images of the comets were captured with an Olympus microscope
(Olympus Corporation, Tokyo, Japan) equipped with a CCD camera
connected to the fluorescent microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA extraction from the MPC5 podocytes was performed
using TRIzol® reagent according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse
transcribed into cDNA, using a 20 µl reaction mixture containing 4
µg of total RNA using M-MLV Reverse Transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.) and oligo dT (15) primers (Fermentas; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
first strand cDNAs served as the template for PCR. The reaction
mixture (25 µl) included 12.5 µl iQ™ SYBR-Green Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), 1 µl cDNA, 300 nM
of each primer, and diethyl pyrocarbonate-treated water to a final
volume of 25 µl. PCR was performed using a DNA engine (ABI 7300;
Thermo Fisher Scientific, Inc.). Amplification conditions were as
follows: Initial denaturation at 95°C for 3 min, followed by 30–40
cycles of denaturation at 95°C for 15 sec, annealing at 56°C for 20
sec and extension at 72°C for 20 sec. PCR was performed using the
following primers: Nephrin, forward 5′-AGCTCGTGTCTCCCAGAGT-3′,
reverse 5′-CGTTCACGTTTGCAGAGATGT-3′; GRP78, forward
5′-AACCCAGATGAGGCTGTAGCA-3′, reverse 5′-ACATCAAGCAGAACCAGGTCAC-3′;
C/EBP-homologous protein (CHOP), forward
5′-CCAGCAGAGGTCACAAGCAC-3′, reverse 5′-CGCACTGACCACTCTGTTTC-3′; and
GAPGH, forward 5′-GGTGGAGGTCGGGAGTCAACGGA-3′ and reverse
5′-GAGGGATCTCGCTCCTGGAGGA-3′. Relative expression levels of the
target genes were normalized to GAPDH, using the 2−ΔΔCq
method (20).
Western blot analysis
The MPC5 podocytes were homo genized in NP-40
buffer, followed by 5–10 min boiling and centrifugation at 12,000 ×
g for 10 min at 4°C to obtain the supernatants. Protein
concentrations were determined using the Bicinchoninic Acid kit for
Protein Determination (cat. no. BCA1-1KT; Sigma-Aldrich; Merck
KGaA). Equal amounts of extracted protein samples (30 µg) were
separated by 10% SDS-PAGE and transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). Following saturation with
5% non-fat dry milk in TBS containing 0.1% Tween-20 (TBST) for 2 h
at room temperature and two washes with PBS, the membranes were
incubated with the following primary antibodies at 4°C overnight:
Anti-nephrin (cat. no. sc-377246; 1:1,000), anti-GRP78 (cat. no.
sc-376768; 1:1,000), anti-cleaved-caspase-3 (cat. no. sc-271028;
1:1,000), anti-β-actin (cat. no. sc-130065; 1:2,000) from Santa
Cruz Biotechnoogy, Inc. (Dallas, TX, USA); and anti-CHOP (cat. no.
AC532; 1:1,000) from Beyotime Institute of Biotechnology. Following
three washes with TBST, the membranes were incubated for 2 h at
37°C with donkey anti-mouse horseradish peroxidase-conjugated
immunoglobulin G (cat. no. sc-2096; 1:10,000) from Santa Cruz
Biotechnology, Inc. Subsequently, membranes were washed three times
with TBST and visualized using an enhanced chemiluminescence kit
(Thermo Fisher Scientific, Inc.). Blots were semi-quantified using
densitometric analysis with the Quantity One® software version 4.5
(Bio-Rad Laboratories, Inc.) and normalized to β-actin expression
to correct for unequal loading.
Statistical analysis
The data from experiments are reported as the mean ±
standard deviation for each group. All statistical analyses were
performed using GraphPad Prism software version 4.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Inter-group differences were
analyzed using one-way analysis of variance, followed by a post hoc
Tukey's test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
HQH protects against HG-induced
podocyte apoptosis and dysfunction
To investigate the potential apoptotic effects of HG
in podocytes, the present study first examined the effect of HG on
cell survival using an MMT assay. The podocytes were treated with
30 mM HG for various periods of time, and the results showed that
HG reduced cell viability in a time-dependent manner, compared with
that of the control group (Fig.
1A). Determination of the cytotoxic effect of HQH was
imperative prior to further experiments. The viability of podocytes
following incubation with different concentrations of HQH for 24 h
was determined using the MTT assay. The podocytes retained almost
the same viability when exposed to HQH at concentrations of 0–2
mg/ml, whereas concentrations of HQH >20 mg/ml markedly altered
cell viability (Fig. 1B).
Therefore, concentrations of HQH <2 mg/ml were suitable for the
selective pharmacological action of the drug without any
interference of normal cell function. The podocyte protein,
nephrin, is essential for maintaining the filtration barrier of the
kidney and preventing albuminuria (21). As shown in Fig. 1C and D, the results indicated that,
compared with the NG-treated group, HG treatment of podocytes
exerted a marked decrease in the mRNA and protein levels of
nephrin, whereas HQH at concentrations of 1 and 2 mg/ml
significantly reversed this effect. Subsequently, TUNEL staining
was performed to examine the effect of the downregulation of HQH on
podocyte cell apoptosis, and the percentage of TUNEL-positive
(apoptotic) cells was calculated. As shown in Fig. 1E, the percentage of apoptotic cells
induced by HG decreased when the podocytes were exposed to HQH at
concentrations of 1 and 2 mg/ml.
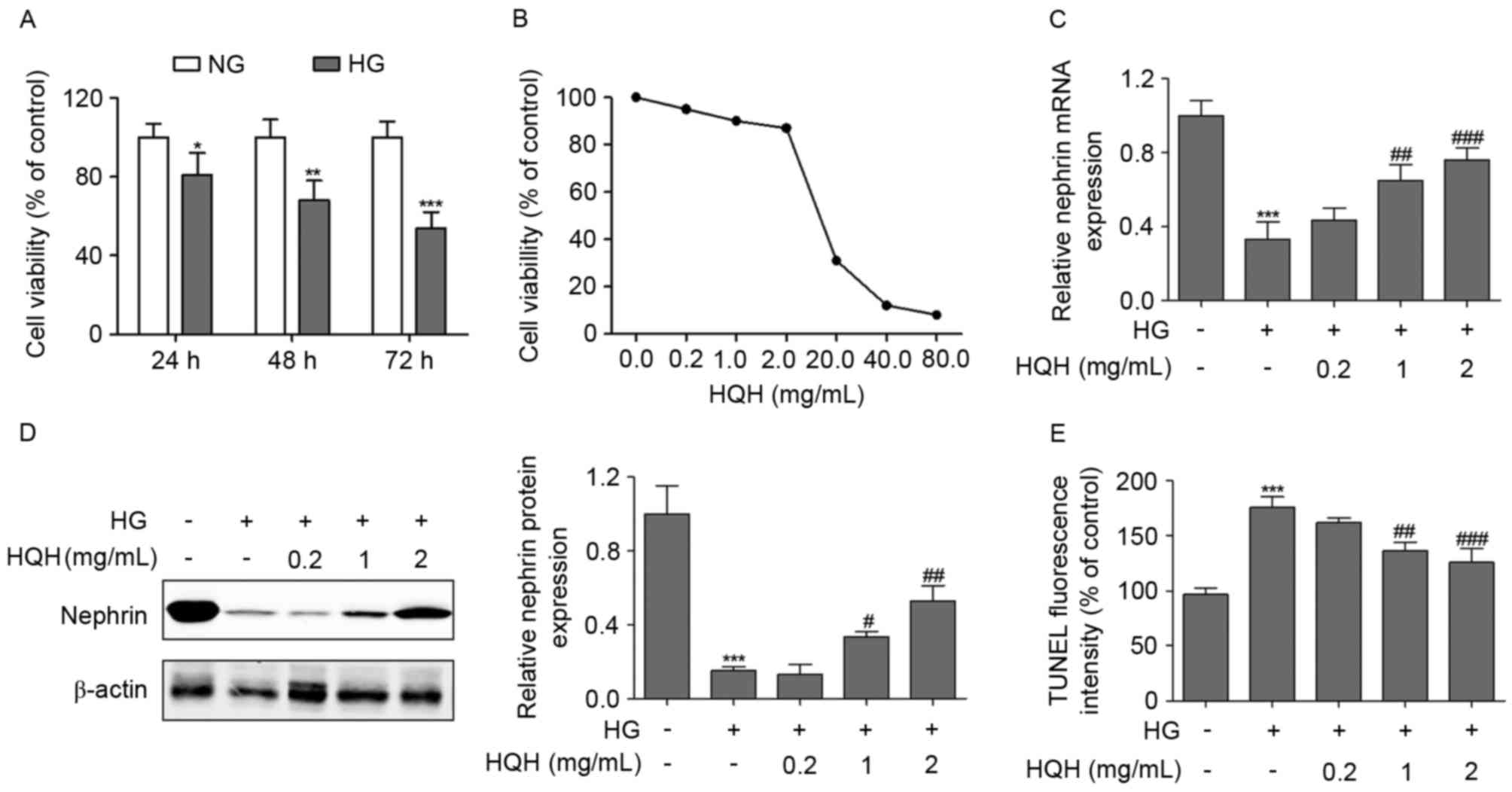 | Figure 1.HQH protects against HG-induced
podocyte apoptosis and dysfunction. (A) MPC5 podocytes were
incubated with HG (30 mM) and HQH, and the cell viability was
examined using an MTT assay. (B) Effect of HQH on the viability of
podocytes (1×104 cells/well) incubated with HQH of
different concentrations for 24 h. Cell viability was determined
using the MTT assay. The (C) mRNA and (D) protein expression levels
of nephrin were measured using reverse transcription-polymerase
chain reaction and western blot analyses, respectively, following
24 h treatment. (E) TUNEL-positive (apoptotic) cells were measured
using flow cytometry following 24 h treatment. Values are expressed
as the mean ± standard deviation (n=3 in each group). *P<0.05,
**P<0.01 and ***P<0.001, vs. control group;
#P<0.05, ##P<0.01 and
###P<0.001, vs. HG only treatment group. HG, high
glucose; HQH, Huaiqihuang; NG, normal glucose; MTT, 3-
(4,5-dimethylthiazol-2-yl) −2,5-diphenyltetrazolium bromide; TUNEL,
terminal deoxynucleotidyl transferase-mediated deoxyuridine
triphosphate nick end labeling. |
HQH inhibits HG-induced ROS and
mitochondrial dysfunction in podocytes
The effects of HQH on HG-induced ROS and
mitochondrial dysfunction were determined in podocytes (Fig. 2). To identify the role of ROS in
podocyte injury, ROS concentrations were measured by flow cytometry
using DCFH-DA. Compared with untreated podocytes, treatment with HG
caused a significant increase in intracellular ROS generation.
Treatment of the podocytes with HQH at concentrations of 1 and 2
mg/ml markedly suppressed the HG-induced ROS generation (Fig. 2A). In addition, treatment of the
podocytes with HQH significantly reversed the HG-induced
upregulation in the production of H2O2
(Fig. 2B), permeability (Fig. 2C) and MDA (Fig. 2F) in podocytes. To further examime
whether HG-induced cell apoptosis was mediated through
mitochondrial dysfunction, the present study determined
mitochondrial membrane potential using the mitochondria-sensitive
dye, DiOC6, with flow cytometry. As shown in Fig. 2D, treatment of podocytes with HG
led to the loss of mitochondrial membrane potential, compared with
that in the control group. HG in combination with HQH significantly
improved mitochondrial membrane potential in the podocytes. The
effect of HG on the mobilization of Ca2+ was then
assessed. When podocytes were treated with HG, the Ca2+
levels were significantly increased, compared with those in the
control group, however, treatment of podocytes with HQH
significantly reversed the HG-induced upregulation of
Ca2+ (Fig. 2E).
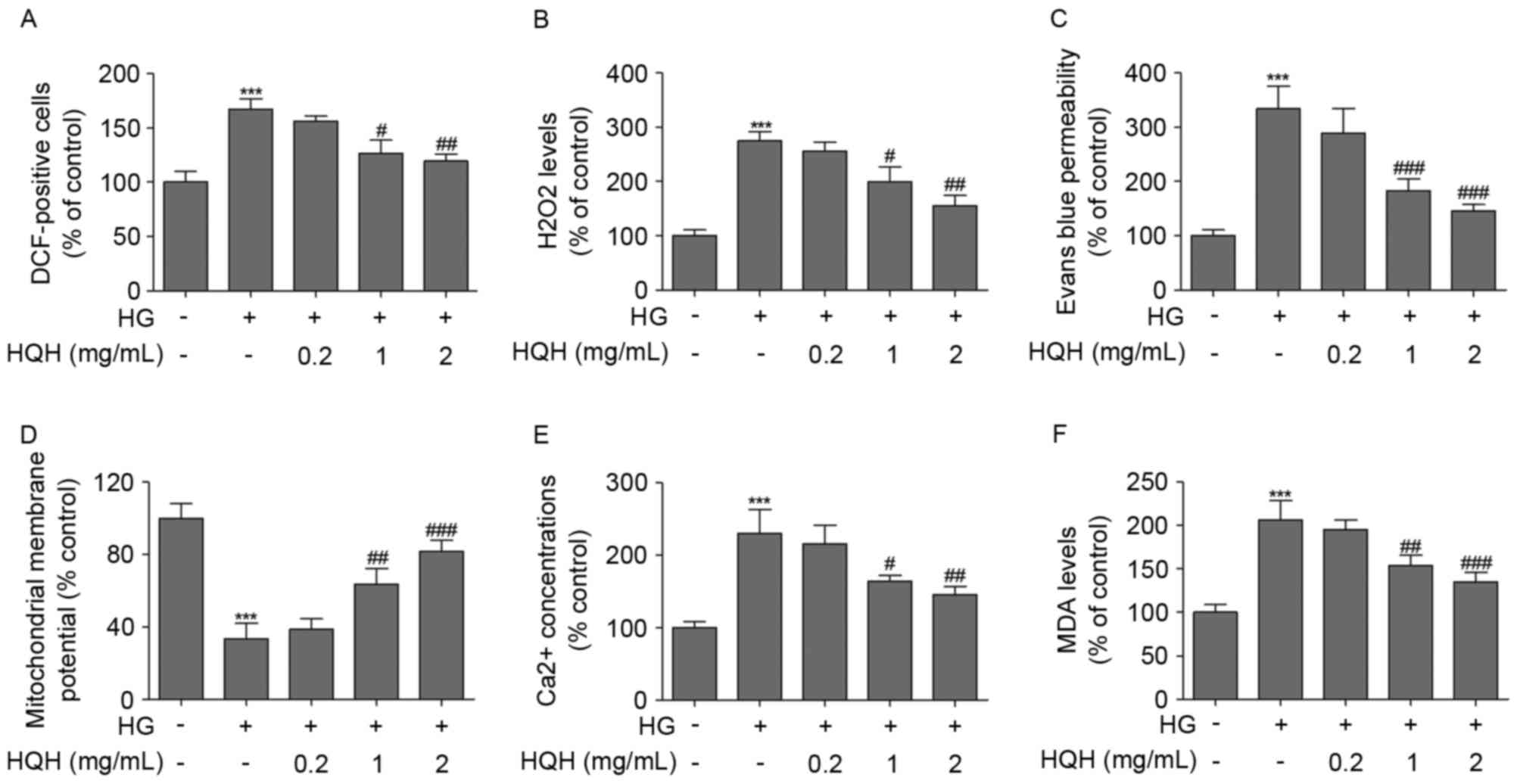 | Figure 2.HQH inhibits HG-induced ROS and
mitochondrial dysfunction in podocytes. (A) Intracellular ROS
production was measured according to changes in the fluorescence
intensity of DCF, the oxidized derivative of DCF-DA, following 12 h
treatment. (B) H2O2 was measured using an
Amplex Red assay following 24 h treatment. (C) Effects of HG and
HQH on permeability in MPC5 podocytes was measured over 24 h. MPC5
podocytes were incubated with HG (30 mM) or HQH for 24 h, and the
(D) mitochondrial membrane potential and (E) release of
Ca2+ were examined using flow cytometry. (F) MDA levels
were measured following exposure of MPC5 podocytes to HG (30 mM) or
HQH for 24 h. Values are expressed as the mean ± standard deviation
(n=3 in each group). ***P<0.001, vs. control group;
#P<0.05, ##P<0.01 and
###P<0.001, vs. HG only treatment group. HG, high
glucose; HQH, Huaiqihuang; DCF-DA, dichlorodihydrofluorescein
diacetate; MDA, malondialdehyde. |
HQH inhibits HG-induced GRP78 and CHOP
in podocytes
GRP78, an important molecular chaperone localized in
the ER, is used as an indicator of ER stress (22). Compared with untreated podocytes,
GRP78 was increased in the HG single treatment group, at the mRNA
and protein levels (Fig. 3A and
B). Previous studies have demonstrated the importance of CHOP
in ER stress-induced cell death (16). Consistent with this, HG treatment
in the present study resulted in a significant increase in the mRNA
and protein expression of CHOP in podocytes (Fig. 3C and D). These results demonstrated
that ER stress was activated in the HG-treated podocytes. Of note,
treatment of the podocytes with HQH significantly reversed the
HG-induced upregulation of GRP78 (Fig.
3A and B) and CHOP (Fig. 3C and
D).
 | Figure 3.HQH inhibits HG-induced GRP78 and CHOP
in podocytes. (A) mRNA and (B) protein expression levels of GRP78
were measured using RT-PCR and western blot analyses, respectively,
following 24 h treatment. The (C) mRNA and (D) protein expression
levels of CHOP were measured using RT-PCR and western blot
analyses, respectively, following 24 h treatment. Values are
expressed as the mean ± standard deviation (n=3 in each group).
***P<0.001, vs. control group; ##P<0.01 and
###P<0.001, vs. HG only treatment group. HG, high
glucose; HQH, Huaiqihuang; RT-PCR, reverse transcription-polymerase
chain reaction; CHOP C/EBP-homologous protein; GRP78,
glucose-related protein 78. |
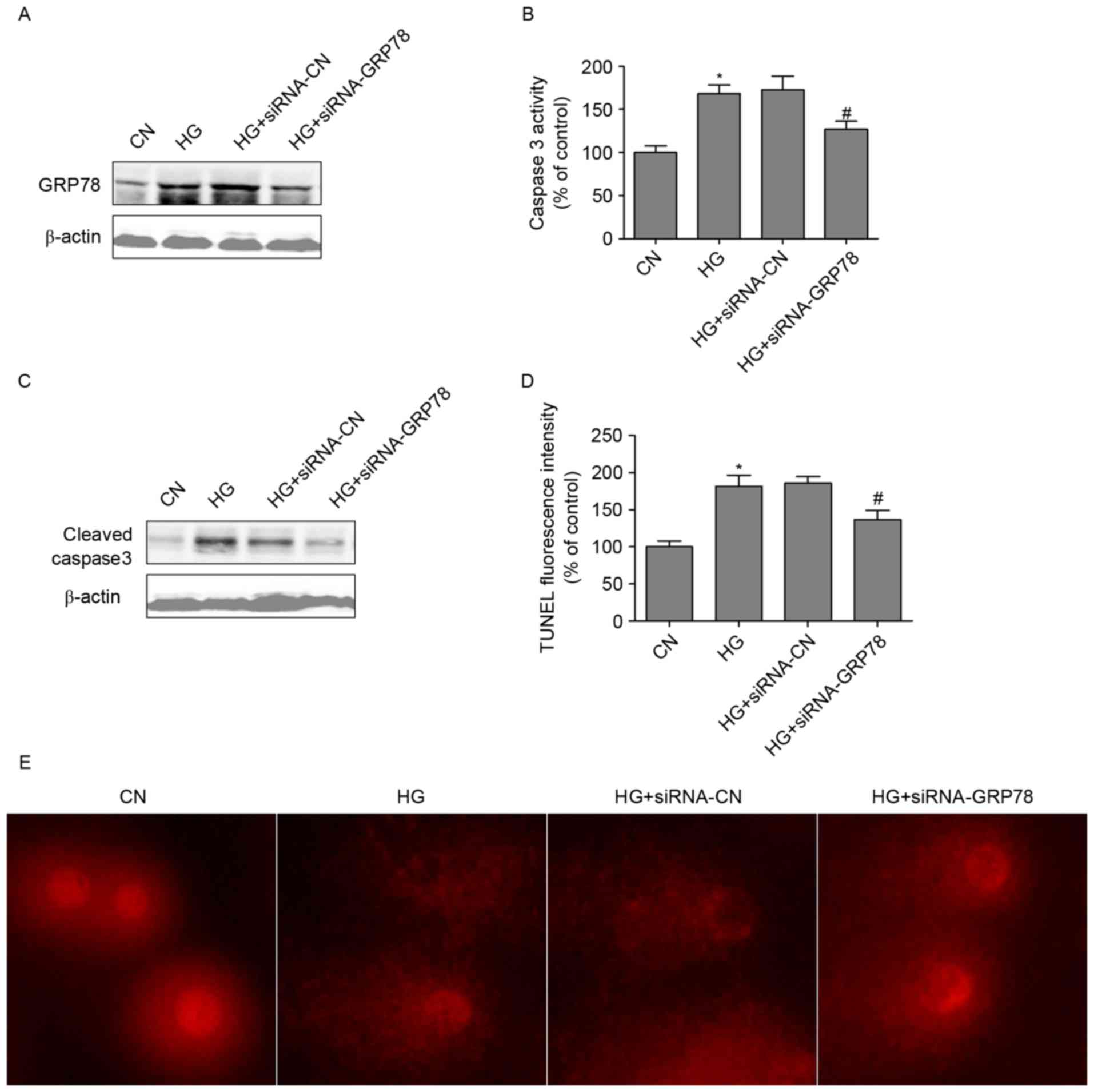
GRP78 loss-of-function attenuates
HG-induced podocyte dysfunction
To further investigate whether HG-induced podocyte
dysfunction occurred due to the activation of GRP78, GRP78 siRNA
was used. The transfection of podocytes with GRP78 siRNA
specifically inhibited the expression of GRP78 (Fig. 4A). In addition, GRP78 siRNA reduced
the HG-induced upregulation of caspase 3 (Fig. 4B) and the protein expression of
cleaved-caspase3 in podocytes (Fig.
4C). Furthermore, GRP78 siRNA inhibited HG-induced cell
apoptosis (Fig. 4D). DNA damage
has been found in podocytes with induced injury (9). In the present study, the tail length
in the HG-treated group was markedly longer, compared with that in
the control group. However, the tail length was significantly
suppressed by GRP78 loss-of-function (Fig. 4E). These results suggested that
GRP78 loss-of-function alleviated the podocyte dysfunction induced
by HG.
Discussion
In the present study, it was determined that cell
viability and the expression of nephrin decreased in cultured
podocytes exposed to HG (30 mM). The results also demonstrated that
HG induced ROS generation, mitochondrial dysfunction and ER stress
in podocytes. Simultaneously, HG treatment resulted in a
significant increase in the mRNA and protein expression of GRP78
and CHOP in podocytes. HQH was found to reverse HG-induced ROS
generation, mitochondrial dysfunction, ER stress and the
upregulated expression of GRP78 and CHOP, and GRP78
loss-of-function alleviate the podocyte dysfunction, which was
induced by HG. It was concluded that HQH may act as a potential
therapeutic drug for HG-induced podocyte dysfunction.
Mitochondrial dysfunction has been implicated in
several major diseases, including glomerular diseases (23). Mitochondria maintain cellular redox
and energy homeostasis, and are a major source of intracellular ROS
production. Mitochondrial ROS accumulation may contribute to
stress-induced mitochondrial dysfunction and apoptosis, and thereby
to glomerulosclerosis (24,25).
Previous studies have indicated that elevated levels of saturated
free fatty acid are harmful to mouse podocytes following
mitochondrial oxidative stress (9). Aldosterone-induced injury has been
found to decrease the expression of peroxisome
proliferator-activated receptor-γ coactivator 1α, and induce
mitochondrial and podocyte damage in a dose- and time-dependent
manner (26). These results
suggest that mitochondrial dysfunction is involved in toxin-induced
podocyte dysfunction. Two major events have been reported in
apoptosis involving mitochondrial dysfunction. One is the
alteration in membrane permeability and the subsequent loss of
membrane potential; the other is the release of apoptotic proteins,
including cytochrome c, from the intermembrane space of
mitochondria into the cytosol (27,28).
In the present study, it was found that treatment of podocytes with
HG induced the loss of the mitochondrial membrane potential.
However, HQH significantly improved mitochondrial membrane
potential in HG-induced podocyte mitochondrial dysfunction. When
the podocytes were treated with HG, the levels of Ca2+
were significantly increased, compared with those in the control
group, whereas treatment of podocytes with HQH significantly
reversed the HG-induced upregulation of Ca2+.
The ER is critical in controlling the fate of cells
and is a dynamic organelle responsible for multiple cellular
functions (9,16). An increasing number of studies have
demonstrated that ER stress is key in the pathogenesis of podocyte
dysfunction (9,11,29).
In diabetic rats, ER stress-induced podocyte apoptosis has been
found to be associated with upregulation of the mRNA and protein
expression levels of GRP78 (30).
Palmitate can induce podocyte apoptosis via ER stress, and the
expression of GRP78 is significantly increased when exposed to
palmitate (31). GRP78, a 78 kDa
glucose-regulated protein, is a major ER chaperone, which is
critical in regulating ER, and its upregulation has been suggested
to increase the capacity to buffer stressful insults initiating
from ER (13). In the present
study, GRP78 was increased in the HG single treatment group, at the
mRNA and protein levels. Treatment of podocytes with HQH
significantly reversed the HG-induced upregulation of GRP78. In
addition, treatment of podocytes with HQH significantly reversed
the HG-induced upregulation of CHOP. CHOP is a nuclear protein,
which forms stable heterodimers with C/EBP family members and is
induced in response to ER stress (32).
In conclusion, the present study demonstrated that
HG was able to exert mitochondrial dysfunction and ER stress in
podocytes. The results showed that HQH suppressed HG-induced cell
apoptosis, mitochondrial dysfunction and ER stress in the
podocytes. These findings provide a novel explanation for the
direct anti-apoptotic effects of HQH, which may have a potential
protective effect against HG-induced podocyte dysfunction.
Acknowledgements
The present study was supported by the Public
Projects of Zhejiang Province (grant no. 2012C33048).
References
|
1
|
Weir MR: Salt, hypertension, and
proteinuria in diabetic nephropathy. Lancet Diabetes Endocrinol.
2:351–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong C, Zheng H, Huang S, You N, Xu J, Ye
X, Zhu Q, Feng Y, You Q, Miao H, et al: Heme oxygenase-1 enhances
autophagy in podocytes as a protective mechanism against high
glucose-induced apoptosis. Exp Cell Res. 337:146–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jefferson JA, Shankland SJ and Pichler RH:
Proteinuria in diabetic kidney disease: A mechanistic viewpoint.
Kidney Int. 74:22–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolf G, Chen S and Ziyadeh FN: From the
periphery of the glomerular capillary wall toward the center of
disease: Podocyte injury comes of age in diabetic nephropathy.
Diabetes. 54:1626–1634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lemley KV, Lafayette RA, Safai M, Derby G,
Blouch K, Squarer A and Myers BD: Podocytopenia and disease
severity in IgA nephropathy. Kidney Int. 61:1475–1485. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyer TW, Bennett PH and Nelson RG:
Podocyte number predicts long-term urinary albumin excretion in
Pima Indians with Type II diabetes and microalbuminuria.
Diabetologia. 42:1341–1344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Menini S, Iacobini C, Oddi G, Ricci C,
Simonelli P, Fallucca S, Grattarola M, Pugliese F, Pesce C and
Pugliese G: Increased glomerular cell (podocyte) apoptosis in rats
with streptozotocin-induced diabetes mellitus: Role in the
development of diabetic glomerular disease. Diabetologia.
50:2591–2599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han SY, Kang YS, Jee YH, Han KH, Cha DR,
Kang SW and Han DS: High glucose and angiotensin II increase beta1
integrin and integrin-linked kinase synthesis in cultured mouse
podocytes. Cell Tissue Res. 323:321–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu S, Nam SM, Kim JH, Das R, Choi SK,
Nguyen TT, Quan X, Choi SJ, Chung CH, Lee EY, et al: Palmitate
induces ER calcium depletion and apoptosis in mouse podocytes
subsequent to mitochondrial oxidative stress. Cell Death Dis.
6:e19762015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pereira ER, Frudd K, Awad W and Hendershot
LM: Endoplasmic reticulum (ER) stress and hypoxia response pathways
interact to potentiate hypoxia-inducible factor 1 (HIF-1)
transcriptional activity on targets like vascular endothelial
growth factor (VEGF). J Biol Chem. 289:3352–3364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zode GS, Sharma AB, Lin X, Searby CC,
Bugge K, Kim GH, Clark AF and Sheffield VC: Ocular-specific ER
stress reduction rescues glaucoma in murine glucocorticoid-induced
glaucoma. J Clin Invest. 124:1956–1965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Q, Guo R, Liu C, Fu D, Liu F, Hu J and
Jiang H: Endoplasmic reticulum stress-mediated apoptosis
contributing to high glucose-induced vascular smooth muscle cell
calcification. J Vasc Res. 52:291–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YJ, Wu CL, Liu JF, Fong YC, Hsu SF,
Li TM, Su YC, Liu SH and Tang CH: Honokiol induces cell apoptosis
in human chondrosarcoma cells through mitochondrial dysfunction and
endoplasmic reticulum stress. Cancer Lett. 291:20–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ha TS, Park HY, Seong SB and Ahn HY:
Angiotensin II induces endoplasmic reticulum stress in podocyte,
which would be further augmented by PI3-kinase inhibition. Clin
Hypertens. 21:132015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W
and Duan H: Role of endoplasmic reticulum stress in apoptosis of
differentiated mouse podocytes induced by high glucose. Int J Mol
Med. 33:809–816. 2014.PubMed/NCBI
|
|
16
|
Wang ZS, Xiong F, Xie XH, Chen D, Pan JH
and Cheng L: Astragaloside IV attenuates proteinuria in
streptozotocin-induced diabetic nephropathy via the inhibition of
endoplasmic reticulum stress. BMC Nephrol. 16:442015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Sun W, Gu LB, Tu Y, Yu BY and Hu H:
Huaiqihuang Granules () reduce proteinuria by enhancing nephrin
expression and regulating necrosis factor κB signaling pathway in
adriamycin-induced nephropathy. Chin J Integr Med. 2015.
|
|
18
|
Sun W, Zhu Z, Yu J, Wang YH, Xiong M, Gao
X, Zhao ZH and Liu XG: Effects of Chinese herbal medicine
Huaiqihuang Granule on nephrin and podocin expressions in renal
tissues of rats with adriamycin-induced nephrosis. Zhong Xi Yi Jie
He Xue Bao. 9:546–552. 2011.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CX, Xia M, Han WQ, Li XX, Zhang C,
Boini KM, Liu XC and Li PL: Reversal by growth hormone of
homocysteine-induced epithelial-to-mesenchymal transition through
membrane raft-redox signaling in podocytes. Cell Physiol Biochem.
27:691–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruotsalainen V, Ljungberg P, Wartiovaara
J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C and Tryggvason K:
Nephrin is specifically located at the slit diaphragm of glomerular
podocytes. Proc Natl Acad Sci USA. 96:7962–7967. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Liu CP, Xu KF, Mao XD, Lu YB, Fang
L, Yang JW and Liu C: Effect of taurine-conjugated ursodeoxycholic
acid on endoplasmic reticulum stress and apoptosis induced by
advanced glycation end products in cultured mouse podocytes. Am J
Nephrol. 28:1014–1022. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Casalena G, Krick S, Daehn I, Yu L, Ju W,
Shi S, Tsai SY, D'Agati V, Lindenmeyer M, Cohen CD, et al: Mpv17 in
mitochondria protects podocytes against mitochondrial dysfunction
and apoptosis in vivo and in vitro. Am J Physiol Renal Physiol.
306:F1372–F1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daehn I, Casalena G, Zhang T, Shi S,
Fenninger F, Barasch N, Yu L, D'Agati V, Schlondorff D, Kriz W, et
al: Endothelial mitochondrial oxidative stress determines podocyte
depletion in segmental glomerulosclerosis. J Clin Invest.
124:1608–1621. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu C, Xuan X, Che R, Ding G, Zhao M, Bai
M, Jia Z, Huang S and Zhang A: Dysfunction of the
PGC-1α-mitochondria axis confers adriamycin-induced podocyte
injury. Am J Physiol Renal Physiol. 306:F1410–F1417. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan Y, Huang S, Wang W, Wang Y, Zhang P,
Zhu C, Ding G, Liu B, Yang T and Zhang A: Activation of peroxisome
proliferator-activated receptor-γ coactivator 1α ameliorates
mitochondrial dysfunction and protects podocytes from
aldosterone-induced injury. Kidney Int. 82:771–789. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zamzami N, Brenner C, Marzo I, Susin SA
and Kroemer G: Subcellular and submitochondrial mode of action of
Bcl-2-like oncoproteins. Oncogene. 16:2265–2282. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
Requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan Y, Xu X, Zhao C, Zhao M, Wang H,
Zhang B, Wang N, Mao H, Zhang A and Xing C: The roles of oxidative
stress, endoplasmic reticulum stress, and autophagy in
aldosterone/mineralocorticoid receptor-induced podocyte injury. Lab
Invest. 95:1374–1386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Gui D, Chen J, He D, Luo Y and
Wang N: Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside
IV is associated with the inhibition of endoplasmic reticulum
stress-induced podocyte apoptosis in diabetic rats. Cell Physiol
Biochem. 33:1975–1987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao JL, Wen YB, Shi BY, Zhang H, Ruan XZ,
Li H, Li XM, Dong WJ and Li XW: Endoplasmic reticulum stress is
involved in podocyte apoptosis induced by saturated fatty acid
palmitate. Chin Med J (Engl). 125:3137–3142. 2012.PubMed/NCBI
|
|
32
|
Wang XZ, Lawson B, Brewer JW, Zinszner H,
Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM and Ron D:
Signals from the stressed endoplasmic reticulum induce
C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol.
16:4273–4280. 1996. View Article : Google Scholar : PubMed/NCBI
|