Introduction
Rats are widely used in experiments based on
surgery, as it is easier to perform surgery in rats, including
spinal cord injury (1), peripheral
nerve system injury (2) and brain
injection (3), compared with mice.
Certain experiments examining nerve injury in young models require
cells from newborn and young rats. It is easier to perform surgery
on newborn rats than mice, for example in the establishment of
models of brachial plexus injury (4). Schwann cells (SCs) are the principal
glial cells of the peripheral nervous system (PNS) and are involved
in a wide range of biological and pathological process. Tissue
engineering of peripheral nerves and ex vivo gene therapy
requires rat SCs of a highly purity (5). Primarily cultured rat SCs are
essential for the investigation of molecular mechanisms regulating
the proliferation, survival, differentiation and myelination of SCs
(6,7). They are also useful for the
development of efficient transplantation for the regeneration of
injury to the spinal cord or PNS (6,7). In
regenerative medicine approaches, the preparation of a
highly-enriched SC population is required in SC transplantation
(8). Minimization of the number of
contaminating fibroblasts, which can affect the biological analysis
and experimentation of SCs, and increase scar tissue formation, is
required. For this purpose, the present study modified the
techniques of several previously published protocols and developed
a method for the isolation and enrichment of rat SCs from sciatic
nerves (9–14).
A problem in preparing SCs is fibroblast
contamination and the overgrowth of SCs by fibroblasts in long-term
culture. Therefore, several methods exist to separately remove
either fibroblast cells from the SC cultures or SCs from
fibroblasts, as a form of purification (15). The use of antimitotic chemicals is
a commonly used technique to inhibit fibroblast growth on the basis
of the higher proliferation rate of fibroblasts (9). Furthermore, the preferential surface
expression of Thy-1 by fibroblast cells can be exploited by using
anti-Thy1 antibodies, in conjunction with complement-mediated cell
lysis (16). Other selective
purification methods include the use of magnetic beads labeled with
low-affinity nerve growth factor receptor (p75NGFR)
antibodies, with physical removal and subsequent isolation
(12–17). Similarly, the use of magnetic beads
labeled with Thy-1 antibody to remove fibroblast cells has been
reported (18). Nonspecific
purification methods are also common and include a ‘cold-jet’
technique, in which ice-cold culture medium is added to impure
cultures followed by a rapid aspiration step (19,20).
This method preferentially removes weakly adherent SCs whereas the
more adherent fibroblast cells remain on the dishes. A method
utilizing immunopanning to deplete macrophages and fibroblasts from
the nerve cell suspension, and to positively select for SCs has
also been used (21,22).
In order to examine the general biology of SCs, the
present study aimed to achieve a method of harvesting SCs rapidly.
Cell biology can be affected by a long duration of culture in
vitro, chemicals, growth factors and serum. Therefore, the
present study aimed to develop a simple protocol to generate highly
purified rat SCs by cell sorting using p75 and oligodendrocyte
marker 4 (O4), which resulted in 98% pure SC cultures. According to
previous studies, p75 and O4 are used as markers for the isolation
of SC (11,19,21,22).
p75 is reported to be expressed in SCs, with the exception of
myelinating SCs (23–25), whereas O4 is expressed in SCs,
including myelinating SCs (23–26).
p75 and O4 are surface markers, which enables labeling of living
SCs and sorting of the cells. Therefore, the two antibodies can be
jointly used to label SCs in vivo to obtain the maximum
number of cells. Following sorting, SCs can be cultured in SC
culture medium to stimulate cell growth and differentiation, or
analyzed immediately. This method is potentially rapid, efficient
and reproducible, thus facilitating SC isolation, and promoting
SC-associated investigations and applications.
Materials and methods
Establishment of rat SC cultures for
fluorescence-activated cell sorting (FACS) and following FACS
The sterile removal of sciatic nerves was performed
on newborn rats (1–3 day old) housed in SPF conditions. The animals
were supplied by the Experimental Animal Center of Nantong
University (Nantong, China) and were maintained at 24°C with a 12-h
light/dark cycle and a routine provision of food and water. All
animal experiments were performed in accordance with the Industrial
Animal Care Guidelines of Nantong University (Nantong, China) and
approved by the Administration Committee of Experimental Animals
(Jiangsu, China). The nerves were pooled and cut into 1 nm sections
in Hibernate E (BrainBits, LLC, Springfield, IL, USA) containing 2%
B27 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on ice. The
tissues were then harvested by centrifugation for 5 min at 139 × g
and 4°C. The supernatant was discarded and 1% collagenase (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added and
incubated at 37°C for 20 min, following which 0.125% trypsin
(Gibco; Thermo Fisher Scientific, Inc.) was added and incubated for
another 10 min. Digestion was terminated with DMEM (Gibco; Thermo
Fisher Scientific, Inc.) and 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.). Dissociation was achieved by
mechanical dissociation through a 1-ml Pasteur pipette. The cells
were centrifuged for 5 min at 210 × g, and 4°C. The cell sediment
was rinsed twice using PBS and suspended in 0.1 M PBS for antibody
labeling. Following FACS, the sorted cells were seeded onto dishes
or slides in DMEM with 10% FBS and 1% penicillin/streptomycin in a
37°C, 5% CO2 incubator and cultured for 4 h. Then, the
culture medium was replaced with SC culture medium consisting of
DMEM with 10% FBS, 1% penicillin/streptomycin, 2 µM forskolin and
10 ng/ml HRG (both from Sigma; Merck KGaA) and the cell culture was
maintained subsequently in a 37°C, 5% CO2 incubator for
24–48 h.
Coating cell culture surfaces
At 24 h prior to seeding of the SCs, plastic or
glass surfaces were coated with poly-L-lysine (Sigma; Merck KGaA)
and incubated for 30 min at room temperature. The dishes and slides
were rinsed three times with sterile water, dried naturally and
then stored at room temperature until use.
Labeling of SCs with p75 and O4 for
FACS
The cells isolated from the sciatic nerves were
prepared for use for FACS. The cells were counted and divided into
four groups: Negative control; positive control for p75 and O4, and
the antibodies labeled separately. The cells for labeling were
incubated with FITC-conjugated anti-p75 (ab62122; Abcam, Cambridge,
MA, USA) diluted 1:100 and PE-conjugated anti-O4 antibodies
(FAB1326P; R&D Systems, Inc., Minneapolis, MN, USA) diluted
1:10 in PBS for 30 min at room temperature. The cells were then
washed twice with PBS to remove the unbounded antibodies. Finally,
the labeled cells were resuspended in 500 µl PBS ready for
FACS.
FACS of p75-and O4-labeled SCs
FACS was performed on a FACSAria cell sorter using
FACSDiva software (BD Biosciences, San Jose, CA, USA). Prior to
sorting, the sample was filtered to remove any columns and
aggregates. FITC and PE fluorescence were excited at a wavelength
of 488 nm and collected through a 520 and 578 bandpass interference
filter. Compensation for FITC and PE were adjusted. The cells
positive for p75 and O4 were collected. Following cell sorting, the
collected cells were reassessed using FACS in order to determine
the sorting purity. The sorted cells were then plated on the dishes
and slides. After 4–6 h, The cells were rechecked and their medium
was replaced with the SC culture medium.
Immunocytochemistry
The cells plated onto coated glass coverslips were
cultured for 24 h or until 80% confluent, and fixed for 30 min in
4% paraformaldehyde (pH 7.2) at room temperature. The cells were
then washed once in PBS, blocked for 2 h in blocking buffer [0.3%
Triton X-100 and 10% goat serum (ZLI-9021; Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) in 0.01 M PBS] for 60 min
at 37°C, and incubated with anti-S100β antibody, anti-glial
fibrillary acidic protein (GFAP) antibody and anti-tubulin 3 β
chain (Tubb3) antibody (ab212816, ab7260 and ab18207; 1:200;
Abcam), respectively at 4°C overnight, followed by incubation with
FITC-conjugated rabbit anti mouse IgG or Cy3-conjugated goat anti
rabbit IgG (ab6724 and ab6939; 1:500; Abcam) for 2 h at room
temperature, respectively. The cells were also stained with 5 µg/ml
Hoechst 33342 dye at 37°C for 20 min. The fluorescence was
visualized under a TCS SP5 confocal microscope (Leica Microsystems
GmbH, Wetzlar, Germany).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the sorted SCs cultured
for 72 h using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
and cDNA was synthesized from the total RNA using the SuperScript
First-Strand Synthesis system (Invitrogen; Thermo Fisher
Scientific, Inc.). The qPCR analysis was performed using FastStart®
SYBR-Green qPCR Master Mix (Roche Diagnostics GmbH, Mannheim,
Germany) according to the manufacturer's protocol. A 50 µl reaction
volume consisted of 1 µl cDNA, 25 µl 2X Fast SYBR-Green Master Mix,
1 µl of each primer and 22 µl of RNase/DNase-free water. A
three-step fast cycle protocol was used, with cycling conditions as
follows: Denaturation at 95°C, 10 sec; annealing at 60°C, 30 sec;
and, extension at 70°C, 10 sec (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The data were analyzed using the software
supplied by the manufacturer (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primer sequences are listed in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) |
|---|
| 18S |
agtccctgccctttgtacaca |
|
|
cgttccgagggcctcact |
| Sox10 |
cccaggtgaagacagaga |
|
|
agactgagggaggtgtagg |
| P0 |
ggacatagtgggcaagac |
|
|
aggtagaagagcaacagca |
| Sox2 |
cagctcgcagacctacat |
|
|
tcggacttgaccacagag |
| Krox-20 |
accacctcaccactcaca |
|
|
actgctcttcctctccttct |
| Pmp22 |
tggctttgcttacatcct |
|
|
ttggttttctggtttcctt |
| Necl-4 |
atggtgtggtgctctgtc |
|
|
ttcttctttccgcttgtg |
| Tubb3 |
gtcaaggtagcggtgtgt |
|
|
gtgaactccatctcatcca |
Results and Discussion
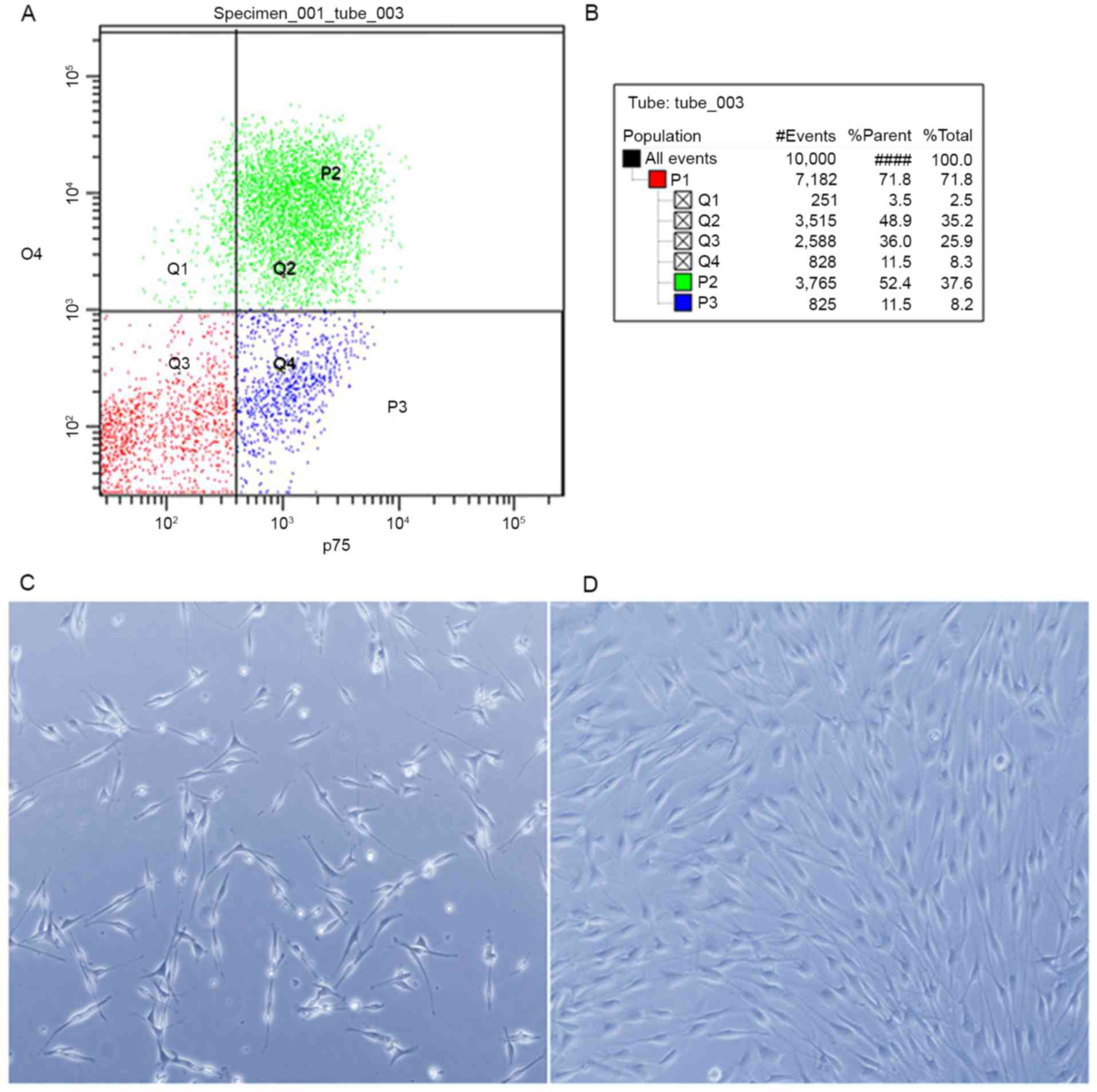
The cell mixture labeled with p75 and O4 antibodies
was analyzed using FACSAria І (Fig.
1A). The p75- and O4-positive populations were sorted, the
percentages of which are demonstrated in Fig. 1B. Following sorting, the cells were
plated on poly-L-lysine coated glass cover slips or dishes. After
24 h, almost all of the cells exhibited the classical morphology of
SCs (Fig. 1C). The sorted cells
were then cultured for 72 h in SC growth medium. The cells
proliferated to confluence (Fig.
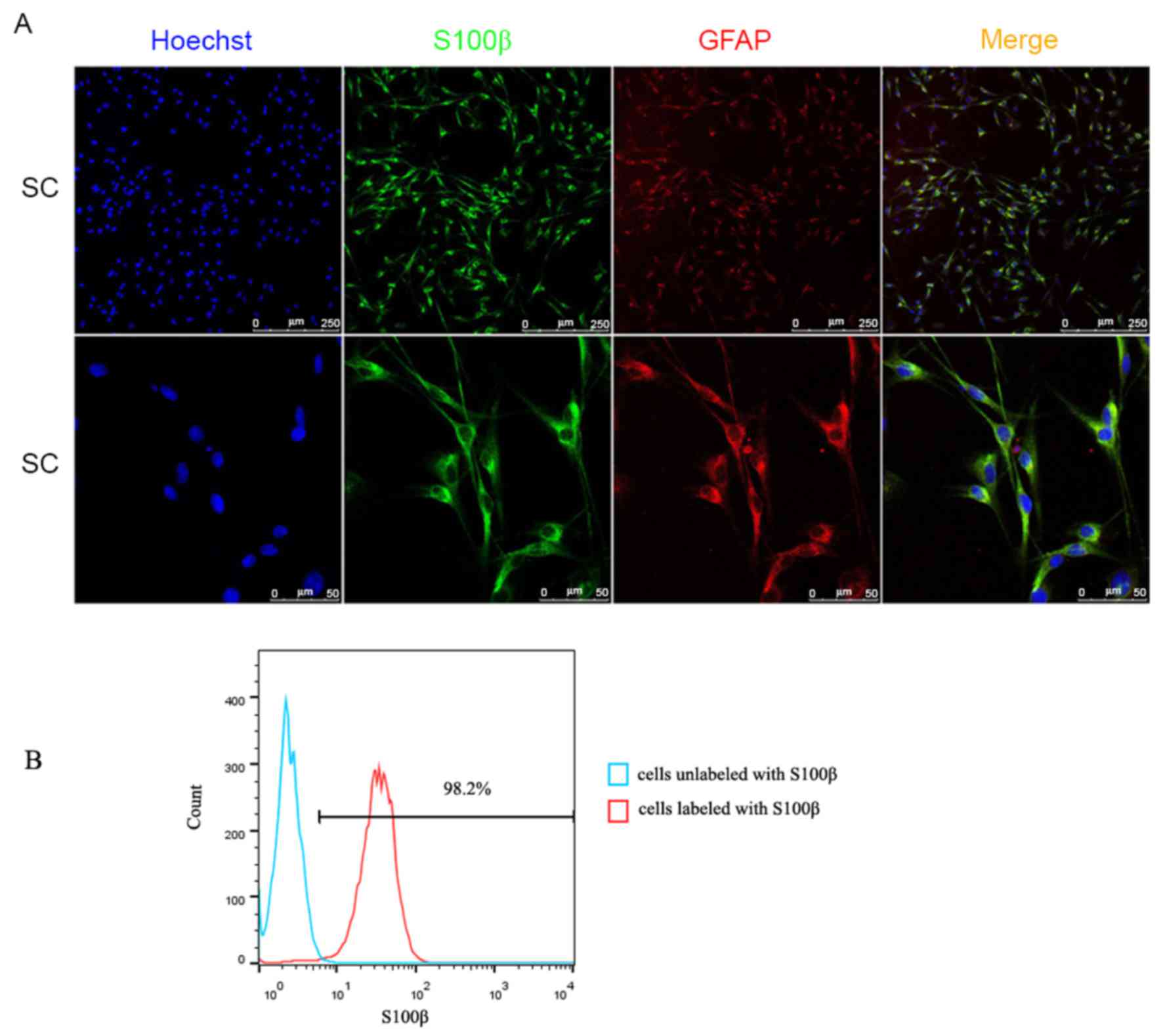
1D). Immunocytochemistry with anti-S100β and anti-GFAP
antibodies, and S100β and GFAP proteins serving as SC markers,
provided further evidence of the cell purity of the FACS cells at
24 h (Fig. 2A). The purity of the
SCs cultured for 72 h was confirmed using flow cytometry (Fig. 2B), which indicated that >98% of
the cell population was S100β-positive. These data indicated that
cell purity was markedly enhanced following sorting, with almost
99% purity achieved. Using RT-qPCR analysis, the mRNA expression
levels of genes expressed in SCs were determined, as demonstrated
in Fig. 3. SCs isolated by sorting
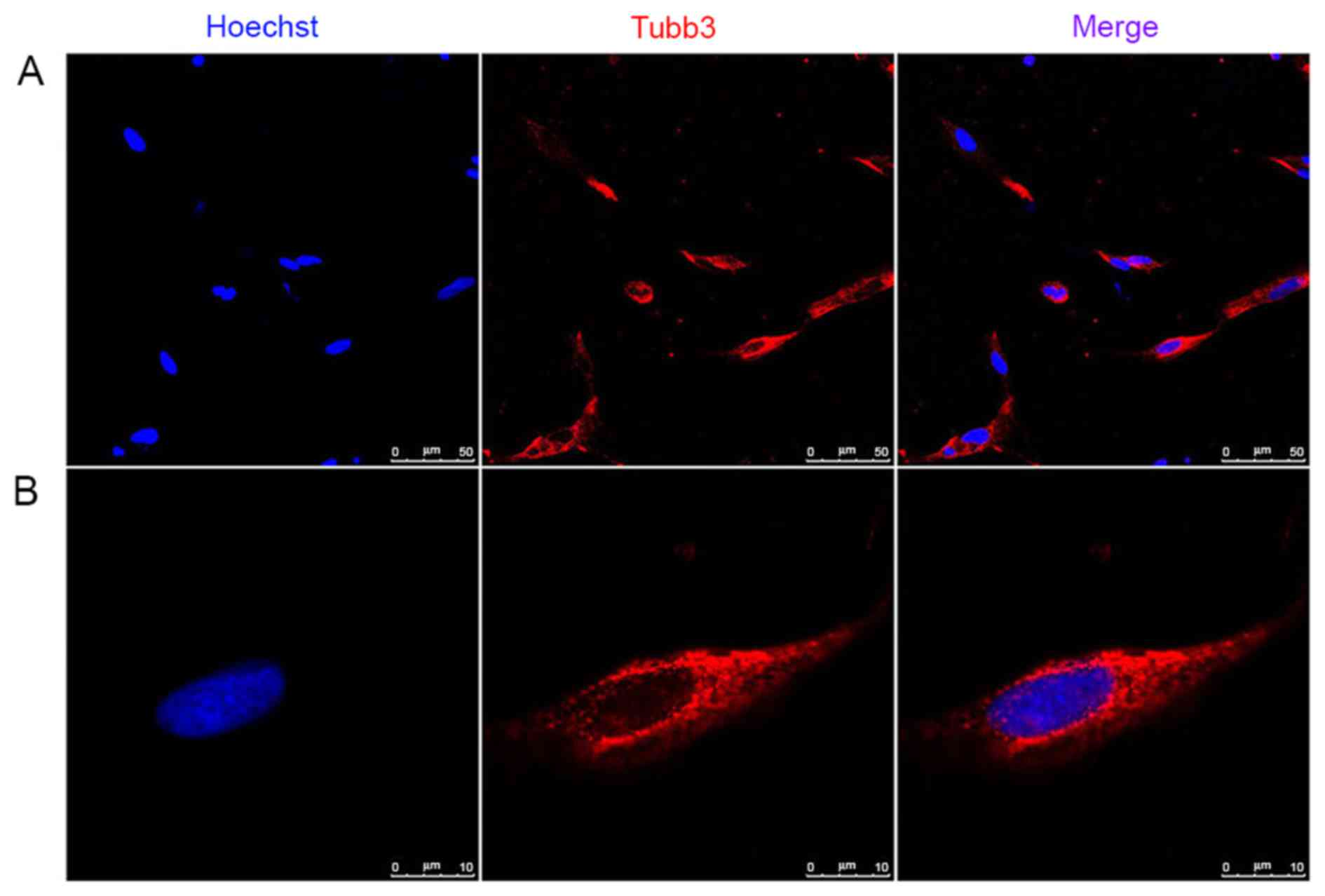
can express myelinating and non-myelinating SC markers (26). Of note, Tubb3, which is expressed
in neurons, was present in the SCs isolated by sorting and
validated using RT-qPCR analysis and immunocytochemistry (Figs. 3 and 4). Our previous study reported that Tubb3
was expressed in primary cultured SCs (9). To a degree, the results of the
present study provide further evidence in support of the previous
study.
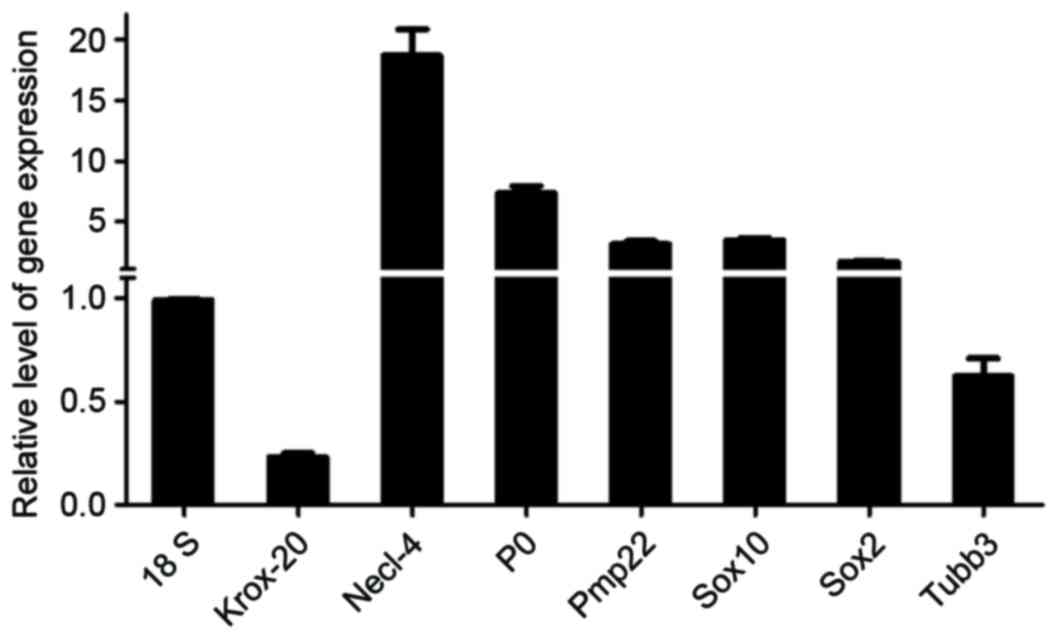 | Figure 3.Validation of SC-associated genes
using RT-qPCR. Histogram showing the mRNA expression levels of
Krox-20, Necl-4, P0, Pmp22, Sox10, Sox2 and Tubb3 relative to that
of 18s, in sorted SCs cultured for 72 h. Results were determined
using RT-qPCR. SCs, Schwann cells; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. Sox10,
SRY-Box 10; P0, myelin protein 0; Sox2, SRY-Box 2; Krox-20, early
growth response 2; Pmp22, peripheral myelin protein 22; Necl-4,
nectin-like molecule 4; Tubb3, tubulin β 3 class III. |
The aim of the present study was to obtain highly
enriched pure SCs from rat peripheral nerves. Several studies have
attempted to improve the purification of SCs in an efficient and
convenient method, one which is less time consuming and reduces
cell loss (10–18,20–22).
These methods can be divided into several approaches: i)
Differential adhesion and differential digestion, or a cold jet
technique, based on the different adherence abilities of SC and
fibroblasts; ii) removal of fibroblasts from the SC cell culture by
complement-mediated cell killing; iii) cell sorting based on
specific cell surface biomarkers of SCs. The method used in the
present study, which employed FACS, may be more convenient and take
less time. In the process of cell sorting, cells can be plated
directly in dishes, wells or tubes according to the requirements of
the subsequent experiment. The cells can be analyzed directly and
rapidly from the in vivo environment. The cells can also be
cultured in SC culture medium containing forskolin and HRG to
improve cell proliferation and differentiation for future use. The
purified SCs can be used immediately for cell transplantation or
passaged and frozen for future use. It may also have less of an
effect on alterations of SC biology, as fewer chemicals are used in
the process. The method presented in the present study enabled the
isolation of SCs from rat peripheral nerves within 24 h, and can
potentially be applied to SCs from other species and other sources
depending on the appropriate antibodies.
FACS has the significant advantage in that cells
with specific marker combinations can be isolated in one step,
provided that each marker is labeled with a different fluoresce.
The protocol presented in the present study is well-suited to
isolating SCs for use in general SC biological analysis and tissue
engineering. In previous studies, either p75 or O4 has been
selected as a surface marker for SC purification. In the present
study, both of these antibodies were used to label SCs in the rat
primary cell mixture derived from sciatic nerve tissues. This
enabled harvesting of the highest number of SCs possible, as cells
positive to p75 alone, to O4 alone and to p75 and O4 combined (as
demonstrated in the Q1, Q2 and Q3 regions of Fig. 1A) were all identified as SCs for
harvest. Therefore, this FACS-based method offered another method
for isolating SCs in vivo.
In conclusion, the novel method used in the present
study for obtaining primary cultured SCs from the sciatic nerves of
rats offers potential for facilitating investigations and
applications of SC biology.
Acknowledgements
This study was supported by a project funded by the
National Natural Science Foundation of China (grant nos. 81371389,
31300942 and 31500927) and the Priority Academic Program
Development of Jiangsu Higher Education Institutions.
References
|
1
|
Aras M, Altas M, Motor S, Dokuyucu R,
Yilmaz A, Ozgiray E, Seraslan Y and Yilmaz N: Protective effects of
minocycline on experimental spinal cord injury in rats. Injury.
46:1471–1474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou S, Zhang S, Wang Y, Yi S, Zhao L,
Tang X, Yu B, Gu X and Ding F: miR-21 and miR-222 inhibit apoptosis
of adult dorsal root ganglion neurons by repressing TIMP3 following
sciatic nerve injury. Neurosci Lett. 586:43–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peelaerts W, Bousset L, van der Perren A,
Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R and
Baekelandt V: α-Synuclein strains cause distinct synucleinopathies
after local and systemic administration. Nature. 522:340–344. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu JX, Chen L, Ding F and Gu YD: A rat
model study of atrophy of denervated musculature of the hand being
faster than that of denervated muscles of the arm. J Muscle Res
Cell Motil. 34:15–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Zhao Y, Sun C, Hu W, Zhao J, Li G,
Zhang L, Liu M, Liu Y, Ding F, et al: Chitosan degradation products
promote nerve regeneration by stimulating schwann cell
proliferation via miR-27a/FOXO1 Axis. Mol Neurobiol. 53:28–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
di Summa PG, Kalbermatten DF, Pralong E,
Raffoul W, Kingham PJ and Terenghi G: Long-term in vivo
regeneration of peripheral nerves through bioengineered nerve
grafts. Neuroscience. 181:278–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moradi F, Bahktiari M, Joghataei MT,
Nobakht M, Soleimani M, Hasanzadeh G, Fallah A, Zarbakhsh S,
Hejazian LB, Shirmohammadi M and Maleki F: BD PuraMatrix peptide
hydrogel as a culture system for human fetal Schwann cells in
spinal cord regeneration. J Neurosci Res. 90:2335–2348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanno H, Pearse DD, Ozawa H, Itoi E and
Bunge MB: Schwann cell transplantation for spinal cord injury
repair: Its significant therapeutic potential and prospectus. Rev
Neurosci. 26:121–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen M, Ji Y, Zhang S, Shi H, Chen G, Gu X
and Ding F: A proteome map of primary cultured rat Schwann cells.
Proteome Sci. 10:202012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weinstein DE and Wu R: Isolation and
purification of primary Schwann cells. Curr Protoc Neurosci:
Chapter 3: Unit 3.17. 2001. View Article : Google Scholar
|
|
11
|
Manent J, Oguievetskaia K, Bayer J, Ratner
N and Giovannini M: Magnetic cell sorting for enriching Schwann
cells from adult mouse peripheral nerves. J Neurosci Methods.
123:167–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haastert K, Mauritz C, Chaturvedi S and
Grothe C: Human and rat adult Schwann cell cultures: Fast and
efficient enrichment and highly effective non-viral transfection
protocol. Nat Protoc. 2:99–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mauritz C, Grothe C and Haastert K:
Comparative study of cell culture and purification methods to
obtain highly enriched cultures of proliferating adult rat Schwann
cells. J Neurosci Res. 77:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spiegel I and Peles E: A novel method for
isolating Schwann cells using the extracellular domain of Necl1. J
Neurosci Res. 87:3288–3296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaewkhaw R, Scutt AM and Haycock JW:
Integrated culture and purification of rat Schwann cells from
freshly isolated adult tissue. Nat Protoc. 7:1996–2004. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morrissey TK, Kleitman N and Bunge RP:
Isolation and functional characterization of Schwann cells derived
from adult peripheral nerve. J Neurosci. 11:2433–2442.
1991.PubMed/NCBI
|
|
17
|
Vroemen M and Weidner N: Purification of
Schwann cells by selection of p75 low affinity nerve growth factor
receptor expressing cells from adult peripheral nerve. J Neurosci
Methods. 124:135–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haastert K, Seef P, Stein VM, Tipold A and
Grothe C: A new cell culture protocol for enrichment and genetic
modification of adult canine Schwann cells suitable for peripheral
nerve tissue engineering. Res Vet Sci. 87:140–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teare KA, Pearson RG, Shakesheff KM and
Haycock JW: Alpha-MSH inhibits inflammatory signalling in Schwann
cells. Neuroreport. 15:493–498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jirsová K, Sodaar P, Mandys V and Bär PR:
Cold jet: A method to obtain pure Schwann cell cultures without the
need for cytotoxic, apoptosis-inducing drug treatment. J Neurosci
Methods. 78:133–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lutz AB: Purification of Schwann cells.
Cold Spring Harb Protoc. 2014:1234–1236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lutz AB: Purification of Schwann cells
from the neonatal and injured adult mouse peripheral nerve. Cold
Spring Harb Protoc. 2014:1312–1319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gordon T: Neurotrophic factor expression
in denervated motor and sensory Schwann cells: Relevance to
specificity of peripheral nerve regeneration. Exp Neurol.
254:99–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirsky R, Woodhoo A, Parkinson DB,
Arthur-Farraj P, Bhaskaran A and Jessen KR: Novel signals
controlling embryonic Schwann cell development, myelination and
dedifferentiation. J Peripher Nerv Syst. 13:122–135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jessen KR and Mirsky R: The origin and
development of glial cells in peripheral nerves. Nat Rev Neurosci.
6:671–682. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dworski S: Comparison of Schwann cells
derived from peripheral nerve with Schwann cells differentiated
from skin-derived precursors. Master. 2011.
|