Introduction
Cancerhas a large influence on human health and
economy. Despite the fact that progress has been made in
understanding cancer, it remains one of the leading causes of
mortality (1,2). Traditional cancer therapies including
surgery, radiotherapy and chemotherapy, aim to destroy the tumor
and leave normal tissue intact as much as is possible (3). However, the emergence of resistance
to these therapies remains a challenge for successful treatment of
cancer. Given the unsatisfactory efficacy and unavoidable systemic
toxicity of traditional treatment, gene therapy has been
investigated as a gene transcription and translation intervention,
serving important roles for tumor growth, spread, survival and
therapy resistance (4).
As a special type of RNA nuclear protease,
telomerase synthesizes telomeric DNA sequences that provide tandem
GT-rich repeats (TTAGGG) to compensate for telomere shortening
(5,6). Activated telomerase can be observed
in more than 90% of cancer cases and beconsidered to permit tumor
cell immortalization and promote cell malignant transformation
(7,8). Therefore, telomerase can be used as a
potential target for gene therapy.
As the most important regulator of telomerase
activity, human telomerase reverse transcriptase (hTERT) is a
catalytic subunit of telomerase and processes telomere ends, which
are over expressed in greater than 90% of tumor cells and
contribute to tumor cell proliferation (8). Previous studies have demonstrated
that antisense oligodeoxynucleotide (ASODN) gene therapy against
hTERT may effectively inhibit telomerase activity and tumor growth
(9,10).
In the current study, a phosphorothioate ASODN
(PS-ASODN) against hTERT was used to treat Walker 256 cells. The
inhibitory effect and the mechanism of the PS-ASODN were explored
in the Walker 256 cells to provide novel strategies for cancer gene
therapy.
Materials and methods
Cell culture
Walker 256 cells were purchased from Shanghai
Institute of Pharmaceutical Industry (Shanghai, China) and cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) that contained 10% (v/v) fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin, within a humidified at mosphere containing
5% CO2 at 37°C.
Cell transfection
The hTERT-targeted PS-ASODN was purchased from
Shanghai Biotechnology Corporation (Shanghai, China). Walker 256
cells were transiently transfected with hTERT-targeted PS-ASODN
using Lipofectamine® 2000 Transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.).
Cell proliferation and viability
assay
Walker 256 cells were seeded onto 96-cell plates and
incubated at 37°C overnight. For the cell proliferation assay,
cells were treated with PS-ASODN at a final concentration of 0, 1,
2, 3, 4 and 5 µmol/l for 1, 3 and 7 days, respectively. For cell
viability assay, cells were treated with 5 µmol/l PS-ASODN for 24 h
and then irradiation (IR) was performed by using an RS 2000 X-ray
Biological Irradiator (Rad Source, Suwanee, GA, USA) at doses of 0,
2, 4, 6, and 8 Gy for 24 h. MTT solution (20 µl) was added into
each well and the plates were incubated for 4 h at 37°C. Then cells
were exposed to 150 µl DMSO and incubated for 10 min. The optical
density at 490 nm was read by an enzyme-linked immunosorbent assay
(ELISA) reader.
Senescence-associated β-gal
staining
Walker 256 cells were treated with 5 µmol/l PS-ASODN
for 5 days. Senescence-associated β-gal (SA-β-gal) activity was
performed using SA-β-gal staining kit (BeyotimeInstitute of
Biotechnology, Haimen, China). Briefly, following removal of the
RPMI-1640 medium, Walker 256 cells were washed with PBS and fixed
with 2% form aldehyde and 0.2% glutaraldehyde for 10 min.
Subsequently, cells were incubated with fresh SA-β-gal stain
solution overnight at 37°C (without CO2) following
washing with PBS. After staining, cells were photographed using a
microscope (IX73; Olympus Corporation, Tokyo, Japan), and the
percentage of senescence cell was determined via counting five
random fields.
Telomerase activity assay
Walker 256 cells were lysed with ice-cold lysis
buffer (Takara Bio, Inc., Otsu, Japan) and centrifuged at 12,000 ×
g for 30 min at 4°C. The Bicinchoninic Acid (BCA) Protein Assay kit
(Takara Bio, Inc.) was used to measure protein concentration.
Telomerase activity assay was measured using the telomeric repeat
amplification protocol (TRAP) with the TeloTAGGG Telomerase PCR
ELISA kit (Roche Diagnostics, Basel, Switzerland) according to the
manufacturer's instructions.
Cell apoptosis assay
Walker 256 cells were treated with PS-ASODN at final
concentrations of 0, 1, 3 and 5 µmol/l for 24 h, respectively, then
IR was performed using the RS 2000 X-ray Biological Irradiator at
doses of 6 Gy for 24 h. Cell apoptosis following radiation
treatment was determined using the Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptotic cell
detection kit (BD Biosciences, Franklin Lakes, NJ, USA) according
to manufacturer's protocol. The percentage of apoptotic cells was
determined via flow cytometry based on Annexin V/PI stain.
Western blot analysis
Walker 256 cells were treated with PS-ASODN at final
concentrations of 0, 1, 3 and 5 µmol/l for 24 h, respectively, then
IR was performed using the RS 2000 X-ray Biological Irradiator at
doses of 6 Gy for 24 h. Cells were collected and lysed by ice-cold
lysis buffer (Takara Bio, Inc.). Protein concentrations were
determined using the BCA Protein Assay kit (Takara Bio, Inc.).
Then, protein extracts were separated using 12% SDS-PAGE and
transferred onto polyvinylidene difluoride (PVDF) membranes.
Following incubated with 5% nonfat milk, the membranes were
incubated with polyclonal rabbit anti-caspase 3 (1:1,000; cat. no.
ab13585; Abcam, Cambridge, MA, USA) or polyclonal rabbit
anti-caspase 9 antibodies (1:1,000; cat. no. 9502; Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C. Membranes
were then incubated with horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat. no. ab181658; Abcam) for 1 h at
room temperature. Finally, the membranes were visualized using ECL
Chemiluminescent Substrate Reagent kit (Thermo Fisher Scientific,
Inc.).
Comet assay
DNA damage was determined using a comet assay.
Walker 256 cells were treated with PS-ASODN at final concentrations
of 0, 1, 3 and 5 µmol/l for 24 h, respectively, then IR was
performed using the RS 2000 X-ray Biological Irradiator at doses of
6 Gy for 24 h. Cells were collected and mixed with 0.5 % low
melting agarose. Following layeringonto agarose-coated slides, the
slides were immersed in lysis buffer overnight at 4°C.
Subsequently, slides were incubated in electrophoresis buffer for
10 h. Following electrophoresis, slides were neutralized with 0.4
mol/l Tris-HCl buffer (pH=7.5) twice per 5 min and then immersed in
100% ethanol for 20 min. After being air-dried, slides were stained
with 2 µg/ml ethidium bromide for 15 min. Finally, slides were
observed under a confocal microscope.
Statistical analysis
Data were expressed as the mean ± standard
deviation. GraphPad Prism 5 software (GraphPad Software, Inc., La
Jolla, CA, USA) was used for the statistical tests. The data were
compared between two groups using the two-tailed Student's t-test
and between multiple groups using two-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of PS-ASODN on Walker 256
cell proliferation
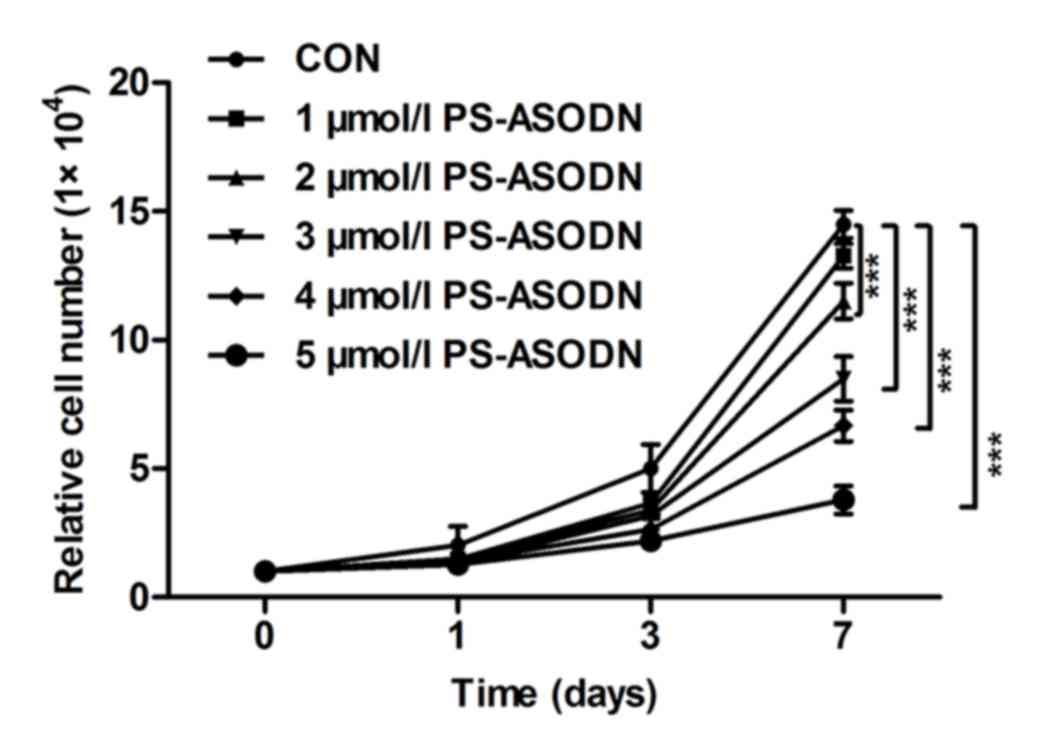
The proliferation of Walker 256 cells was inhibited
by treating with PS-ASODN (1–5 µM) on days 0, 1, 3 and 7 (Fig. 1). The proliferation rate between
the PS-ASODN group and control group was significant. The
proliferation rate decreased as the time and PS-ASODN concentration
increased, which indicated that PS-ASODN inhibited Walker 256 cells
proliferation in a dose- and time-dependent manner.
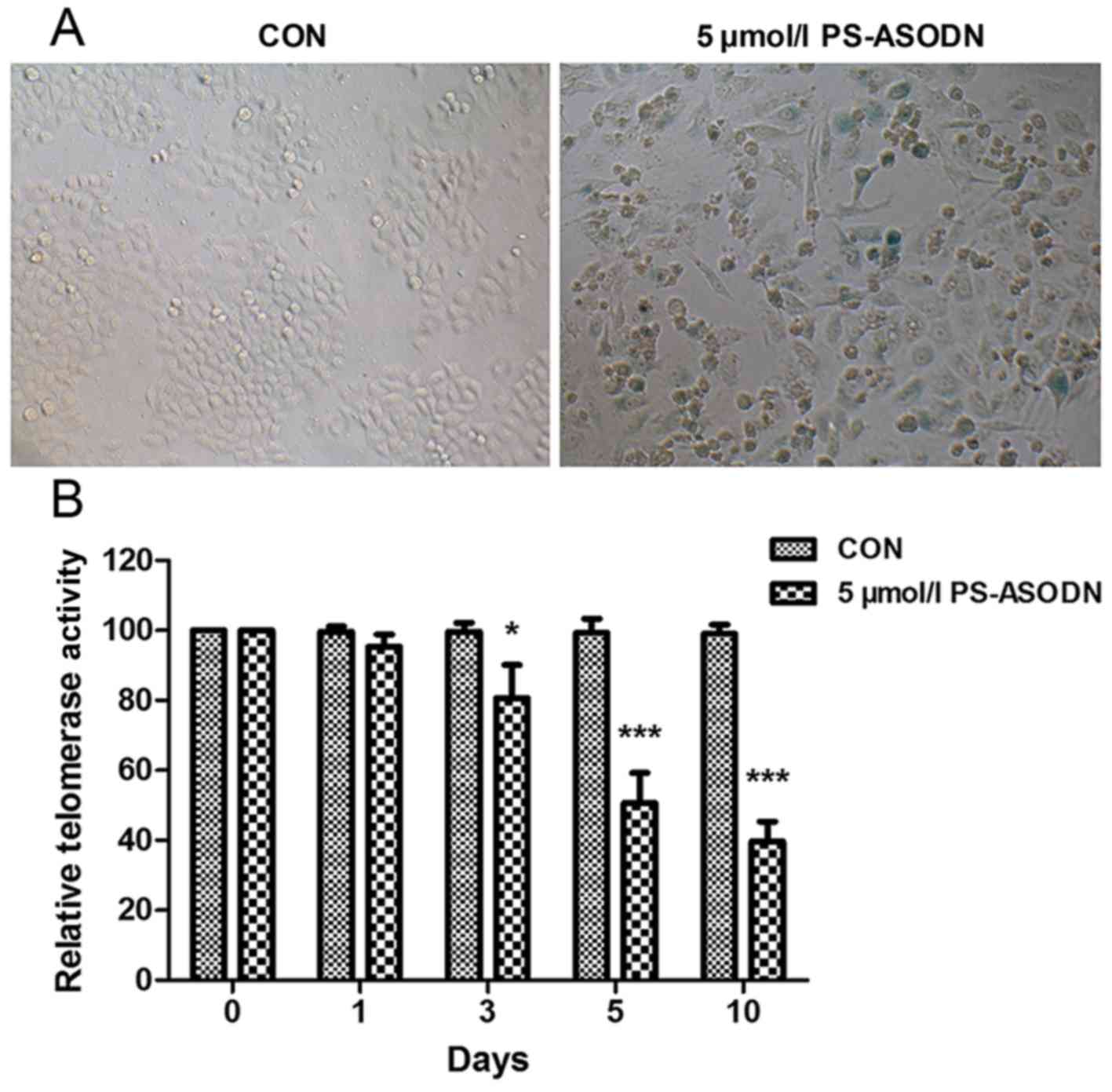
PS-ASODN induced acceleration of cell
senescence by inhibiting telomerase activity
SA-β-gal commonly acts as a biomarker of replicative
senescence due to its overexpression in pre-senescent and senescent
cells. In order to study the effect of PS-ASODN on senescence of
Walker 256 cells, SA-β-gal activity was measured after treating
Walker 256 cells with PS-ASODN (5 µmol/l) for 5 days. As presented
in Fig. 2A, PS-ASODN accelerated
cell senescence.
Telomere shortening and the accumulation of
dysfunctional telomeres are associated with cell senescence
(11,12). Therefore, following treatment of
Walker 256 cells with PS-ASODN (5 µmol/l), the effect of PS-ASODN
on telomerase activity was determined. As presented in Fig. 2B, PS-ASODN resulted in a
time-dependent decrease in telomerase activity, indicating that
PS-ASODN promoted cell senescence by inhibiting telomerase
activity.
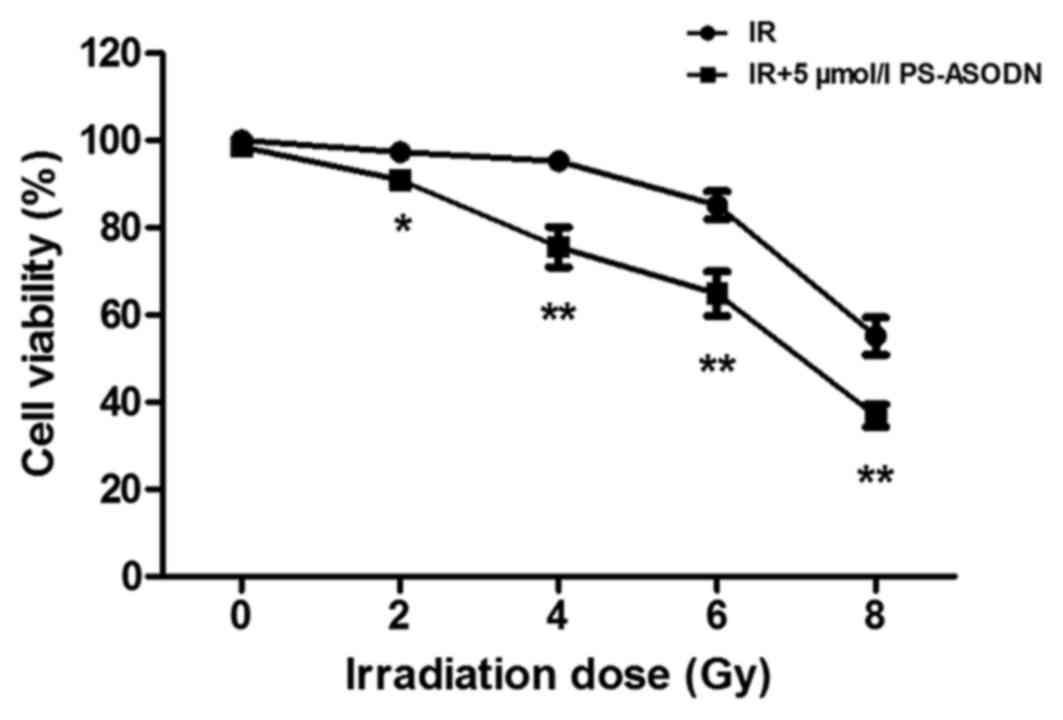
Enhancement of PS-ASODN on cell
sensitivity to IR
In order to evaluate the effect of PS-ASODN on radio
sensitization of cells, the cell viability was examined by MTT
assay. As presented in Fig. 3,
PS-ASODN enhanced the inhibition of IRon cell viability. In
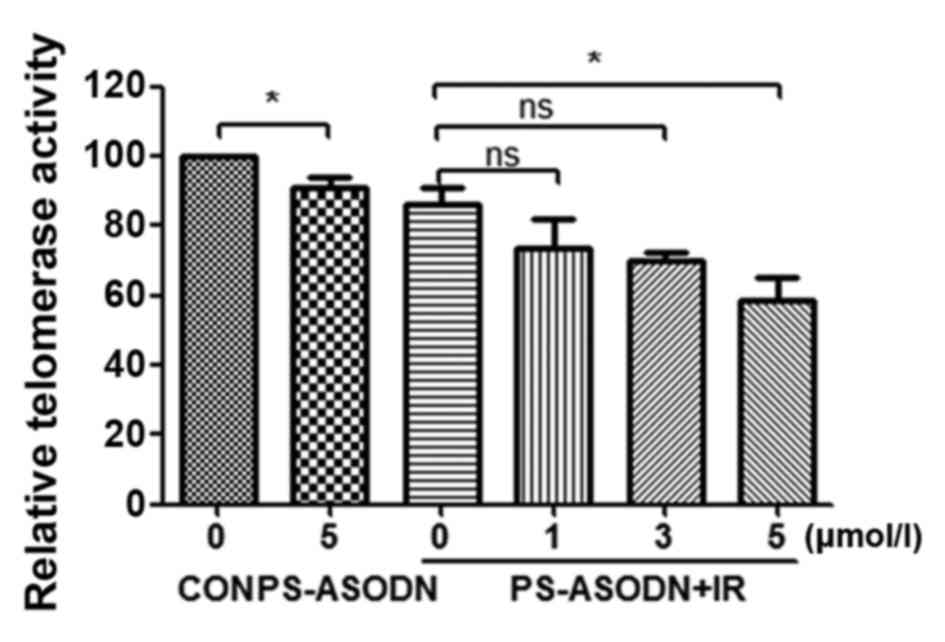
addition, PS-ASODN was also identified to improve the inhibition of
IR on telomerase activity (Fig.
4).
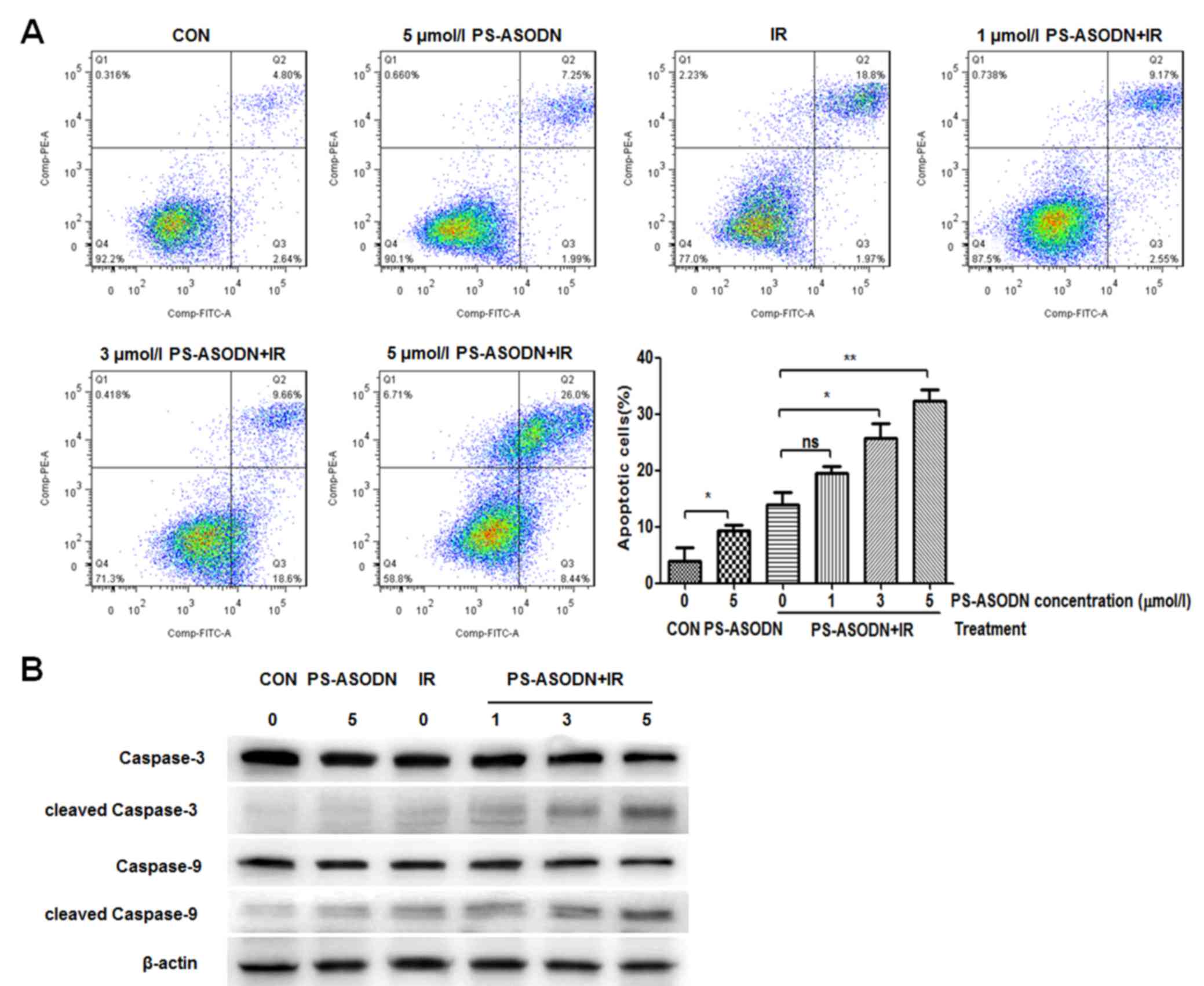
The apoptosis of Walker 256 cells was further
analyzed by flow cytometry. Compared with the control group, the
apoptosis rate of PS-ASODN treatment group increased significantly
(Fig. 5A), indicating that
PS-ASODN may induce cell apoptosis. Compared with the IR treatment
group, the apoptosis rate of Walker 256 cells treated with PS-ASODN
and IR increased as the PS-ASODN concentration increased (Fig. 5A), suggesting that PS-ASODN
promoted IR-induced cell apoptosis. In addition, the results of
western blotting indicated that combined treatment of PS-ASODN with
IR resulted in increased protein expression levels of cleaved
caspase-3 and cleaved caspase-9 in the Walker 256 cells (Fig. 5B), thus indicating that combined
treatment of PS-ASODN with IR promoted activation of cleaved
caspase-3/9 in the apoptotic signaling pathway.
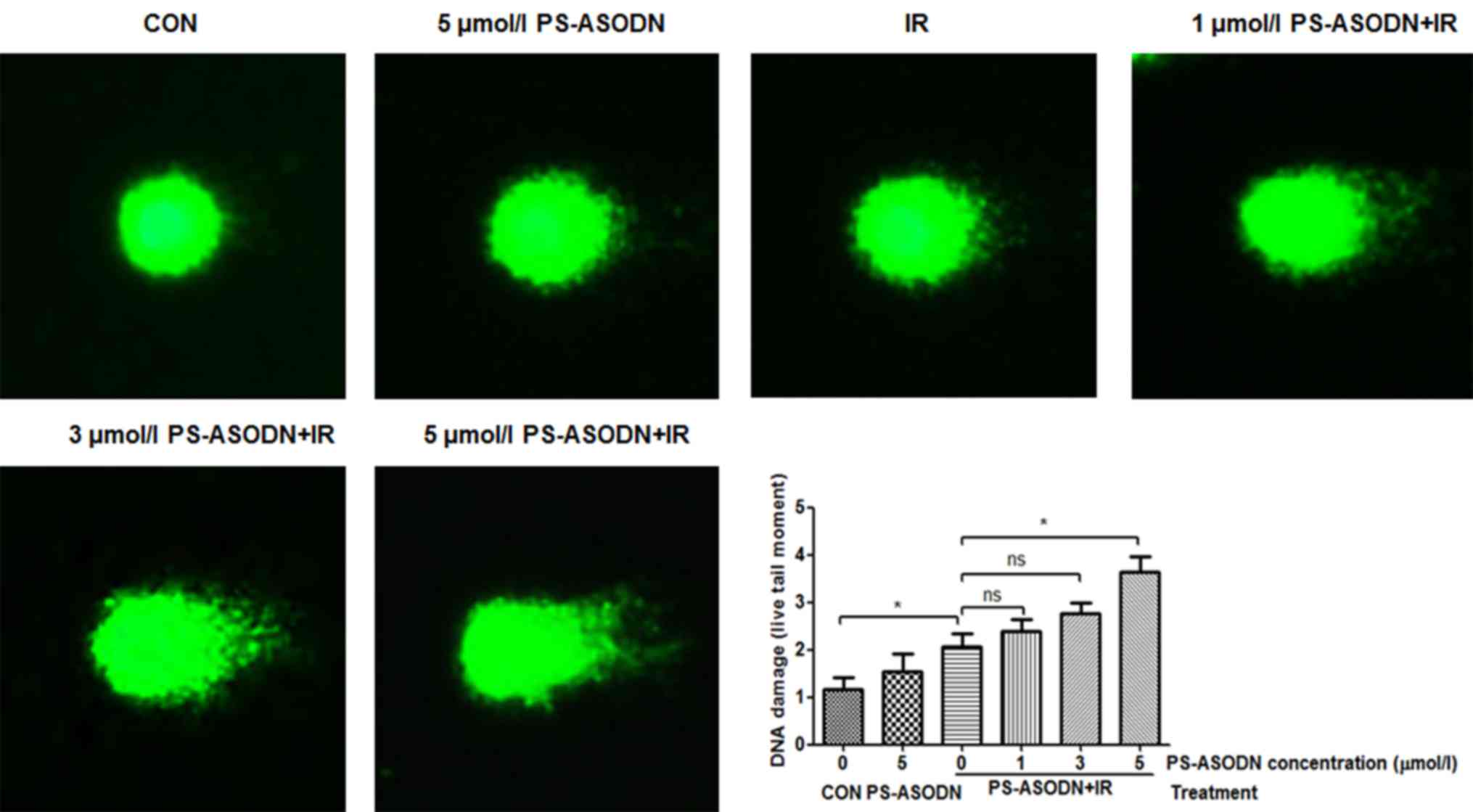
To confirm the effect of PS-ASODN on IR-induced DNA
damage, the comet assay was performed to assess degree of DNA
damage. Compared with IR treatment group, the DNA damage of Walker
256 cells treated with PS-ASODN and IR increased as the PS-ASODN
concentration increased at 5 µmol/l (Fig. 6), suggesting that PS-ASODN enhanced
IR-induced DNA damage.
Taken together, these observations suggested that
effect of PS-ASODN may improve Walker 256 cell sensitivity to
IR.
Discussion
As a special type of RNA nuclear protease,
telomerase maintains the function of the telomere and servesan
important role in cell senescence and carcinogenesis. It was
reported that high telomerase activity has been identifiedin
malignant tumors, and low telomerase activity has been identifiedin
the majority of normal tissues and benign tumors (13). hTERT is a subunit of telomerase and
its expression levels are indicated to be associated with
telomerase activity (14–16). Therefore, it is suggested that
hTERT may be an appropriate target for anti-sense therapy.
ASODN has become a research focus due to its high
specificity, targeting and low toxicity, and may be applied to gene
therapy (7). ASODNs are short DNA
sequences that hybridize to complementary mRNA sequences based on
Watson-Crick base pairing. ASODN gene therapy has been identifiedto
effectively target the expression of genes involved in causing
cancer, tumor growth inhibition, radiosensitization or
chemosensitization (17–20). However, because ASODNs exhibit poor
solubility, low affinity for their target complementary RNA
sequences (21), phosphorothioate
modification was adopted for ASODN in the current study. PS-ASODN
presents with good aqueous solubility, increased resistance to
degradation of oligodeoxynucleotide by nuclease, increased
stability and liposome encapsulation (7,22).
Previous studies have demonstrated that PS-ASOND suppressed the
activity of telomerase and tumor growth (23–27).
In the current study, PS-ASODN was demonstrated to
have an inhibitory effect on cell proliferation in a time-dependent
manner. PS-ASODN accelerated cell senescence and resulted in a
time-dependent decrease in telomerase activity, indicating that
PS-ASODN improved cell senescence by suppressing telomerase
activity. Through treating Walker 256 cells with PS-ASODN and IR in
combination, it was identified that PS-ASODN may enhance the
inhibition of IRon cell viability and telomerase activity. Besides,
PS-ASODN also promoted the induction of IRon cell apoptosis by
activating apoptosis-associated proteins, and enhanced the
induction of IRon DNA damage.
In conclusion, it is suggested that hTERT PS-ASODN,
the inhibitor of telomerase activity, can inhibit cell
proliferation, accelerate cell senescence by down-regulating
telomerase activity, and enhance the inhibition of IRon cell
viability and induction of IRon cell apoptosis and DNA damage, thus
resulting in increased sensitivity to IR. These observations
suggest that combination of PS-ASODN and IR is of significance in
providing an experimental foundation for gene therapy and guiding
the radio therapeutic strategy for cancer treatment.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 81171435).
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lebedeva IV and Stein CA: Antisense
oligonucleotides in cancer: Recent advances. BioDrugs. 13:195–216.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walther W and Schlag PM: Current status of
gene therapy for cancer. Curr Opin Oncol. 25:659–664. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nittis T, Guittat L and Stewart SA:
Alternative lengthening of telomeres (ALT) and chromatin: Is there
a connection? Biochimie. 90:5–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rao YK, Kao TY, Wu MF, Ko JL and Tzeng YM:
Identification of small molecule inhibitors of telomerase activity
through transcriptional regulation of hTERT and calcium induction
pathway in human lung adenocarcinoma A549 cells. Bioorg Med Chem.
18:6987–6994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan XK, Yan RH, Li BJ, Chen XM, Wei L and
Wang Z: Antisense oligodeoxynucleotide against human telomerase
reverse transcriptase inhibits the proliferation of Eca-109
esophageal carcinoma cells. Exp Ther Med. 8:1247–1252.
2014.PubMed/NCBI
|
|
8
|
Ponnala S, Chetty C, Veeravalli KK, Dinh
DH, Klopfenstein JD and Rao JS: MMP-9 silencing regulates hTERT
expression via β1 integrin-mediated FAK signaling and induces
senescence in glioma xenograft cells. Cell Signal. 23:2065–2075.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folini M, Brambilla C, Villa R, Gandellini
P, Vignati S, Paduano F, Daidone MG and Zaffaroni N: Antisense
oligonucleotide-mediated inhibition of hTERT, but not hTERC,
induces rapid cell growth decline and apoptosis in the absence of
telomere shortening in human prostate cancer cells. Eur J Cancer.
41:624–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kraemer K, Fuessel S, Schmidt U, Kotzsch
M, Schwenzer B, Wirth MP and Meye A: Antisense-mediated hTERT
inhibition specifically reduces the growth of human bladder cancer
cells. Clin Cancer Res. 9:3794–3800. 2003.PubMed/NCBI
|
|
11
|
Sekoguchi S, Nakajima T, Moriguchi M, Jo
M, Nishikawa T, Katagishi T, Kimura H, Minami M, Itoh Y, Kagawa K,
et al: Role of cell-cycle turnover and oxidative stress in telomere
shortening and cellular senescence in patients with chronic
hepatitis C. J Gastroenterol Hepatol. 22:182–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaul Z, Cesare AJ, Huschtscha LI, Neumann
AA and Reddel RR: Five dysfunctional telomeres predict onset of
senescence in human cells. EMBO Rep. 13:52–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sekaran VG, Soares J and Jarstfer MB:
Structures of telomerase subunits provide functional insights.
Biochim Biophys Acta. 1804:1190–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donate LE and Blasco MA: Telomeres in
cancer and ageing. Philos Trans R Soc Lond B Biol Sci. 366:76–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zvereva MI, Shcherbakova DM and Dontsova
OA: Telomerase: Structure, functions, and activity regulation.
Biochemistry (Mosc). 75:1563–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agrawal A, Dang S and Gabrani R: Recent
patents on anti-telomerase cancer therapy. Recent Pat Anticancer
Drug Discov. 7:102–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakhlband A, Barar J, Bidmeshkipour A,
Heidari HR and Omidi Y: Bioimpacts of anti epidermal growth factor
receptor antisense complexed with polyamidoamine dendrimers in
human lung epithelial adenocarcinoma cells. J Biomed Nanotechnol.
6:360–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao FJ, Zhang SL, Ma L, Gao H and Zong
ZH: Inhibitory effects of c-erbB-2 antisense oligonucleotide
transfection on uterine endometrial cancer Ishikawa cell lines. Eur
J Gynaecol Oncol. 30:54–59. 2009.PubMed/NCBI
|
|
20
|
Loriot Y, Mordant P, Brown BD, Bourhis J,
Soria JC and Deutsch E: Inhibition of BCL-2 in small cell lung
cancer cell lines with oblimersen, an antisense BCL-2
oligodeoxynucleotide (ODN): In vitro and in vivo enhancement of
radiation response. Anticancer Res. 30:3869–3878. 2010.PubMed/NCBI
|
|
21
|
Zhang KZ, Xu JH, Huang XW, Wu LX, Su Y and
Chen YZ: Curcumin synergistically augments bcr/abl phosphorothioate
antisense oligonucleotides to inhibit growth of chronic myelogenous
leukemia cells. Acta Pharmacol Sin. 28:105–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan Y, Cai H, Yang XJ, Li W, He J, Guo TK
and Chen YR: Liposome-mediated induction of apoptosis of human
hepatoma cells by c-myc antisense phosphorothioate
oligodeoxynucleotide and 5-fluorouracil. Asian Pac J Cancer Prev.
15:5529–5533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Svinareva LV, Glukhov AI, Moskaleva EY and
Shvets VI: Effect of modified DNA and RNA oligonucleotides on
telomerase activity and tumor cell survival in vitro. Appl Biochem
Micro. 47:718–722. 2011. View Article : Google Scholar
|
|
24
|
Wang XS, Wang K, Li X and Fu SB: Effects
of phosphorothioate anti-sense oligodeoxynucleotides on colorectal
cancer cell growth and telomerase activity. World J Gastroenterol.
10:3455–3458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao XD and Chen YR: Inhibition of
telomerase with human telomerase reverse transcriptase antisense
increases the sensitivity of tumor necrosis factor-alpha-induced
apoptosis in prostate cancer cells. Asian J Androl. 9:697–704.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji XM, Xie CH, Fang MH, Zhou FX, Zhang WJ,
Zhang MS and Zhou YF: Efficient inhibition of human telomerase
activity by antisense oligonucleotides sensitizes cancer cells to
radiotherapy. Acta Pharmacol Sin. 27:1185–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan XK, Yan RH, Li BJ, Chen XM, Wei L and
Wang Z: Antisense oligodeoxynucleotide against human telomerase
reverse transcriptase inhibits the proliferation of Eca-109
esophageal carcinoma cells. Exp Ther Med. 8:1247–1252.
2014.PubMed/NCBI
|