|
1
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of poly
(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Issaeva N, Thomas HD, Djureinovic T,
Jaspers JE, Stoimenov I, Kyle S, Pedley N, Gottipati P, Zur R,
Sleeth K, et al: 6-thioguanine selectively kills BRCA2-defective
tumors and overcomes PARP inhibitor resistance. Cancer Res.
70:6268–6276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCabe N, Turner NC, Lord CJ, Kluzek K,
Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka
MZ, et al: Deficiency in the repair of DNA damage by homologous
recombination and sensitivity to poly (ADP-ribose) polymerase
inhibition. Cancer Res. 66:8109–8115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson LH, Brookman KW, Weber CA,
Salazar EP, Reardon JT, Sancar A, Deng Z and Siciliano MJ:
Molecular cloning of the human nucleotide-excision-repair gene
ERCC4. Proc Natl Acad Sci USA. 91:6855–6859. 1994. View Article : Google Scholar : PubMed/NCBI
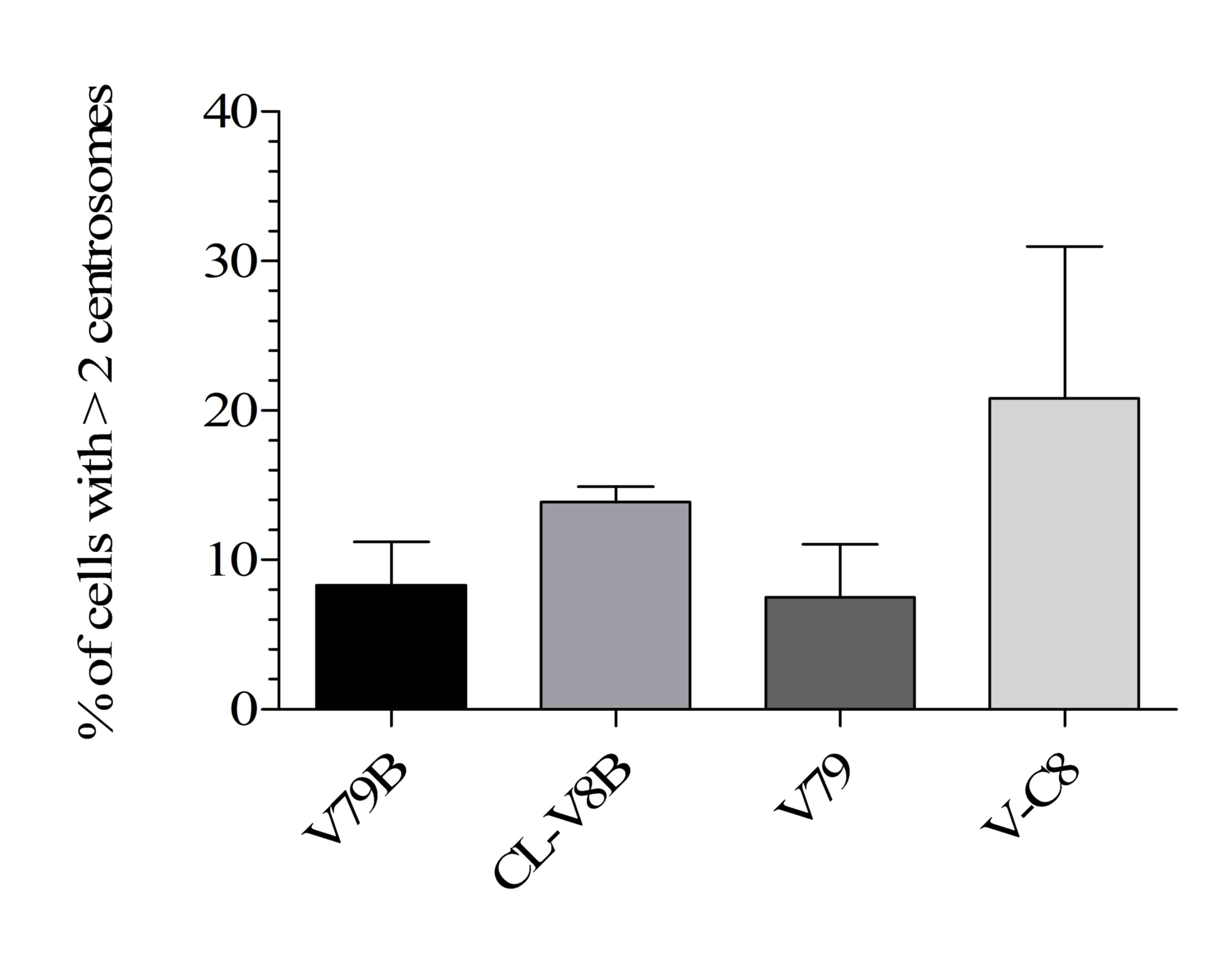
|
|
5
|
Weeda G, Hoeijmakers JH and Bootsma D:
Genes controlling nucleotide excision repair in eukaryotic cells.
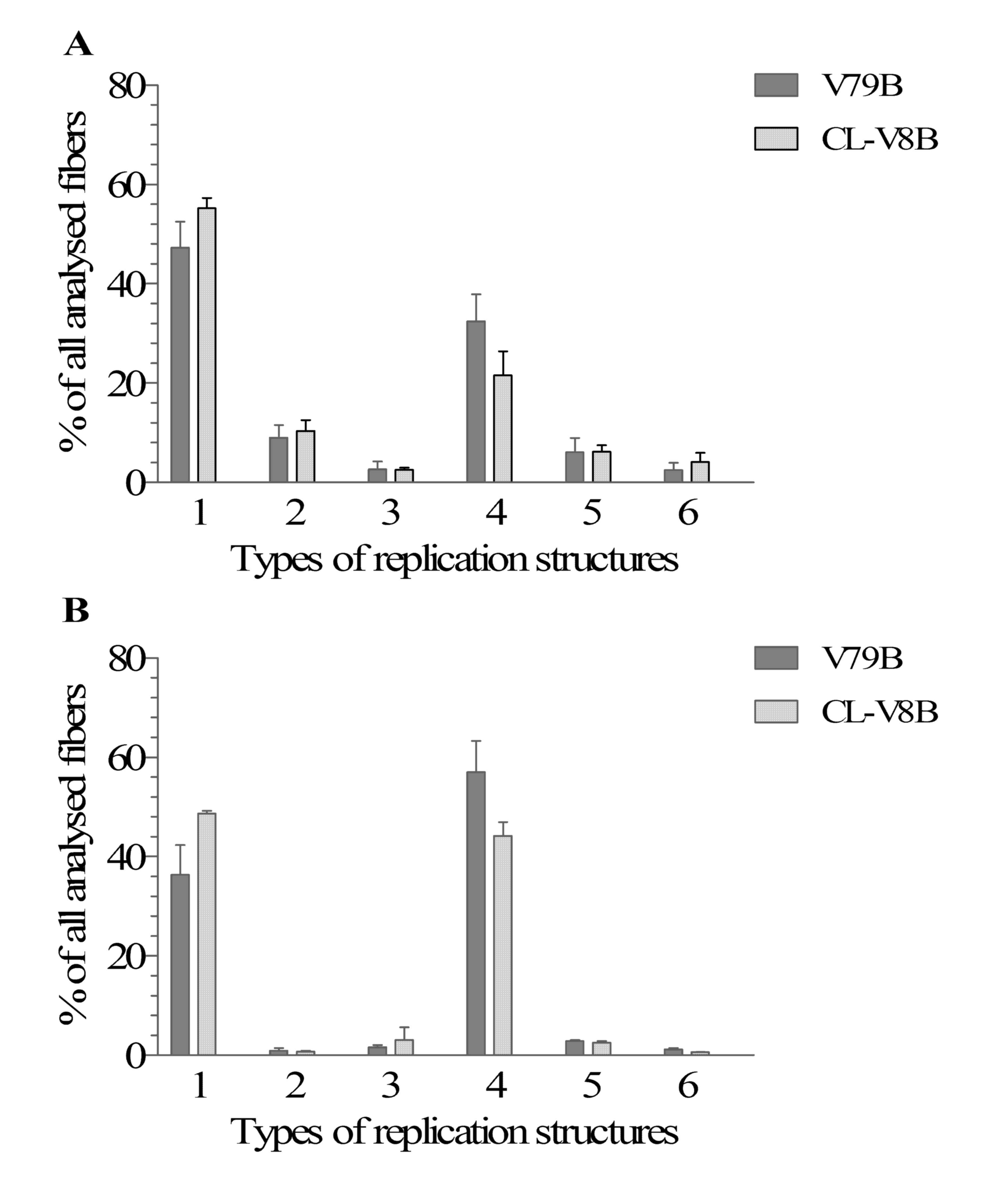
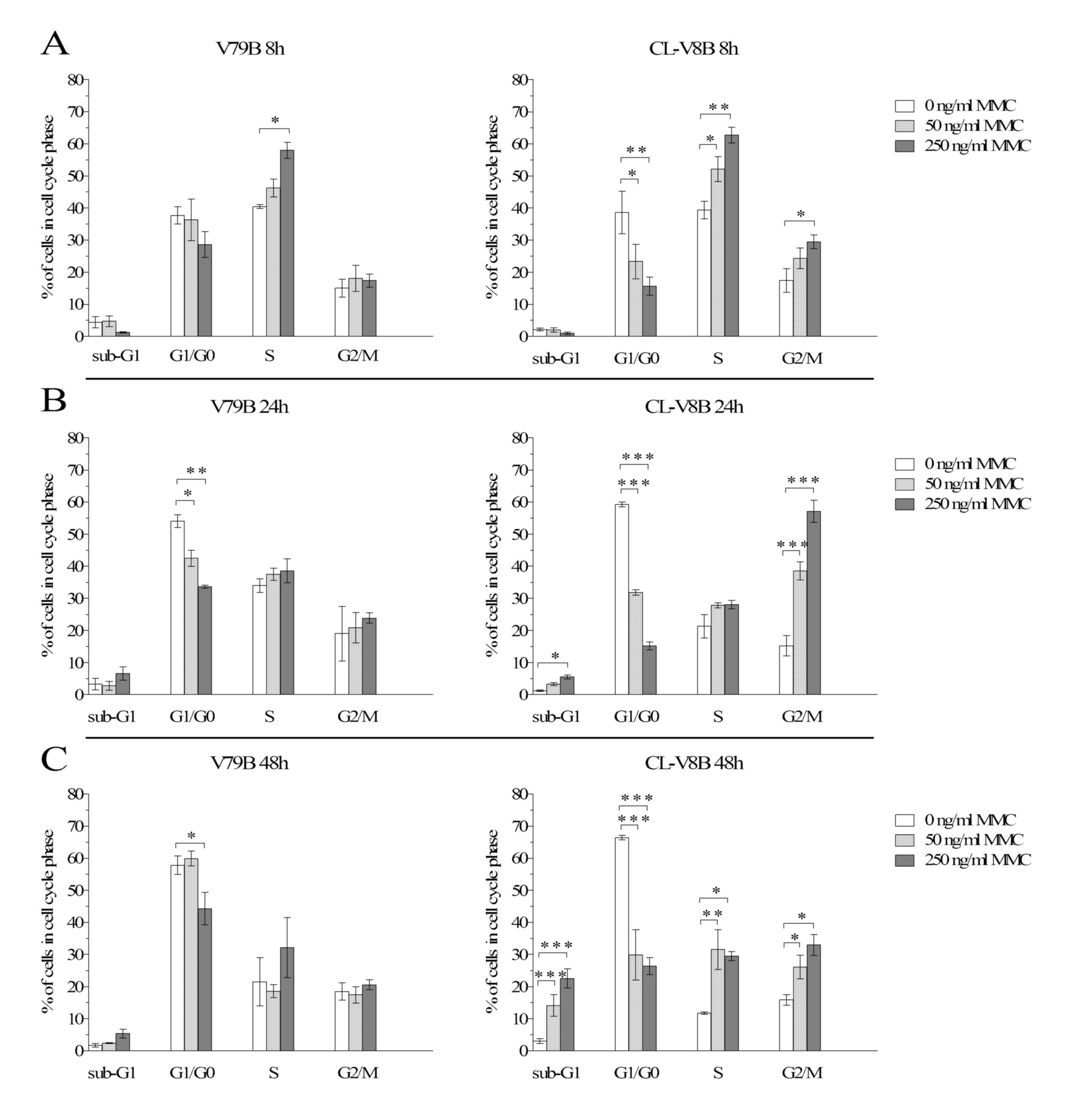
Bioessays. 15:249–258. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thacker J and Zdzienicka MZ: The mammalian
XRCC genes: Their roles in DNA repair and genetic stability. DNA
Repair (Amst). 2:655–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rolig RL, Lowery MP, Adair GM and Nairn
RS: Characterization and analysis of Chinese hamster ovary cell
ERCC1 mutant alleles. Mutagenesis. 13:357–365. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Godthelp BC, Wiegant WW, van
Duijn-Goedhart A, Schärer OD, van Buul PP, Kanaar R and Zdzienicka
MZ: Mammalian Rad51C contributes to DNA cross-link resistance,
sister chromatid cohesion and genomic stability. Nucleic Acids Res.
30:2172–2182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaz F, Hanenberg H, Schuster B, Barker K,
Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F, et al:
Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat
Genet. 42:406–409. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bracalente C, Ibañez IL, Molinari B,
Palmieri M, Kreiner A, Valda A, Davidson J and Durán H: Induction
and persistence of large γH2AX foci by high linear energy transfer
radiation in DNA-dependent protein kinase-deficient cells. Int J
Radiat Oncol Biol Phys. 87:785–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maeda J, Bell JJ, Genet SC, Fujii Y, Genet
MD, Brents CA, Genik PC and Kato TA: Potentially lethal damage
repair in drug arrested G2-phase cells after radiation exposure.
Radiat Res. 182:448–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deans AJ and West SC: DNA interstrand
crosslink repair and cancer. Nat Rev Cancer. 11:467–480. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jaspers NG, Raams A, Silengo MC, Wijgers
N, Niedernhofer LJ, Robinson AR, Giglia-Mari G, Hoogstraten D,
Kleijer WJ, Hoeijmakers JH and Vermeulen W: First reported patient
with human ERCC1 deficiency has cerebro-oculo-facio-skeletal
syndrome with a mild defect in nucleotide excision repair and
severe developmental failure. Am J Hum Genet. 80:457–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vrouwe MG, Elghalbzouri-Maghrani E,
Meijers M, Schouten P, Godthelp BC, Bhuiyan ZA, Redeker EJ, Mannens
MM, Mullenders LH, Pastink A and Darroudi F: Increased DNA damage
sensitivity of Cornelia de Lange syndrome cells: Evidence for
impaired recombinational repair. Hum Mol Genet. 16:1478–1487. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der LP, Oostra AB, Rooimans MA, Joenje
H and de Winter JP: Diagnostic overlap between fanconi anemia and
the cohesinopathies: Roberts syndrome and warsaw breakage syndrome.
Anemia. 2010:5652682010.PubMed/NCBI
|
|
16
|
Kim H and D'Andrea AD: Regulation of DNA
cross-link repair by the Fanconi anemia/BRCA pathway. Genes Dev.
26:1393–1408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong MW, Nordfors C, Mossman D,
Pecenpetelovska G, Avery-Kiejda KA, Talseth-Palmer B, Bowden NA and
Scott RJ: BRIP1, PALB2, and RAD51C mutation analysis reveals their
relative importance as genetic susceptibility factors for breast
cancer. Breast Cancer Res Treat. 127:853–859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J and Krantz ID: Cornelia de Lange
syndrome, cohesin, and beyond. Clin Genet. 76:303–314. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zdzienicka MZ, Arwert F, Neuteboom I,
Rooimans M and Simons JW: The Chinese hamster V79 cell mutant V-H4
is phenotypically like Fanconi anemia cells. Somat Cell Mol Genet.
16:575–581. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bishop DK, Ear U, Bhattacharyya A,
Calderone C, Beckett M, Weichselbaum RR and Shinohara A: Xrcc3 is
required for assembly of Rad51 complexes in vivo. J Biol Chem.
273:21482–21488. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kraakman-van der Zwet M, Overkamp WJ, van
Lange RE, Essers J, van Duijn-Goedhart A, Wiggers I, Swaminathan S,
van Buul PP, Errami A, Tan RT, et al: Brca2 (XRCC11) deficiency
results in radioresistant DNA synthesis and a higher frequency of
spontaneous deletions. Mol Cell Biol. 22:669–679. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Regan P, Wilson C, Townsend S and
Thacker J: XRCC2 is a nuclear RAD51-like protein required for
damage-dependent RAD51 focus formation without the need for ATP
binding. J Biol Chem. 276:22148–22153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zdzienicka MZ, Jaspers NG, van der Schans
GP, Natarajan AT and Simons JW: Ataxia-telangiectasia-like Chinese
hamster V79 cell mutants with radioresistant DNA synthesis,
chromosomal instability, and normal DNA strand break repair. Cancer
Res. 49:1481–1485. 1989.PubMed/NCBI
|
|
24
|
Jackson DA and Pombo A: Replicon clusters
are stable units of chromosome structure: Evidence that nuclear
organization contributes to the efficient activation and
propagation of S phase in human cells. J Cell Biol. 140:1285–1295.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zdzienicka MZ and Simons JW:
Mutagen-sensitive cell lines are obtained with a high frequency in
V79 Chinese hamster cells. Mutat Res. 178:235–244. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bargonetti J, Champeil E and Tomasz M:
Differential toxicity of DNA adducts of mitomycin C. J Nucleic
Acids. 2010:pii: 6989602010. View Article : Google Scholar
|
|
27
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Godthelp BC, Artwert F, Joenje H and
Zdzienicka MZ: Impaired DNA damage-induced nuclear Rad51 foci
formation uniquely characterizes Fanconi anemia group D1. Oncogene.
21:5002–5005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hinz JM, Tebbs RS, Wilson PF, Nham PB,
Salazar EP, Nagasawa H, Urbin SS, Bedford JS and Thompson LH:
Repression of mutagenesis by Rad51D-mediated homologous
recombination. Nucleic Acids Res. 34:1358–1368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smiraldo PG, Gruver AM, Osborn JC and
Pittman DL: Extensive chromosomal instability in Rad51d-deficient
mouse cells. Cancer Res. 65:2089–2096. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sonoda E, Sasaki MS, Morrison C,
Yamaguchi-Iwai Y, Takata M and Takeda S: Sister chromatid exchanges
are mediated by homologous recombination in vertebrate cells. Mol
Cell Biol. 19:5166–5169. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindh A Renglin, Schultz N, Saleh-Gohari N
and Helleday T: RAD51C (RAD51L2) is involved in maintaining
centrosome number in mitosis. Cytogenet Genome Res. 116:38–45.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nigg EA: Centrosome aberrations: Cause or
consequence of cancer progression? Nat Rev Cancer. 2:815–825. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Henry-Mowatt J, Jackson D, Masson JY,
Johnson PA, Clements PM, Benson FE, Thompson LH, Takeda S, West SC
and Caldecott KW: XRCC3 and Rad51 modulate replication fork
progression on damaged vertebrate chromosomes. Mol Cell.
11:1109–1117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schwab RA, Blackford AN and Niedzwiedz W:
ATR activation and replication fork restart are defective in
FANCM-deficient cells. EMBO J. 29:806–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schwab RA, Nieminuszczy J, Shin-ya K and
Niedzwiedz W: FANCJ couples replication past natural fork barriers
with maintenance of chromatin structure. J Cell Biol. 201:33–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koç A, Wheeler LJ, Mathews CK and Merrill
GF: Hydroxyurea arrests DNA replication by a mechanism that
preserves basal dNTP pools. J Biol Chem. 279:223–230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mladenov E, Tsaneva I and Anachkova B:
Activation of the S phase DNA damage checkpoint by mitomycin C. J
Cell Physiol. 211:468–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang SG, Chung H, Yoo YD, Lee JG, Choi YI
and Yu YS: Mechanism of growth inhibitory effect of Mitomycin-C on
cultured human retinal pigment epithelial cells: Apoptosis and cell
cycle arrest. Curr Eye Res. 22:174–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Somyajit K, Subramanya S and Nagaraju G:
Distinct roles of FANCO/RAD51C protein in DNA damage signaling and
repair: Implications for Fanconi anemia and breast cancer
susceptibility. J Biol Chem. 287:3366–3380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rocca CJ, Soares DG, Bouzid H, Henriques
JA, Larsen AK and Escargueil AE: BRCA2 is needed for both repair
and cell cycle arrest in mammalian cells exposed to S23906, an
anticancer monofunctional DNA binder. Cell Cycle. 14:2080–2090.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaina B: DNA damage-triggered apoptosis:
Critical role of DNA repair, double-strand breaks, cell
proliferation and signaling. Biochem Pharmacol. 66:1547–1554. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wilson JB, Johnson MA, Stuckert AP,
Trueman KL, May S, Bryant PE, Meyn RE, D'Andrea AD and Jones NJ:
The Chinese hamster FANCG/XRCC9 mutant NM3 fails to express the
monoubiquitinated form of the FANCD2 protein, is hypersensitive to
a range of DNA damaging agents and exhibits a normal level of
spontaneous sister chromatid exchange. Carcinogenesis.
22:1939–1946. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jones NJ, Ellard S, Waters R and Parry EM:
Cellular and chromosomal hypersensitivity to DNA crosslinking
agents and topoisomerase inhibitors in the radiosensitive Chinese
hamster irs mutants: Phenotypic similarities to ataxia
telangiectasia and Fanconi's anaemia cells. Carcinogenesis.
14:2487–2494. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wiltshire TD, Lovejoy CA, Wang T, Xia F,
O'Connor MJ and Cortez D: Sensitivity to poly (ADP-ribose)
polymerase (PARP) inhibition identifies ubiquitin-specific
peptidase 11 (USP11) as a regulator of DNA double-strand break
repair. J Biol Chem. 285:14565–14571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Somyajit K, Mishra A, Jameei A and
Nagaraju G: Enhanced non-homologous end joining contributes toward
synthetic lethality of pathological RAD51C mutants with poly
(ADP-ribose) polymerase. Carcinogenesis. 36:13–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan SS, Chang HL and Lee EY: Ionizing
radiation-induced Rad51 nuclear focus formation is cell
cycle-regulated and defective in both ATM(−/−) and c-Abl(−/−)
cells. Mutat Res. 525:85–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gudmundsdottir K, Lord CJ, Witt E, Tutt AN
and Ashworth A: DSS1 is required for RAD51 focus formation and
genomic stability in mammalian cells. EMBO Rep. 5:989–993. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xia B, Dorsman JC, Ameziane N, de Vries Y,
Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, Llera J, et al:
Fanconi anemia is associated with a defect in the BRCA2 partner
PALB2. Nat Genet. 39:159–161. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dong Z, Zhong Q and Chen PL: The Nijmegen
breakage syndrome protein is essential for Mre11 phosphorylation
upon DNA damage. J Biol Chem. 274:19513–19516. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van Veelen LR, Essers J, van de Rakt MW,
Odijk H, Pastink A, Zdzienicka MZ, Paulusma CC and Kanaar R:
Ionizing radiation-induced foci formation of mammalian Rad51 and
Rad54 depends on the Rad51 paralogs, but not on Rad52. Mutat Res.
574:34–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shimada M, Sagae R, Kobayashi J, Habu T
and Komatsu K: Inactivation of the Nijmegen breakage syndrome gene
leads to excess centrosome duplication via the ATR/BRCA1 pathway.
Cancer Res. 69:1768–1775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dronkert ML, Beverloo HB, Johnson RD,
Hoeijmakers JH, Jasin M and Kanaar R: Mouse RAD54 affects DNA
double-strand break repair and sister chromatid exchange. Mol Cell
Biol. 20:3147–3156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dong Z and Fasullo M: Multiple
recombination pathways for sister chromatid exchange in
Saccharomyces cerevisiae: Role of RAD1 and the RAD52 epistasis
group genes. Nucleic Acids Res. 31:2576–2585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Brugmans L, Verkaik NS, Kunen M, van
Drunen E, Williams BR, Petrini JH, Kanaar R, Essers J and van Gent
DC: NBS1 cooperates with homologous recombination to counteract
chromosome breakage during replication. DNA Repair (Amst).
8:1363–1370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu X, Weaver Z, Linke SP, Li C, Gotay J,
Wang XW, Harris CC, Ried T and Deng CX: Centrosome amplification
and a defective G2-M cell cycle checkpoint induce genetic
instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell.
3:389–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bertrand P, Lambert S, Joubert C and Lopez
BS: Overexpression of mammalian Rad51 does not stimulate
tumorigenesis while a dominant-negative Rad51 affects centrosome
fragmentation, ploidy and stimulates tumorigenesis, in
p53-defective CHO cells. Oncogene. 22:7587–7592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Date O, Katsura M, Ishida M, Yoshihara T,
Kinomura A, Sueda T and Miyagawa K: Haploinsufficiency of RAD51B
causes centrosome fragmentation and aneuploidy in human cells.
Cancer Res. 66:6018–6024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Godthelp BC, van Buul PP, Jaspers NG,
Elghalbzouri-Maghrani E, van Duijn-Goedhart A, Arwert F, Joenje H
and Zdzienicka MZ: Cellular characterization of cells from the
Fanconi anemia complementation group, FA-D1/BRCA2. Mutat Res.
601:191–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Beamish H, Williams R, Chen P and Lavin
MF: Defect in multiple cell cycle checkpoints in
ataxia-telangiectasia postirradiation. J Biol Chem.
271:20486–20493. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Eggleston A: Convergence of DNA repair and
cell-cycle checkpoint control. Nat Cell Biol. 2:E952000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bakr A, Oing C, Köcher S, Borgmann K,
Dornreiter I, Petersen C, Dikomey E and Mansour WY: Involvement of
ATM in homologous recombination after end resection and RAD51
nucleofilament formation. Nucleic Acids Res. 43:3154–3166. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhao S, Weng YC, Yuan SS, Lin YT, Hsu HC,
Lin SC, Gerbino E, Song MH, Zdzienicka MZ, Gatti RA, et al:
Functional link between ataxia-telangiectasia and Nijmegen breakage
syndrome gene products. Nature. 405:473–477. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sala-Trepat M, Rouillard D, Escarceller M,
Laquerbe A, Moustacchi E and Papadopoulo D: Arrest of S-phase
progression is impaired in Fanconi anemia cells. Exp Cell Res.
260:208–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Heinrich MC, Hoatlin ME, Zigler AJ, Silvey
KV, Bakke AC, Keeble WW, Zhi Y, Reifsteck CA, Grompe M, Brown MG,
et al: DNA cross-linker-induced G2/M arrest in group C Fanconi
anemia lymphoblasts reflects normal checkpoint function. Blood.
91:275–287. 1998.PubMed/NCBI
|
|
67
|
Rodrigue A, Coulombe Y, Jacquet K, Gagné
JP, Roques C, Gobeil S, Poirier G and Masson JY: The RAD51 paralogs
ensure cellular protection against mitotic defects and aneuploidy.
J Cell Sci. 126:348–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yun J, Zhong Q, Kwak JY and Lee WH:
Hypersensitivity of Brca1-deficient MEF to the DNA interstrand
crosslinking agent mitomycin C is associated with defect in
homologous recombination repair and aberrant S-phase arrest.
Oncogene. 24:4009–4016. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pirnia F, Schneider E, Betticher DC and
Borner MM: Mitomycin C induces apoptosis and caspase-8 and −9
processing through a caspase-3 and Fas-independent pathway. Cell
Death Differ. 9:905–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hinz JM, Helleday T and Meuth M: Reduced
apoptotic response to camptothecin in CHO cells deficient in XRCC3.
Carcinogenesis. 24:249–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bryant PE: The signal model: A possible
explanation for the conversion of DNA double-strand breaks into
chromatid breaks. Int J Radiat Biol. 73:243–251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Parshad R, Sanford KK and Jones GM:
Chromatid damage induced by fluorescent light during G2 phase in
normal and Gardner syndrome fibroblasts. Interpretation in terms of
deficient DNA repair. Mutat Res. 151:57–63. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rey JP, Scott R and Müller H: Apoptosis is
not involved in the hypersensitivity of Fanconi anemia cells to
mitomycin C. Cancer Genet Cytogenet. 75:67–71. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kruyt FA, Dijkmans LM, van den Berg TK and
Joenje H: Fanconi anemia genes act to suppress a
cross-linker-inducible p53-independent apoptosis pathway in
lymphoblastoid cell lines. Blood. 87:938–948. 1996.PubMed/NCBI
|