Introduction
Breast cancer is the most common malignant disease
in women (1) and accounts for ~25%
of cancer cases (2,3). Despite the number of breast cancer
medicines, the prognosis of breast cancer remains poor (4). The main approaches used to treat
breast cancer are surgery, radiotherapy, chemotherapy, hormone
therapy and targeted therapy (5).
However, recovery rates following application of these conventional
methods are only 60–80% for primary cancer and ~50% for metastatic
cancer (5,6).
Traditional Chinese medicine (TCM) has been used to
treat various cancers (7).
Scutellaria baicalensis Georgi (SBG) is a well-known TCM and
is traditionally described as possessing ‘heat-clearing,
dampness-drying, fire-purging, detoxicating and
hemostasis-maintaining’ properties (8). In the modern era, it has been
demonstrated to possess various properties, including antioxidant
and anti-inflammatory effects (9,10),
and to inhibit the proliferations of several tumors via apoptosis
pathways (11–15). For example, SBG induces the
apoptosis of human bladder 5637 cancer cells via the reactive
oxygen species (ROS)-dependent activation of caspases (11), inhibits the spread of B16F10 mouse
melanoma cells by inactivating the phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) signaling pathway (12), suppresses the proliferation of
ovarian cancer cells by suppressing the p38 mitogen-activated
protein kinases (MAPK)-dependent pathway (13), inhibits the growth of human MCF10A
cells via transforming epithelial-mesenchymal transition (14) and has been reported to inhibit
pulmonary tumor metastasis by impairing the activations of MAPK and
PI3K-Akt (15).
Apoptosis is one of the processes of programmed cell
death with specific morphologic characteristics and biochemical
features, including membrane blebbing, intracellular fragmentation
associated with membrane enclosed cellular fragments (apoptotic
bodies) and cellular shrinkage (16,17).
Apoptosis-associated molecules serve key roles in the maintenance
of physiological homeostasis, but can be abnormally expressed
during tumor development (18,19).
However, the effects of SBG on MCF-7 human breast adenocarcinoma
cells have not been previously investigated. Therefore, the present
study aimed to identify the mechanism responsible for the
SBG-induced apoptosis of MCF-7 human breast cancer cells.
Materials and methods
Preparation of SBG extract (SBGE)
Baicalin and wogonin were purchased from Wako Pure
Chemical Industries Ltd. (Osaka, Japan). Acetonitrile, methanol and
water of high performance liquid chromatography (HPLC)-grade were
obtained from Avantor Performance Materials (Center Valley, PA,
USA). Trifluoroacetic acid was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Accurately weighed standard compounds
were dissolved in methanol and diluted. SBGE was obtained from the
plant extract bank at the Korea Research Institute of Bioscience
and Biotechnology (cat. no. CA04-087; Daejeon, Republic of Korea).
SBGE was dissolved in methanol and then filtered through a 0.2 mm
syringe filter (Biofact Co., Ltd., Daejeon, Korea) prior to
injection into the HPLC apparatus. The HPLC system used was an
Agilent 1200 (Agilent Technologies, Inc., Santa Clara, CA, USA)
equipped with a quaternary pump, autosampler, column oven and
diode-array detector. HPLC data were acquired using Chemstation
software (version B.03.02; Agilent Technologies, Inc.).
Chromatographic separation was performed on a XDB C18
column (4.6×150 mm, 5 µm; Agilent Technologies, Inc.) at 35°C. The
mobile phase consisted of water containing 0.1% trifluoroacetic
acid and acetonitrile and the chromatographic gradient program was:
20% acetonitrile for 2 min, 20–60% acetonitrile from 2 to 10 min
and held for 1 min. The column was then re-equilibrated with 20%
acetonitrile. The flow rate was set at 0.8 ml/min and the injection
volume used was 5 µl. Baicalin and wogonin were detected at 280
nm.
Cell culture and reagents
MCF-7 cells (human breast adenocarcinoma cells) were
established at the Cancer Research Center, Seoul National
University College of Medicine (Seoul, Republic of Korea). Cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and
20 µg/ml penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) in a 5% CO2 atmosphere at 37°C.
SP600125 and PD98059 were purchased from Tocris Bioscience
(Bristol, UK). All other reagents were supplied by Sigma-Aldrich
(Merck KGaA).
Cell viability assay
Cell viability was checked using an assay. MCF-7
cells were treated with 100 µl MTT solution (5 mg/ml in PBS per
well) and incubated for 4 h at 37°C. The absorbance was measured at
a wavelength of 570 nm using a microplate reader (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Measurement of cell cycle
MCF-7 cells were placed in an Eppendorf tube and
ethyl alcohol was slowly added with vortexing. Tubes were then
sealed with parafilm and incubated at 4°C overnight. Samples were
centrifuged for 5 min at 110 × g at 4°C and supernatants was
aspirated and discarded. Cell pellets were resuspended in 200 µl
propidium iodine (PI) staining solution [PI (5 mg/ml; 2 µl)
containing RNase (2 µl in PBS 196 µl)] (20,21)
and then centrifuged at 20,000 × g for 10 sec at 4°C. Following an
incubation for 30 min in the dark at room temperature, samples were
analyzed using a fluorescence-activated cell sorter (FACScan; BD
Biosciences, Franklin Lakes, NJ, USA) at 488 nm using Cell-Quest
Pro software (version 5.1; BD Biosciences).
Tetramethylrhodamine (TMRM)
mitochondrial membrane potential (MMP) assay
To measure MMP, MCF-7 cells were incubated with 25
nM TMRM for 1 h, mounted on a coverslide in a chamber filled with
complete culture medium and incubated at 37°C in a cell culture
incubator. Fluorescent images of samples were observed and captured
using a fluorescence microscope at excitation/emission wavelengths
of 549/575 nm. The fluorescent intensities of images were measured
using ImageJ v1.62 software (National Institutes of Health,
Bethesda, MD, USA) and values were expressed as percentages of
controls.
Western blot analysis
Western blotting was performed using the lysates of
5×106 MCF-7 cells. Briefly, total proteins were extracted using
radioimmunoprecipitation assay buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA) containing a protease inhibitor cocktail
(cat. no. #5871; Cell Signaling Technology, Inc.). The samples were
centrifuged at 18,000 × g and 4°C for 1 min, and protein
concentrations were determined using the Bio-Rad Protein Assay kit
II (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal amounts
(20 µg) of protein from each sample were separated by 10% SDS-PAGE,
transferred to a PVDF membrane and blocked with 5% non-fat dry milk
at room temperature for 1 h. The membranes were washed twice with
Tris-buffered saline containing 0.1% Tween-20 (TBS-T) and incubated
with specific primary antibodies against target proteins, including
B-cell lymphoma 2 (Bcl-2; cat. no. NB100-56098; Novus Biologicals,
LLC, Littleton, CO, USA), Bcl-2 X associated protein (Bax; cat. no.
NB100-65095; Novus Biologicals, LLC), GAPDH (cat. no. sc-32233;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and β-actin (cat.
no. A2066; Sigma-Aldrich; Merck KGaA) antibodies, diluted to 1:100
with 5% skim milk at 4°C overnight. The membranes were rinsed twice
with TBS-T and incubated with the appropriate secondary horseradish
peroxidase-conjugated antibodies, including rat anti-rabbit IgG
(cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) and rabbit
anti-mouse IgG (cat. no. Ab6728; Abcam, Cambridge, MA, USA) diluted
to 1:1,000 with 5% skim milk, at room temperature for 1 h. The
bands for proteins of interest were detected using the ECL Plus
Western Blotting Detection Reagent (GE Healthcare Life Sciences,
Pittsburgh, PA, USA) according to manufacturer's instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNAs were isolated from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and equal
amounts of RNA (2 µg) were converted to cDNA using AccuPower
RT-PreMix (Bioneer Corporation, Daejeon, Korea) at 70°C for 5 min,
42°C for 1 h and 94°C for 5 min using oligo-dT primers. Specific
DNA sequences were amplified using AccuPower PCR-PreMix (Bioneer
Corporation) under the following thermocycling conditions: An
initial denaturation at 95°C for 5 min, followed by 30 cycles of
denaturation for 30 sec at 95°C, annealing for 30 sec at 60°C and
extension for 30 sec at 72°C, with a final extension for 10 min at
72°C. The PCR primers used in this study were as follows: oligo-dT,
5′-TTTTTTTTTTTTTTTTTTTT-3′ reverse; Fas,
5′-ATGCTGGGCATCTGGACCCTCCTA-3′ forward and
5′-TCTGCACTTGGTATTCTGGGTCCG-3′ reverse; Fas ligand (FasL),
5′-ACTTCCGGGGTCAATCTTGC-3′ forward and 5′-TAGAACATCTCGGTGCCTGTA-3′
reverse; tumor necrosis factor (TNF)-α, 5′-GTGGACGAGAGTGTCTGACT-3′
forward and 5′-AGCCATCCTGCTTCCTTCCA-3′ reverse; and β-actin
5′-CAAGAGATGGCCACGGCTGCT-3′ forward and 5′-TCCTTCTGCATCCTGTCGGCA-3′
reverse. Amplified products were analyzed in 1.0% agarose gels
under UV light and images were captured using the GelDoc-It TS
Imaging system (UVP, Inc., Upland, CA, USA).
Caspase assay
Caspase 3 and 9 assay kits (BioMol Cellular Activity
Assay Kit Plus, Enzo Life Sciences, Inc., Farmingdale, NY, USA)
were used. Cells were centrifuged at 1,000 × g at 4°C for 10 min,
washed with PBS and resuspended in ice-cold cell lysis buffer.
Samples were then centrifuged at 10,000 × g for 10 min at 4°C and
supernatants were removed. Supernatant samples (10 µl) were then
incubated with 50 µl substrate (400-lM Ac-DEVD-pNA) in 40 µl assay
buffer at 37°C. Absorbances at 405 nm were read at several
time-points for each sample. pNA concentrations in samples were
determined using a standard plot of absorbance vs. pNA
concentration. zVAD-fmk (Calbiochem; EMD Millipore, Billerica, MA,
USA) was used as the pan-caspase inhibitor.
Measurement of ROS production
ROS generation in MCF-7 cells was quantified using
2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; Molecular
Probes; Thermo Fisher Scientific, Inc.). Briefly, following the
various pharmacological treatments, cells were treated with 10 µl
DCF-DA at 37°C for 30 min and washed with PBS. Fluorescence was
measured using a FACScan system (BD Biosciences) at
excitation/emission wavelengths of 488/525 nm.
Statistical analysis
Data are expressed as the mean ± standard error.
One-way analysis of variation followed by Tukey's post hoc test was
used for multiple comparisons. The statistical analysis was
performed using Prism version 6.0 (GraphPad Software Inc., La
Jolla, CA, USA) and Origin version 8.0 (OriginLab, Northampton, MA,
USA) software. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification of standard compounds
in SBGE
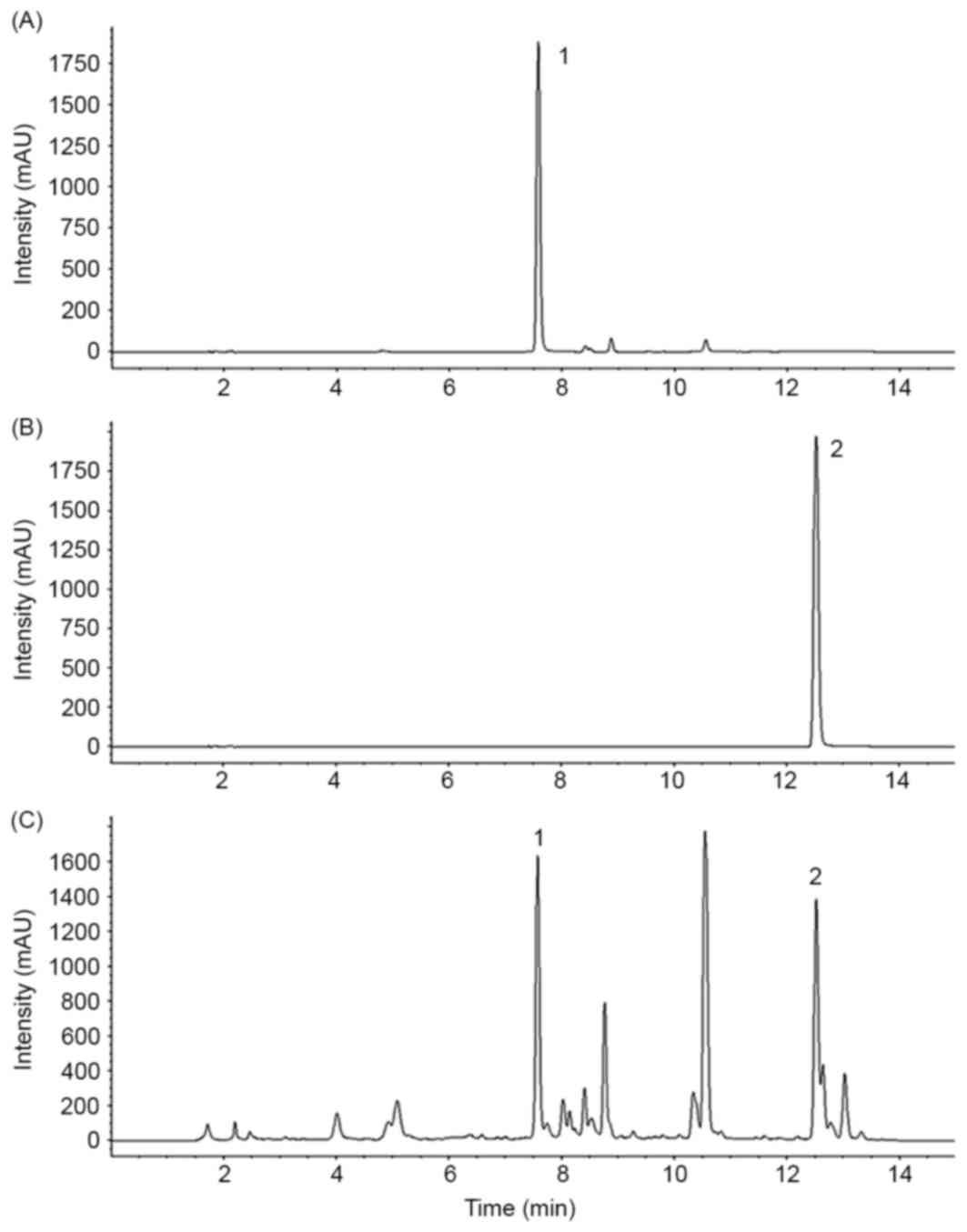
Baicalin and wogonin were detected on the HPLC
chromatogram of SBGE at retention times of 7.5 and 12.5 min,
respectively (Fig. 1).
Apoptosis by SBGE in MCF-7 cells
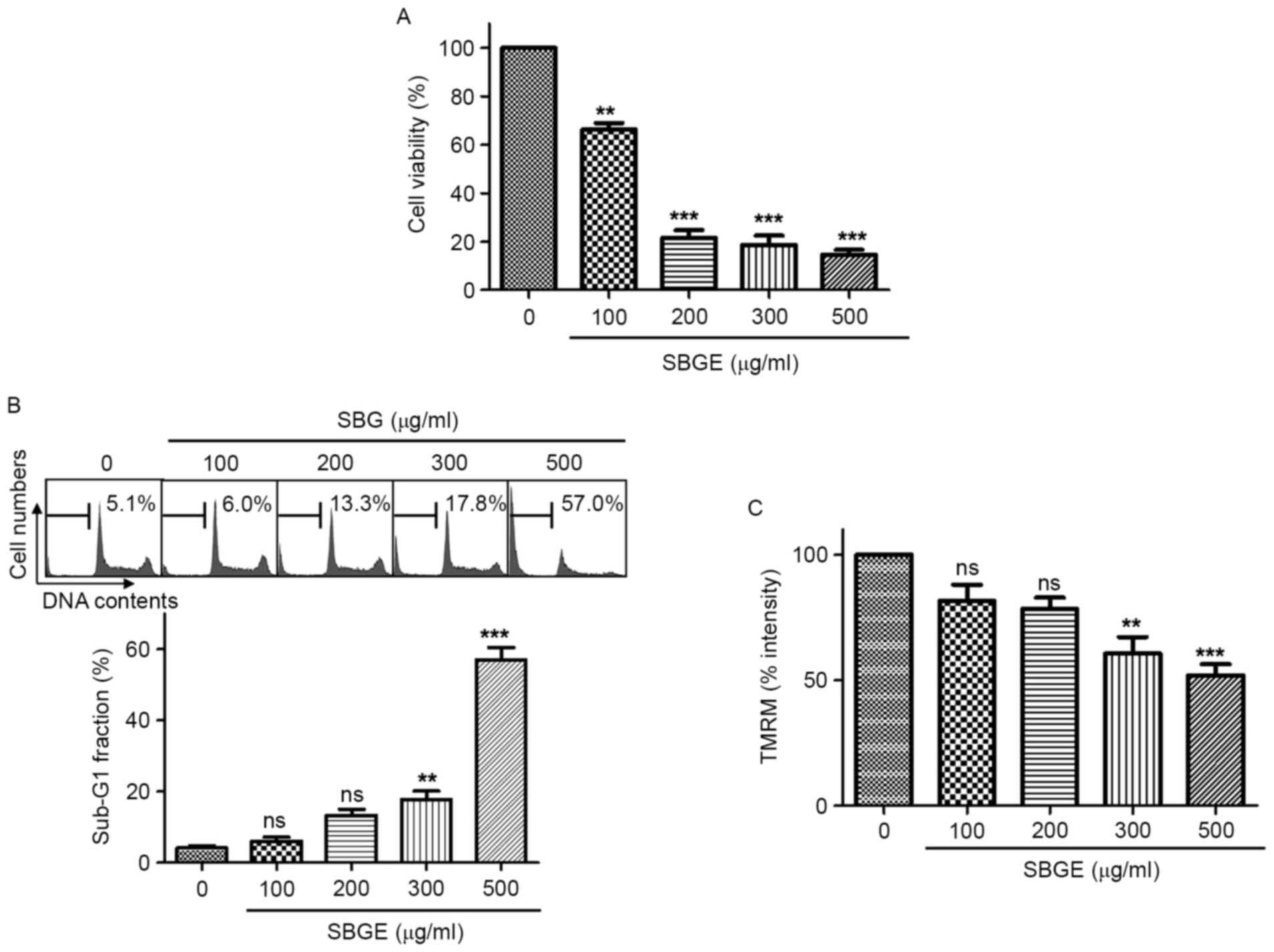
To determine whether SBGE suppresses MCF-7 cell
growth, MTT assays were performed following the culture for 24 h of
cells containing different concentrations of SBGE. Cell viabilities
were identified to be markedly reduced by SBGE treatment. Culture
for 24 h in the presence of 100, 200, 300 or 500 µg/ml of SBGE in
culture medium inhibited cell survival by 33.7±7.3, 78.4±5.4,
81.4±4.5 and 85.5±7.0%, respectively, as determined by MTT assay
(n=5; Fig. 2A). In addition, to
determine whether SBGE induces apoptosis, cell cycle and
mitochondrial membrane potential analysis were conducted by flow
cytometry and fluorescence microscopy, respectively. Cells were
treated with SBGE for 24 h (at concentrations between 100 and 500
µg/ml; Fig. 2B and C). The sub-G1
phase ratio was significantly and dose-dependently increased by
SBGE. More specifically, the sub-G1 phase was markedly increased by
6.2±2.1% at 100 µg/ml, 13.3±3.5% at 200 µg/ml, 17.8±4.1% at 300
µg/ml and 57.1±6.2% at 500 µg/ml compared with untreated cells
(n=5; Fig. 2B). In addition,
mitochondrial membrane potentials were suppressed by SBGE.
SBGE-induced mitochondrial membrane potential was decreased by
18.5±11.3% at 100 µg/ml, 21.7±7.7% at 200 µg/ml, 39.5±11.6% at 300
µg/ml and 48.2±8.0% at 500 µg/ml compared with untreated cells
(n=5; Fig. 2C). These results
suggested that SBGE has an anti-cancer effect and that this effect
is closely associated with apoptotic induction.
SBGE induces apoptosis via a
mitochondrial- and caspase-dependent signaling pathway in MCF-7
cells
To determine whether SBGE-induced MCF-7 cell
apoptosis is regulated by Bcl-2 (anti-apoptotic) and Bax
(pro-apoptotic), western blotting was used following the exposure
of the cells to various concentrations (between 100 and 500 µg/ml)
of SBGE for 24 h. The results revealed that Bcl-2 expression was
markedly inhibited by SBGE, whereas that of Bax was upregulated
(Fig. 3A).
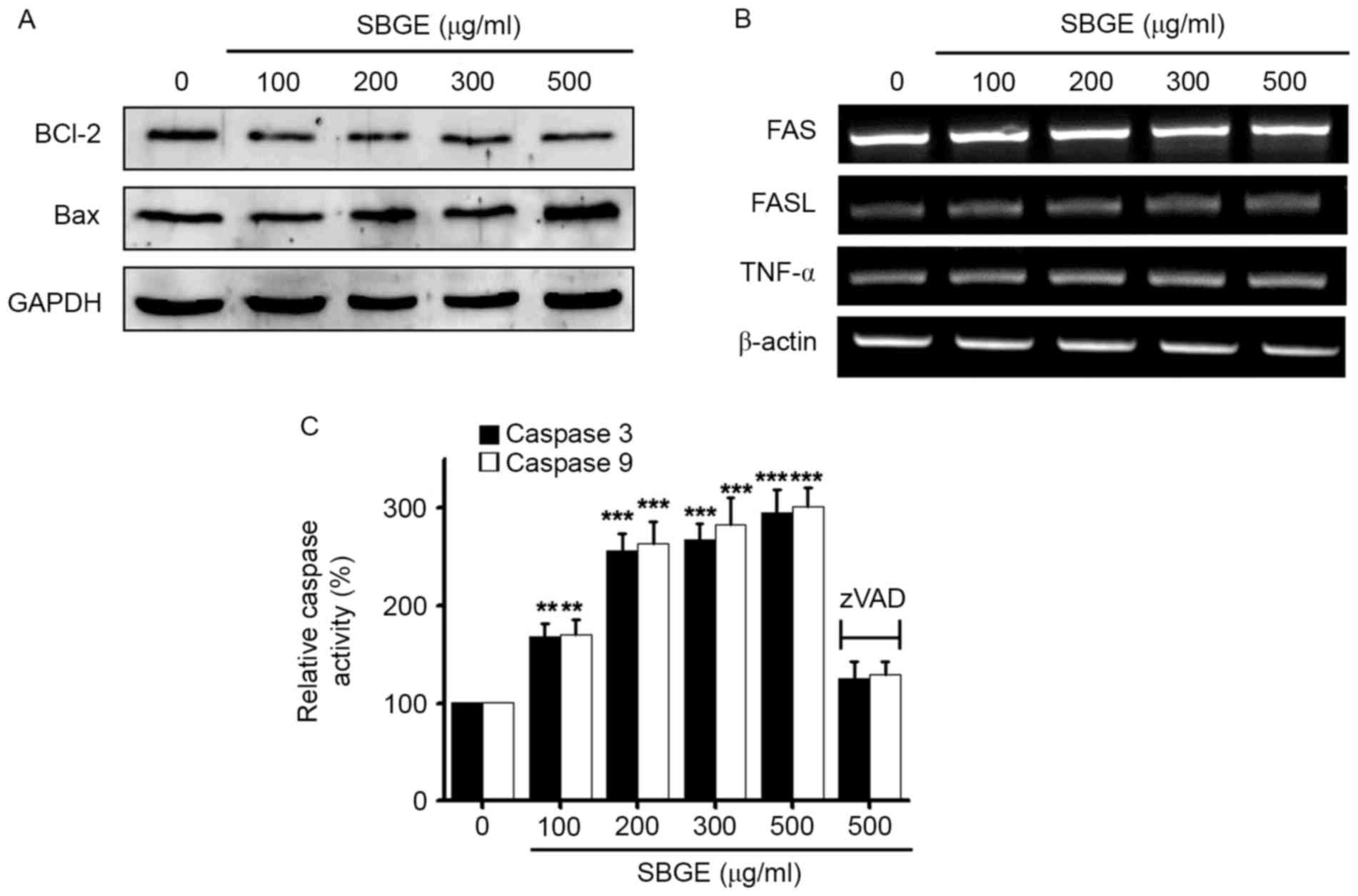 | Figure 3.Bcl-2 protein downregulation, Bax
protein upregulation and caspases 3 and 9 activities in MCF-7
cells. (A) Western blot analysis was performed on MCF-7 cells
treated with different SBGE concentrations for 24 h. Bcl-2
expression was downregulated by SBGE, whereas Bax expression was
upregulated. (B) RT-PCR was performed on MCF-7 cells treated with
different SBGE concentrations for 24 h. The expression levels of
Fas, FasL and TNF-α were unaffected by SBGE. (C) Caspase assays
were conducted following the indicated SBGE concentrations for
cells cultured for 24 h. The caspase activity of untreated cells
was taken to be 100%. Cells were treated with 20 µM zVAD-fm as a
pan-caspase inhibitor. Data are presented as the mean ± standard
error. **P<0.01, ***P<0.001 vs. untreated cells. GAPDH and
β-actin served as a loading control. Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2 X-associated protein; SBGE, Scutellaria baicalensis
Georgi extract; RT-PCR, reverse transcription-polymerase chain
reaction; FasL, Fas ligand; TNF, tumor necrosis factor. |
As the Fas/FasL system serves a key function in
death receptor-mediated apoptosis, the involvement of the
Fas/FasL/TNF-α system was examined in SBGE-treated cells by RT-PCR.
The results demonstrated that Fas/FasL and TNF-α expression levels
were unchanged by SBGE (Fig. 3B).
In addition, as caspase activation is required for apoptosis,
caspase activity assays were performed to assess the activities of
caspases 3 and 9 in MCF-7 cells. It was identified that caspase
activities were increased following treatment with SBGE (between
100 and 500 µg/ml) for 24 h, and that these activities were
suppressed by zVAD-fmk (Fig. 3C).
These results suggested that SBGE-induced apoptosis is mediated by
a mitochondrial- and caspase-dependent signaling pathway in MCF-7
cells.
SBGE induces apoptosis via the c-Jun
N-terminal kinase (JNK) and MAPK signaling pathway in MCF-7
cells
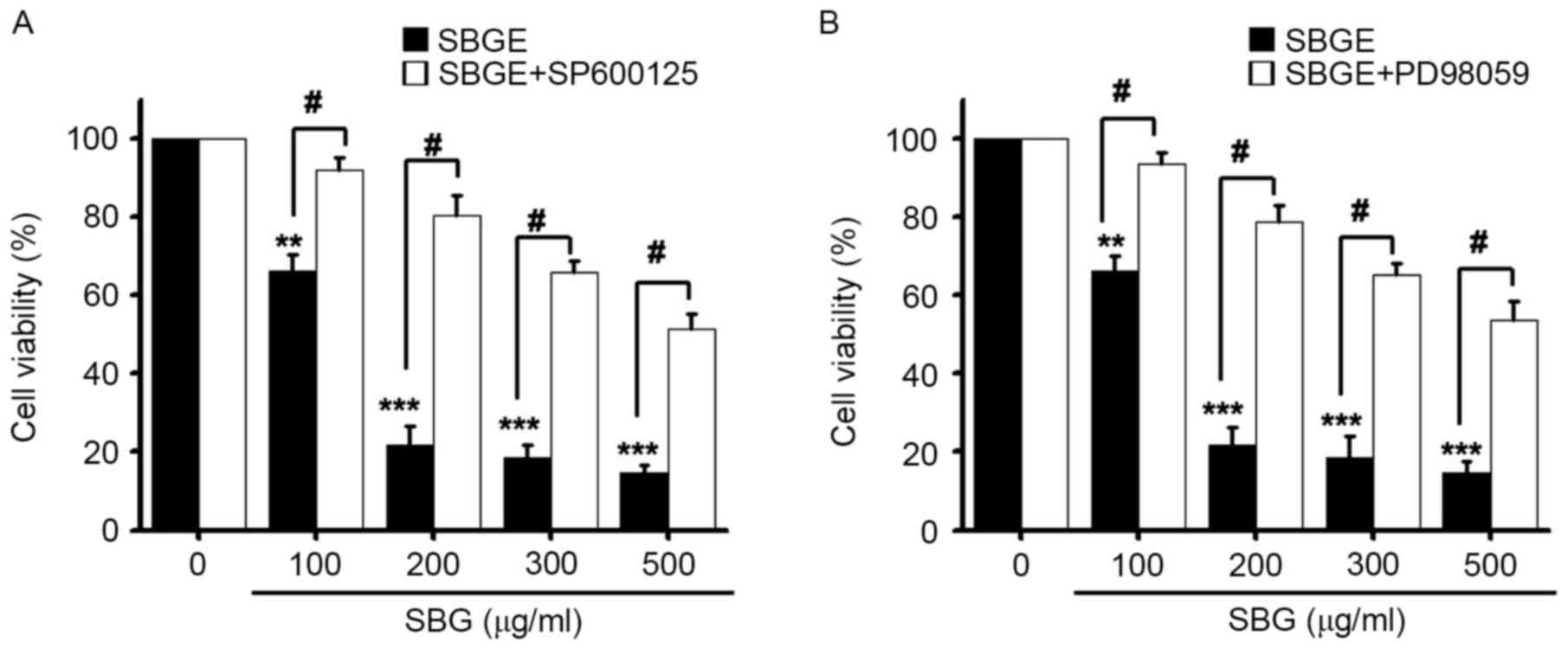
To investigate the association between the
regulation of MAPK pathways and the inhibition of MCF-7 cell
proliferation by SBGE, cell viabilities were measured following the
treatment of cells with SBGE at various concentrations (between 100
and 500 µg/ml) for 24 h with or without SP600125 (a JNK inhibitor)
or PD98059 (a MAPK inhibitor) using the MTT assay. Co-treatment
with 10 µM SP600125 or PD98059 markedly inhibited SBGE-induced cell
death, particularly when cells were co-treated with 200 µg/ml SBGE.
More specifically, co-treatment with 100, 200, 300 or 500 µg/ml
SBGE and SP600125 inhibited cell survival by 7.9±3.5, 19.5±4.9,
34.2±2.8 and 48.7±3.5, respectively, as determined by the MTT assay
(n=6; Fig. 4A). Co-treatment with
100, 200, 300 or 500 µg/ml of SBGE and PD98059 in culture medium
inhibited cell survival by 6.4±2.8, 21.1±3.5, 34.8±2.7 and
46.4±4.5%, respectively (n=6; Fig.
4B). These results suggested that JNK and MAPK are involved in
SBGE-induced apoptosis of MCF-7 cells.
SBGE-induced apoptosis is mediated by
the generation of intracellular ROS in MCF-7 cells
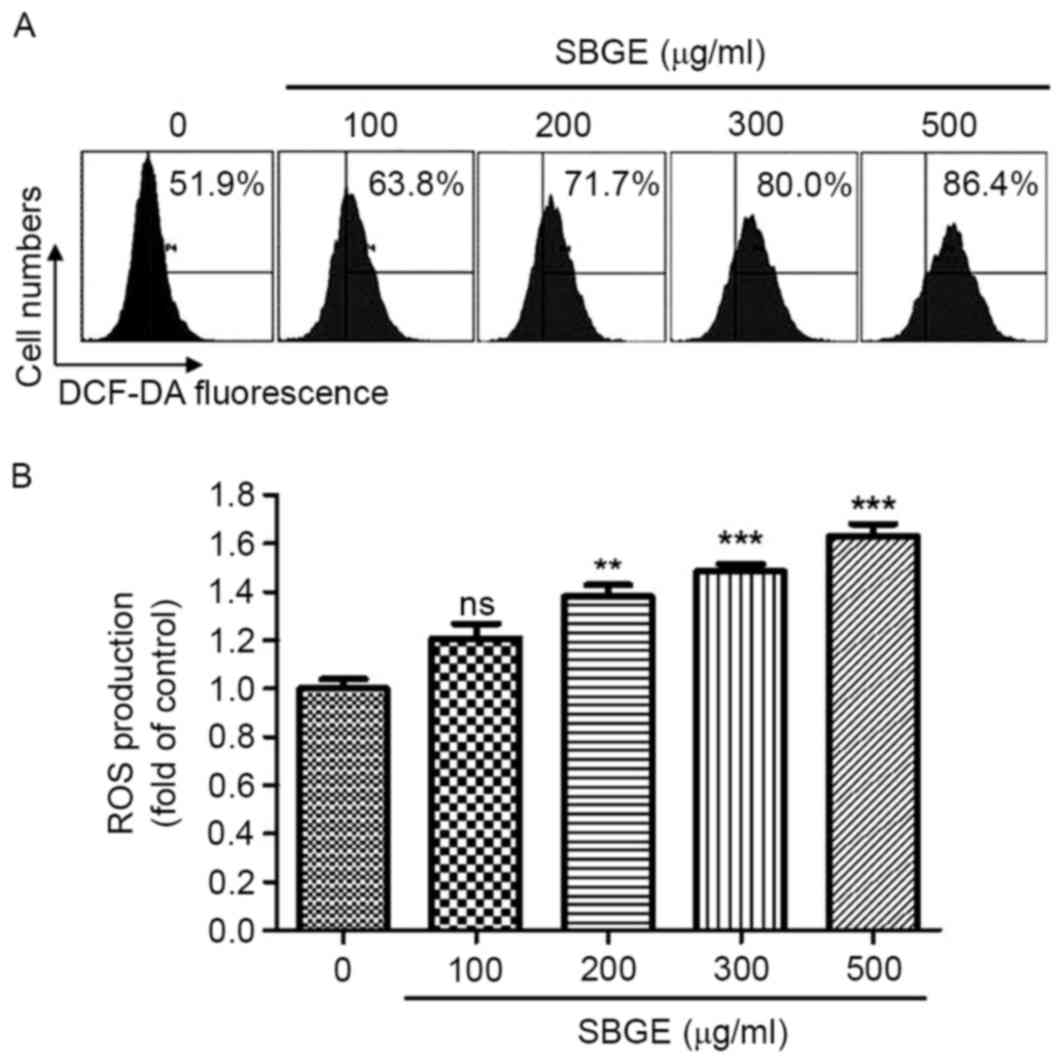
AS intracellular ROS serve a key role in apoptosis,
whether SBGE is able to generate ROS in MCF-7 cells was studied. To
investigate whether ROS generation was directly associated with
SBGE-induced apoptosis, intracellular ROS were investigated using
the fluorescent dye DCF-DA. As indicated in Fig. 5A, when the cells were exposed to
SBGE, levels of intracellular ROS increased. Furthermore, when
cells were treated with various concentrations (between 100 and 500
µg/ml) of SBGE for 24 h, ROS generation significantly and
dose-dependently increased, as demonstrated by flow cytometry
(Fig. 5B).
Discussion
SBG is used as medicinal plant in traditional herbal
medicine, particularly in China (22). The major materials isolated from
S. baicalensis are flavonoids, glycosides and the
glucuronides of flavonoids, including baicalin, baicalein and
wogonin. (22). Certain flavonoids
possess various pharmacological activities, including anti-oxidant,
anti-apoptotic, anti-allergic and anti-inflammatory effects
(22–24). In addition, S. baicalensis
and its flavonoids have been demonstrated to exhibit anticancer
effects in various types of cancer cells (11–15,25–30).
Although it has been reported that S. baicalensis inhibits
the proliferations of several cancer cell lines in vitro
(11–15,25–30),
its anti-cancer activity and associated mechanisms in human breast
adenocarcinoma MCF-7 cells remain unclear. Therefore, the present
study investigated the underlying mechanisms of this phenomenon and
identified SBGE-induced apoptotic signaling via mitochondrial- and
caspase-dependent pathways via ROS generation in human breast
cancer cells.
Apoptosis can be initiated through two signaling
pathways: Extrinsic (death receptor-mediated) and intrinsic
(mitochondria-mediated) pathways (31,32).
The extrinsic pathway is initiated by extracellular signals,
including FasL (33). The
intrinsic pathway is regulated by Bcl-2 family proteins
(anti-apoptotic proteins) (34).
Notably, Bax protein shares regions of homology with Bcl-2
(35,36). Additionally, the downregulation of
Bcl-2 proteins and the upregulation of Bax proteins may induce the
destruction of the mitochondrial outer membrane (37–39).
The caspases are major executors of apoptotic processes and belong
to a group of enzymes called cysteine proteases (40). In the current study, Bcl-2
expression was inhibited by SBGE, whereas Bax expression was
upregulated. However, Fas/FasL and TNF-α expression levels were
unchanged, which suggested that SBGE induces cell death via the
intrinsic pathway in MCF-7 human breast cancer cells. There are a
number of breast cancer cell line types including, MCF-7, HBL100,
MDAMB231, BT-20 and SKBR3 (41).
In the present study, however, the effects of SBGE were
investigated only in MCF-7 cells. The authors are now investigating
the effects of SBGE on other breast cancer cell line types.
Transient receptor potential (TRP) channels were
first cloned from Drosophila species and constitute a
superfamily of proteins that encode a diverse group of
Ca2+-permeable nonselective cation channels (NSCCs)
(42,43). Among the TRP channels, TRP
melastatin type 7 (TRPM7) channel expression is essential for cell
survival in breast adenocarcinoma cells and thus is also a
potential therapeutic target in breast cancer (44). Therefore, the effects of SBGE on
TRPM7 channels were investigated. However, no effects were observed
on overexpressed TRPM7 channels (data not shown). Thus, it is
hypothesized that the TRPM7 channel is not involved in SBGE-induced
anti-cancer effects in MCF-7 cells.
A number of cancer-associated components of the MAPK
signaling pathways have been identified in Ras and B-Raf, which
participate in the extracellular signal regulated kinase (ERK)
signaling pathway (45,46). This kinase cascade presents novel
opportunities for the development of novel cancer therapies
designed to be less toxic than conventional chemotherapeutic drugs
(47). The ERK signaling pathway
serves a function in several steps of tumor development and also
induces the expression of matrix metalloproteinases, thereby
promoting the degradation of extracellular matrix proteins and
consequent tumor invasion (48).
Therefore, components of signaling pathways activated by MAPK have
received great attention as potential targets for the development
of novel therapeutic drugs for cancer (49).
There is considerable interest among oncologists to
find anticancer drugs in TCM. In the past, clinical data
demonstrated that certain herbs possessed anticancer properties
(50,51); however, western scientists have
doubted the validity of TCM due to the lack of scientific evidence
(52). Recently, experiments have
demonstrated that elements of TCM may possess an anticancer role,
and clinical trials have demonstrated that TCM could improve
survival, increase tumor response, improve the quality of life and
reduce chemotherapy toxicity (52–56).
Therefore, the authors of the present study suggest that when
combined with chemotherapy, TCM could raise the efficacy level and
lower toxic reactions.
In conclusion, the present study demonstrated that
SBGE induces Bcl-2 protein downregulation and Bax protein
upregulation, and activates caspases 3 and 9, leading to apoptosis.
It was also identified that SBGE-induced cell death depends on the
ROS-mediated JNK/MAPK signaling pathway. Therefore, SBEG may cause
cell death via the intrinsic pathway in human breast cancer MCF-7
cells. These findings implicate SBEG as a useful potential
anticancer agent.
Acknowledgements
The present study was supported by a Korean National
Research Foundation (NRF) Grant funded by the Korean Government
(MSIP; grant no. 2014R1A5A2009936).
References
|
1
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Pham P: Breast Cancer Stem Cells &
Therapy Resistance. Springer; Berlin, Germany: 2015, View Article : Google Scholar
|
|
3
|
Stewart B and Wild C: World Cancer Report
2014International Agency for Research on Cancer. World Health
Organization; Geneva, Switzerland: 2014, View Article : Google Scholar
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartsch R, Wenzel C and Steger GG:
Trastuzumab in the management of early and advanced stage breast
cancer. Biologics. 1:19–31. 2007.PubMed/NCBI
|
|
6
|
Vici P, Colucci G, Gebbia V, Amodio A,
Giotta F, Belli F, Conti F, Gebbia N, Pezzella G, Valerio MR, et
al: First-line treatment with epirubicin and vinorelbine in
metastatic breast cancer. J Clin Oncol. 20:2689–2694. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Li C, Zheng H, Chen G and Hua B:
Therapeutic targets of traditional Chinese medicine for colorectal
cancer. J Tradit Chin Med. 36:243–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HL: Research progress of Scutellaria
baicalensis. Chem Eng Equip. 4:100–102. 2008.
|
|
9
|
Zhang L, Ravipati AS, Koyyalamudi SR,
Jeong SC, Reddy N, Smith PT, Bartlett J, Shanmugam K, Münch G and
Wu MJ: Antioxidant and anti-inflammatory activities of selected
medicinal plants containing phenolic and flavonoid compounds. J
Agric Food Chem. 59:12361–12367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HP, Son KH, Chang HW and Kang SS:
Anti-inflammatory plant flavonoids and cellular action mechanisms.
J Pharmacol Sci. 96:229–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi EO, Park C, Hwang HJ, Hong SH, Kim
GY, Cho EJ, Kim WJ and Choi YH: Baicalein induces apoptosis via
ROS-dependent activation of caspases in human bladder cancer 5637
cells. Int J Oncol. 49:1009–1018. 2016.PubMed/NCBI
|
|
12
|
Choi EO, Cho EJ, Jeong JW, Park C, Hong
SH, Hwang HJ, Moon SK, Son CG, Kim WJ and Choi YH: Baicalein
inhibits the migration and invasion of B16F10 mouse melanoma cells
through inactivation of the PI3K/Akt signaling pathway. Biomol Ther
(Seoul). 25:2013–221. 2017. View Article : Google Scholar
|
|
13
|
Yan H, Xin S, Wang H, Ma J, Zhang H and
Wei H: Baicalein inhibits MMP-2 expression in human ovarian cancer
cells by suppressing the p38 MAPK-dependent NF-κB signaling
pathway. Anticancer Drugs. 26:649–656. 2015.PubMed/NCBI
|
|
14
|
Chung H, Choi HS, Seo EK, Kang DH and Oh
ES: Baicalin and baicalein inhibit transforming growth
factor-β1-mediated epithelial-mesenchymal transition in human
breast epithelial cells. Biochem Biophys Res Commun. 458:707–713.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SD, Lee YJ, Baik JS, Han JY, Lee CG,
Heo K, Park YS, Kim JS, Ji HD, Park SI, et al: Baicalein inhibits
agonist- and tumor cell-induced platelet aggregation while
suppressing pulmonary tumor metastasis via cAMP-mediated VASP
phosphorylation along with impaired MAPKs and PI3K-Akt activation.
Biochem Pharmacol. 92:251–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saraste A and Pulkki K: Morphologic and
biochemical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuno T, Tsukamoto T, Hara A and Tanaka T:
Cancer chemoprevention through the induction of apoptosis by
natural compounds. J Biophys Chem. 3:156–173. 2012. View Article : Google Scholar
|
|
20
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang BJ, Won SJ, Yu ZR and Su CL: Free
radical scavenging and apoptotic effects of cordycepin sinensis
ractionated by supercritical carbon dioxide. Food Chem Toxicol.
43:543–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaire BP, Moon SK and Kim H: Scutellaria
baicalensis in stroke management: Nature's blessing in traditional
Eastern medicine. Chin J Integr Med. 20:712–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen WP, Xiong Y, Hu PF, Bao JP and Wu LD:
Baicalein inhibits MMPs expression via a MAPK-dependent mechanism
in chondrocytes. Cell Physiol Biochem. 36:325–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Lin G and Zuo Z: Pharmacological
effects and pharmacokinetics properties of Radix Scutellariae and
its bioactive flavones. Biopharm Drug Dispos. 32:427–445. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HZ, Leung HW, Lai MY and Wu CH:
Baicalein induced cell cycle arrest and apoptosis in human lung
squamous carcinoma CH27 cells. Anticancer Res. 25:959–964.
2005.PubMed/NCBI
|
|
26
|
Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD,
Choi CS, Nam JS and Jung JY: Antitumor actions of baicalein and
wogonin in HT-29 human colorectal cancer cells. Mol Med Rep.
6:1443–1449. 2012.PubMed/NCBI
|
|
27
|
Li HL, Zhang S, Wang Y, Liang RR, Li J, An
P, Wang ZM, Yang J and Li ZF: Baicalein induces apoptosis via a
mitochondrial-dependent caspase activation pathway in T24 bladder
cancer cells. Mol Med Rep. 7:266–270. 2013.PubMed/NCBI
|
|
28
|
Wang Z, Jiang C, Chen W, Zhang G, Luo D,
Cao Y, Wu J, Ding Y and Liu B: Baicalein induces apoptosis and
autophagy via endoplasmic reticulum stress in hepatocellular
carcinoma cells. Biomed Res Int. 2014:7325162014.PubMed/NCBI
|
|
29
|
Liu-Smith F and Meyskens FL: Molecular
mechanisms of flavonoids in melanin synthesis and the potential for
the prevention and treatment of melanoma. Mol Nutr Food Res.
60:1264–1274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M,
Dong Q, Liu Y and Xu H: The traditional Chinese medicine baicalein
potently inhibits gastric cancer cells. J Cancer. 7:453–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wajant H: The Fas signaling pathway: More
than a paradigm. Science. 296:1635–1636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moldoveanu T, Liu Q, Tocilj A, Watson M,
Shore G and Gehring K: The X-ray structure of a BAK homodimer
reveals an inhibitory zinc binding site. Mol Cell. 24:677–688.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suzuki M, Youle RJ and Tjandra N:
Structure of Bax: Coregulation of dimer formation and intracellular
localization. Cell. 103:645–654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Green SR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spierings D, McStay G, Saleh M, Bender C,
Chipuk J, Maurer U and Green DR: Connected to death: The
(unexpurgated) mitochondrial pathway of apoptosis. Science.
310:66–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim HJ, Wie J, So I, Jung MH, Ha KT and
Kim BJ: Menthol modulates pacemaker potentials through TRPA1
channels in cultured interstitial cells of cajal from murine small
intestine. Cell Physiol Biochem. 38:1869–1882. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim BJ, Lee GS and Kim HW: Involvement of
transient receptor potential melastatin type 7 channels on Poncirus
fructus-induced depolarizations of pacemaking activity in
interstitial cells of Cajal from murine small intestine. Integr Med
Res. 2:62–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guilbert A, Gautier M, Dhennin-Duthille I,
Haren N, Sevestre H and Ouadid-Ahidouch H: Evidence that TRPM7 is
required for breast cancer cell proliferation. Am J Physiol Cell
Physiol. 297:C493–C502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boutros T, Chevet E and Metrakos P:
Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase
regulation: Roles in cell growth, death, and cancer. Pharmacol Rev.
60:261–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sebolt-Leopold JS: Development of
anticancer drugs targeting the MAP kinase pathway. Oncogene.
19:6594–6599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: an
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheng YL, Chang WL, Lee SC, Liu YG, Lin
HC, Chen CJ, Yen CY, Yu DS, Lin SZ and Harn HJ: Acetone extract of
Bupleurum scorzonerifolium inhibits proliferation of A549 human
lung cancer cells via inducing apoptosis and suppressing telomerase
activity. Life Sci. 73:2383–2394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wartenberg M, Budde P, de Mareés M,
Grünheck F, Tsang SY, Huang Y, Chen ZY, Hescheler J and Sauer H:
Inhibition of tumor-induced angiogenesis and
matrix-metalloproteinase expression in confrontation cultures of
embryoid bodies and tumor spheroids by plant ingredients used in
traditional chinese medicine. Lab Invest. 83:87–98. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ruan WJ, Lai MD and Zhou JG: Anticancer
effects of Chinese herbal medicine, science or myth? J Zhejiang
Univ Sci B. 7:1006–1014. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mabed M, El-Helw L and Shamaa S: Phase II
study of viscum fraxini-2 in patients with advanced hepatocellular
carcinoma. Br J Cancer. 90:65–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu
YM, Shi JY, Zheng PZ, Yan H, Liu YF, et al: All-trans retinoic
acid/As2O3 combination yields a high quality remission and survival
in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci
USA. 101:5328–5335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Piao BK, Wang YX, Xie GR, Mansmann U,
Matthes H, Beuth J and Lin HS: Impact of complementary mistletoe
extract treatment on quality of life in breast, ovarian and
non-small cell lung cancer patients. A prospective randomized
controlled clinical trial. Anticancer Res. 24:303–309.
2004.PubMed/NCBI
|
|
56
|
Yan X, Shen H, Jiang H, Hu D, Zhang C,
Wang J and Wu X: External Qi of Yan Xin Qigong Induces apoptosis
and inhibits migration and invasion of estrogen-independent breast
cancer cells through suppression of Akt/NF-kB signaling. Cell
Physiol Biochem. 25:263–270. 2010. View Article : Google Scholar : PubMed/NCBI
|