Introduction
Spinal cord injury (SCI) is a highly disabling
insult of the central nervous system (CNS), which can be caused by
traffic accidents, falls, violence or sports-related injuries. It
can result in the damage of sensory and motor function, autonomic
nervous dysfunction, and can have effects on the mental health of
patients (1). Recovery following
SCI is difficult, due to the complexity of injury pathogenesis,
which can include primary and secondary injuries (2). The secondary injury can last for
several weeks or months following the initial spinal cord insult,
and disturbances in microcirculation (3), neuronal apoptosis and necrosis
(4–6), inflammatory response (7) and glial scar (8) may appear during this period. These
complications result from neuronal damage, demyelination of axons
and dysfunction of neuroglial cells, as the CNS is characterized by
limited capability for repair and regeneration (9).
The Wnt/β-catenin signaling pathway has been
identified as a crucial regulator of the growth and differentiation
and survival of neurons (10). Wnt
proteins are a large family of signaling proteins, which have been
implicated in the regulation of axonal growth, including the long
axons of spinal cord neurons (10). β-catenin is a critical component of
the canonical Wnt signaling pathway, which, when activated, can
translocate to the nucleus, where it can interact with
transcription factors to induce alterations in the expression of
target genes (11). The neuronal
nuclear antigen (NeuN) is a protein specifically expressed in
post-mitotic neurons. NeuN has been used as a marker of maturing
neurons, and has been applied in neuropathological studies to
investigate neuronal pathophysiology, as healthy neurons are
characterized by strong NeuN expression, whereas weak NeuN
expression in indicative of degeneration of differentiated neurons
(12).
Novel therapeutic approaches are currently being
developed for the treatment of patients with SCI, including stem
cell therapy (13,14), and electroacupuncture (EA)
treatment (15,16). Acupuncture has a long history of
use in traditional Chinese medicine, and EA has been reported to
promote the proliferation and differentiation of neuronal stem
cells (17). Several acupuncture
points for EA therapy have been evaluated for their effects on SCI
recovery. Choi et al (18)
reported that acupuncture targeted at Shuigou (GV26) and
Yanglingquan (GB34) points significantly alleviated neuronal
apoptosis and enhanced their recovery following SCI. Jiang et
al (16) compared different
modalities of acupuncture at Shuigou (DU26) and Fengfu (DU16)
points, and reported anti-inflammatory, antioxidative and
anti-apoptotic effects for EA.
Although EA has garnered much attention as a
potential therapeutic strategy for the treatment of patients with
SCI, its use remains limited, as the mechanisms underlying its
beneficial effects have yet to be elucidated. Therefore, the
present study aimed to investigate the effects of EA therapy on
SCI, and explore the involvement of the Wnt/β-catenin pathway in
the molecular mechanisms underlying EA-associated neuronal
recovery.
Materials and methods
Animal experiments
Specific pathogen-free male Sprague-Dawley (SD) rats
(age, 8 weeks; weight, 250±20 g) were obtained from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). All rats were
acclimated in sterile polypropylene cages under an ambient
temperature of 25±2°C, a relative humidity of 50–60% and a 12/12 h-
dark/light cycle with free access to food and water. Following 1
week of acclimation, rats were randomized into experimental groups.
The study was approved by the Ethics Committee of The Sixth
People's Hospital Affiliated to Shanghai Jiaotong University
(Shanghai, China).
After acclimation, 54 rats were randomly assigned to
three groups (n=18/group): The SCI group, which included rats that
underwent injury at the T9-T11 spinal segment; the sham group,
which included rats that received a laminectomy; and the normal
group, which included rats that were left untreated. SCI group rats
were randomly assigned into day 1, 7 and 14 subgroups (n=6/group).
Rats in the SCI, sham and normal groups were sacrificed on days 1,
3, 7 or 14 post-SCI surgery, and spinal cord tissue samples were
obtained to assess Wnt1 and Nestin expression.
In EA experiments, 96 rats received SCI surgery and
were then randomized into four groups (n=24/group): The SCI group,
which included rats that were left untreated following SCI surgery;
the SCI + EA group, which included rats that received EA treatment
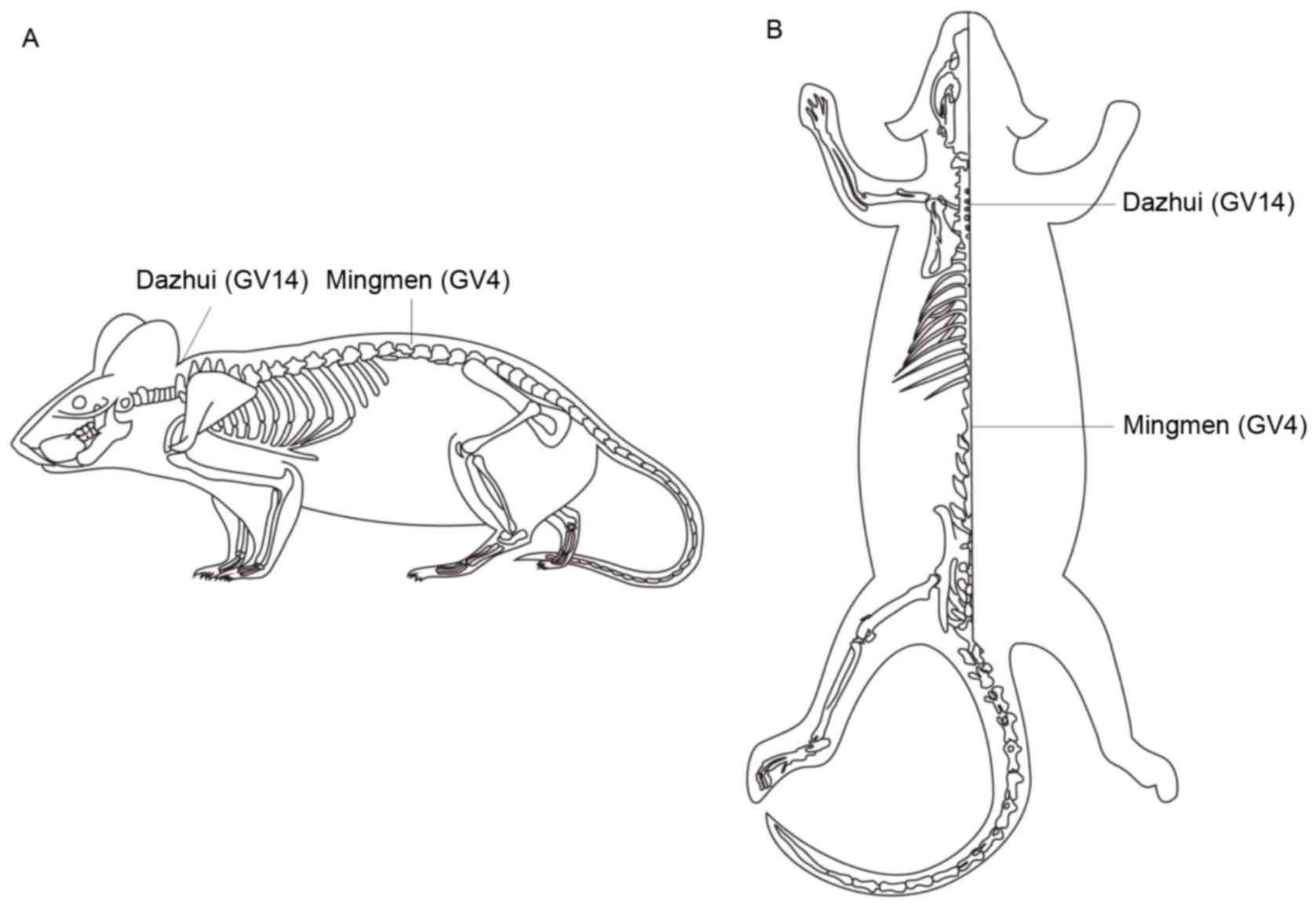
lasting 20 min at Dazhui (GV14) and Mingmen (GV4) acupoints every
day for 2 weeks following SCI surgery; the SCI + LiCl group, which
included rats that received an injection of 0.05 ml lithium
chloride (LiCl) (1 mol/l) at the injured spinal segment every 3
days for 2 weeks following SCI surgery; and the SCI + LiCl + EA
group, which included rats that received a LiCl injection and EA
treatment for 2 weeks following SCI surgery. Tissue samples from
the area of SCI were collected from 9 rats in each group and were
used to assess the expression of Wnt1, nuclear β-catenin and Nestin
on day 3 of treatment. Tissue samples were collected from another 9
rats in each group and were used to assess the expression of
Neuronal Nuclei (NeuN) on day 21 of treatment. The remaining 6 rats
in each group were used to evaluate the Basso, Beattie and
Bresnahan (BBB) score on day 28 of treatment.
SCI
Moderate SCI was established using the modified
Allen method, as previously described (19). Briefly, rats were anesthetized with
10% chloral hydrate (3.5 ml/kg, intraperitoneally), then a
laminectomy was performed at the T9-T11 level and the spinal cord
was exposed without disrupting the dura. Spinous processes of T9
and T11 were stabilized by clamps, and the exposed dorsal surface
of the spinal cord was subjected to contusion injury. The force of
contusion was 40 g × cm and was generated by a free drop, using
Allen's impactor (Peking Union Medical College Microcirculation
Institute, Beijing, China). Rats in the sham group only received
laminectomy without further intervention. In order to avoid direct
contact of the spinal cord wound with air, the wound was covered
with cotton soaked in saline. All surgical interventions and
postoperative animal care were in line with the guidelines and
rules provided by The Sixth People's Hospital Affiliated to
Shanghai Jiaotong University.
EA therapy
Rats belonging to the SCI + EA and SCI + LiCl + EA
groups received EA therapy lasting 20 min each day for 2 weeks. The
acupuncture points GV14 (Dazhui) and GV4 (Mingmen) were selected on
the basis of clinical acupuncture experience and were located as
described in Fig. 1. Stainless
steel Hwato brand acupuncture needles (0.3×25 mm; Suzhou Medical
Appliance Factory, Suzhou, China) were inserted at the selected
points to a depth of 5–7 mm and were connected to the output
terminals of a G6805-2 EA apparatus (Shanghai Medical Equipment
Works Co., Ltd., Shanghai, China). An operating frequency of 2 Hz
and a working current of 1 mA were used.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following the last EA treatment, rats were deeply
anesthetized with 10% chloral hydrate and sacrificed. T9-T11 spinal
cord segments, containing the injury sites, were dissected and
maintained at −80°C until used.
Total RNA was extracted from spinal cord samples
using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The concentration of extracted RNA was
determined using an ultraviolet spectrophotometer. Total RNA (500
ng) was reverse transcribed into cDNA using PrimeScript™
RT reagent (Takara Bio, Inc., Otsu, Japan). The reaction volume was
20 µl and the temperature protocol was as follows: Incubation at
25°C for 10 min, at 42°C for 30 min, at 95°C for 5 min and at 4°C
for 5 min. The primers used for PCR were as follows: Wnt1, forward
5′-TACCTCCAGTCACACTCCCC-3′, reverse 5′-CCATGGCAGGAGAATAGGAA-3′;
Nestin, forward 5′-GCGGGGCGGTGCGTGACTAC-3′, reverse
5′-AGGCAAGGGGGAAGAGAGAAGGATGT-3′; and GAPDH, forward
5′-ACAGCAACAGGGTGGTGGAC-3′ and reverse 5′-TTTGAGGGTGCAGCGAACTT-3′.
qPCR was performed using the SYBR PrimeScript RT-PCR kit (Takara
Bio, Inc.) with an ABI 7500 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction volume
was 25 µl, and contained 12.5 µl SYBR-Green mix, 0.5 µl of each
forward and reverse primer, 2 µl cDNA and 9.5 µl PCR grade sterile
water. Thermocycling conditions were as follows: Initial
denaturation at 95°C for 3 min, followed by 35 cycles at 95°C for
15 sec, at 62°C for 1 min, and at 72°C for 1 min. Each experiment
was performed three times. The relative expression levels of each
gene were normalized to GAPDH and were calculated using the
2−∆∆Cq method (20).
Western blot analysis
Total proteins from spinal cord tissue samples were
extracted using radioimmunoprecipitation assay lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and protein concentrations were determined using
bicinchoninic acid assay kits according to the manufacturer's
protocol (Thermo Fisher Scientific, Inc.). Equal amounts of protein
(30 µg) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. Membranes were blocked with 5%
non-fat milk in TBS containing 0.1% Tween-20 for 2 h at room
temperature and then incubated with the following primary
antibodies obtained from Abcam (Cambridge, UK): Anti-Wnt1 (cat. no.
ab15251; 1:1,000), anti-Nestin (cat. no. ab6142; 1:1,000),
anti-β-catenin (cat. no. ab6302; 1:5,000), anti-NeuN (cat. no.
ab177487; 1:1,000) and anti-GAPDH (cat. no. ab8245; 1:1,000) for 12
h at 4°C. Membranes were then incubated with goat anti-rabbit
horseradish peroxidase-conjugated immunoglobulin G secondary
antibody (cat. no. BA1055; 1:5,000; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) for 2 h at room temperature.
Protein bands were visualized using enhanced chemiluminescence
detection reagents (Thermo Fisher Scientific, Inc.). The optical
densities of the protein bands were semi-quantified using the image
analysis system ImageQuant™ LAS4000mini (GE Healthcare Life
Sciences, Chalfont, UK).
BBB score evaluation
In order to examine the functional deficits of SD
rats following SCI, the BBB method was employed. Normal male SD
rats without functional deficits (BBB score=21), were selected as a
control. Following spinal cord surgery, two trained observers who
were blind to the experimental conditions evaluated the grade of
each rat according to the BBB open field locomotion test (21). Hindlimb movement, body weight
support, foot placement and coordination were observed for 5 min to
determine the BBB score of each animal.
Statistical analysis
The statistical significance of the difference
between groups was assessed by one-way analysis of variance,
followed by a post hoc least significant difference range test.
Data are expressed as the mean ± standard error of the mean of at
least 3 independent experiments. P<0.05 was considered to
indicate a statistically significant difference. The analysis was
performed using GraphPad Prism software version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
Alterations in BBB score, and Wnt1 and
Nestin levels following SCI
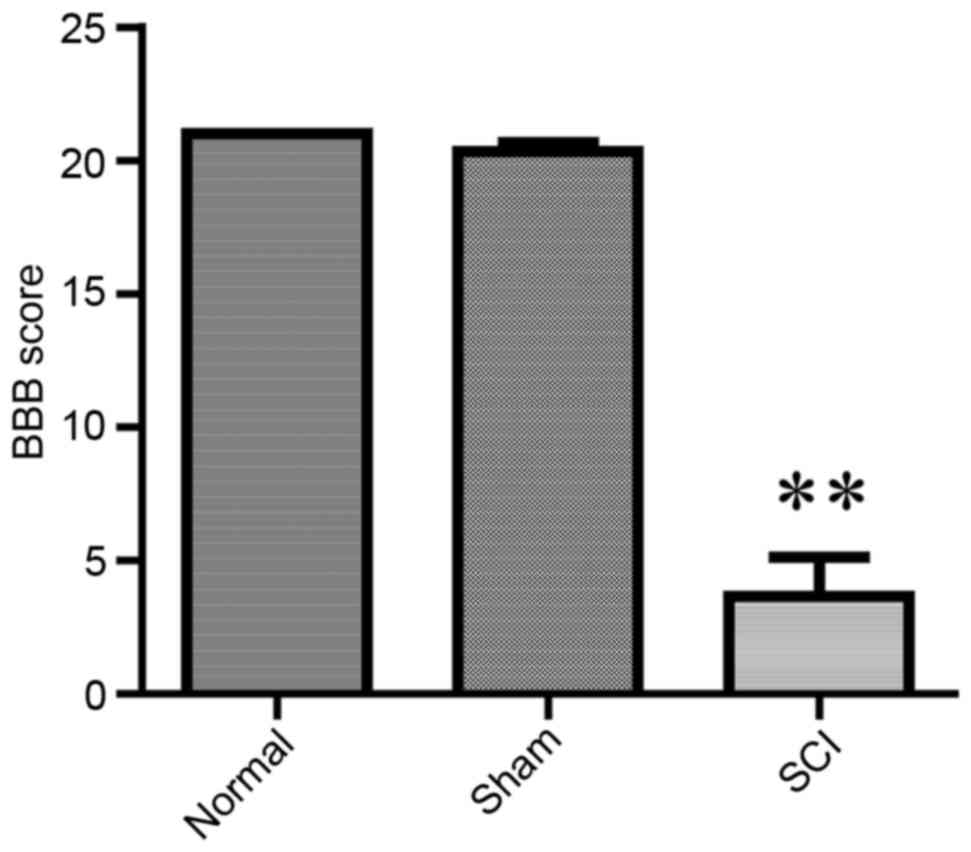
The BBB score has been widely used as a neurological
evaluation method, to assess recovery of functionality following
SCI (18,22). BBB scores were significantly lower
in SCI-treated rats compared with in control and sham rats
(P<0.01), indicating that the rat SCI model was established
successfully (Fig. 2).
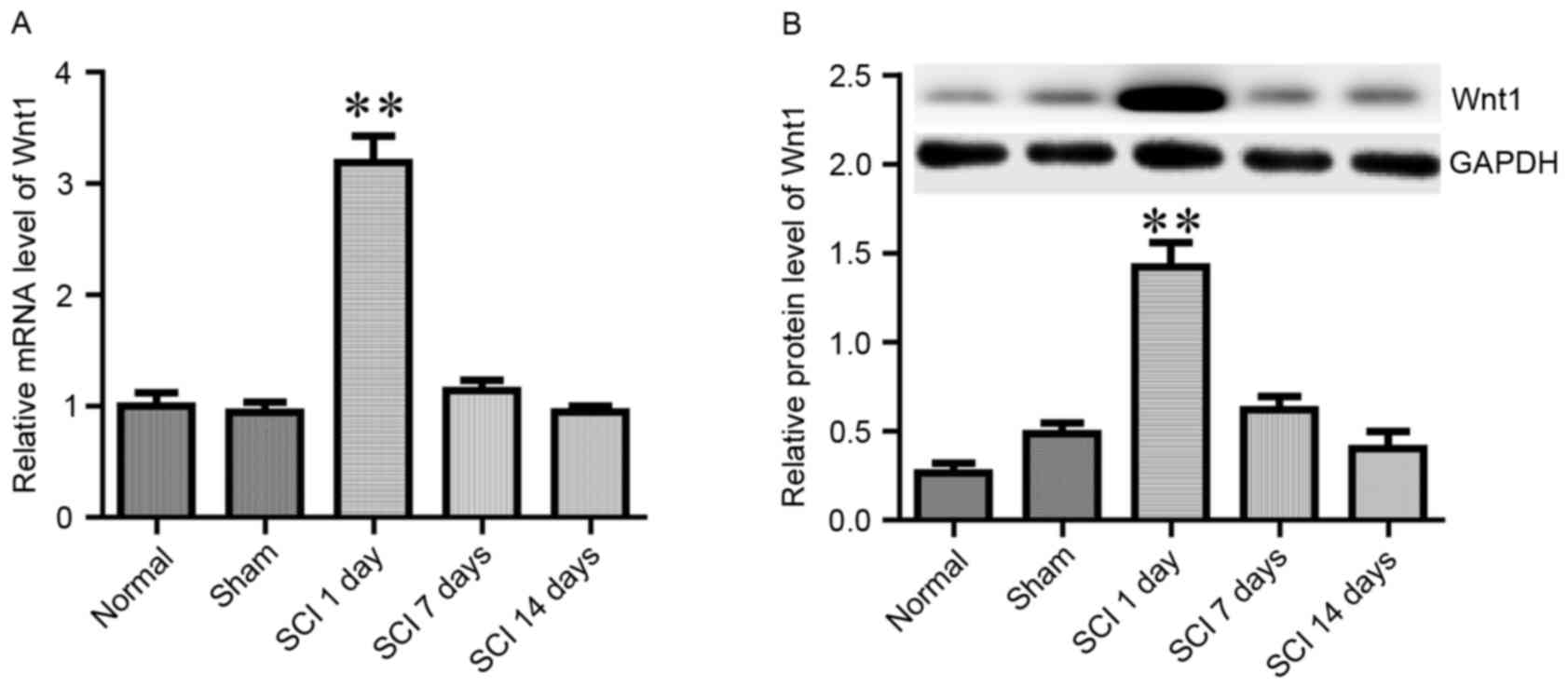
It has previously been reported that Wnt1 was
rapidly induced in the site of injury following SCI, and appeared
to serve a role in the regulation of axonal regeneration (23). In the present study, the mRNA and
protein expression levels of Wnt1 were assessed on day 1, 7 and 14
following SCI (Fig. 3). When
compared with normal and sham rats, the expression of Wnt1 was
revealed to be potently and rapidly induced on day 1 following SCI;
however, it returned to baseline during the following 2 weeks
(Fig. 3). These results are in
agreement with a previously published report by Liu et al
(23).
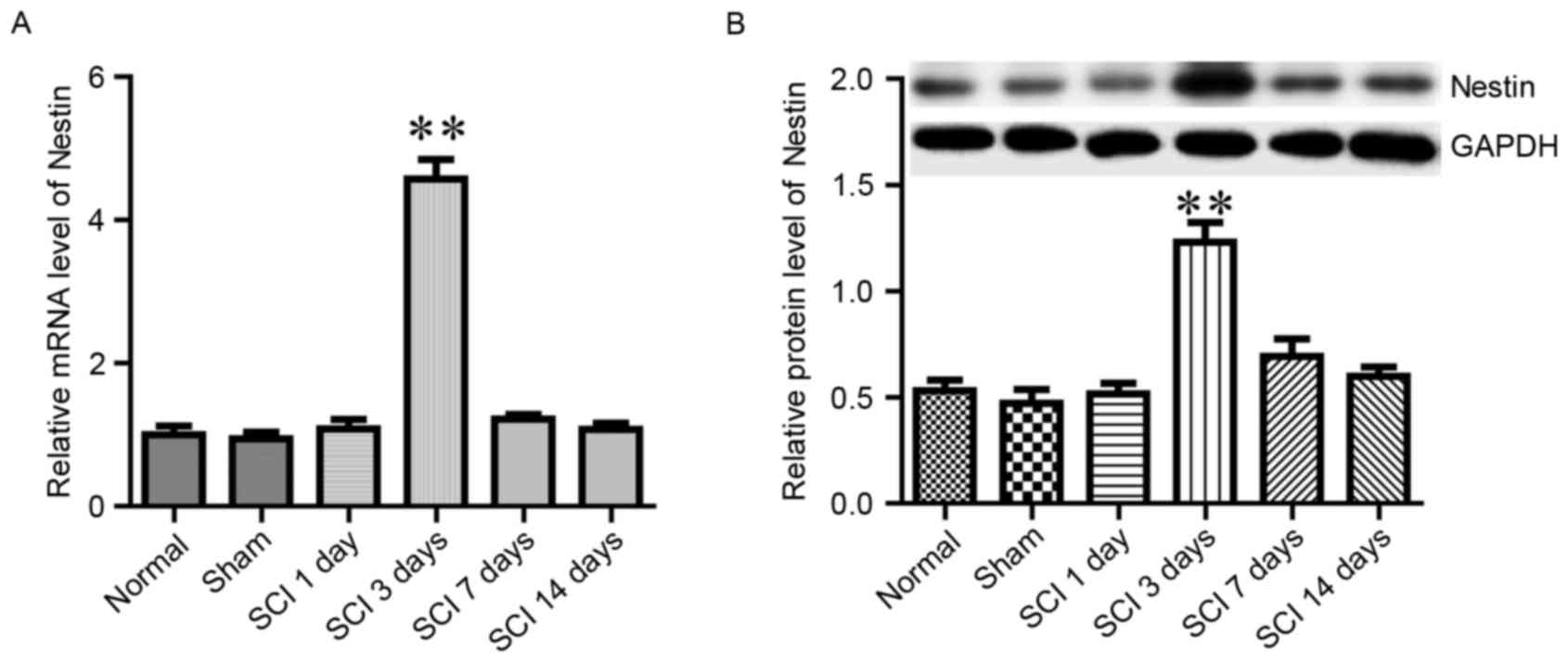
Nestin is a member of the intermediate filament
protein family, and it has been reported to promote the
proliferation and self-renewal of neural stem cells (24). The present study examined whether
Nestin mRNA and protein expression levels in spinal tissue were
altered in response to SCI. In the normal and sham groups, the
expression of Nestin remained at low levels (Fig. 4). However, in the SCI group, the
expression of Nestin was significantly increased on day 3 following
SCI, then returned to baseline levels during the following 2 weeks
(Fig. 4). The aforementioned
alterations in BBB score, as well as Wnt1 and Nestin levels,
demonstrated that the SCI model was successfully established.
Effects of EA on Wnt1, nuclear
β-catenin and Nestin levels on day 3 following SCI
Wnt1 and nuclear β-catenin protein expression levels
in rats that received EA therapy were significantly increased
compared with in untreated rats following SCI (P<0.01; Fig. 5), thus suggesting that EA may
enhance Wnt/β-catenin signaling. In order to test this hypothesis,
SCI rats were treated with LiCl, which inhibits glycogen synthase
kinase-3β and thus can potentiate Wnt signaling. Nuclear β-catenin
in the SCI + LiCl group appeared significantly upregulated compared
with in the SCI group. Furthermore, Wnt1 and nuclear β-catenin
protein levels were significantly higher in SCI rats treated with
EA and LiCl compared with in rats treated with LiCl alone (Fig. 5). These results indicated that the
mechanism underlying the beneficial effects of EA treatment on SCI
may involve the enhancement of Wnt/β-catenin signaling.
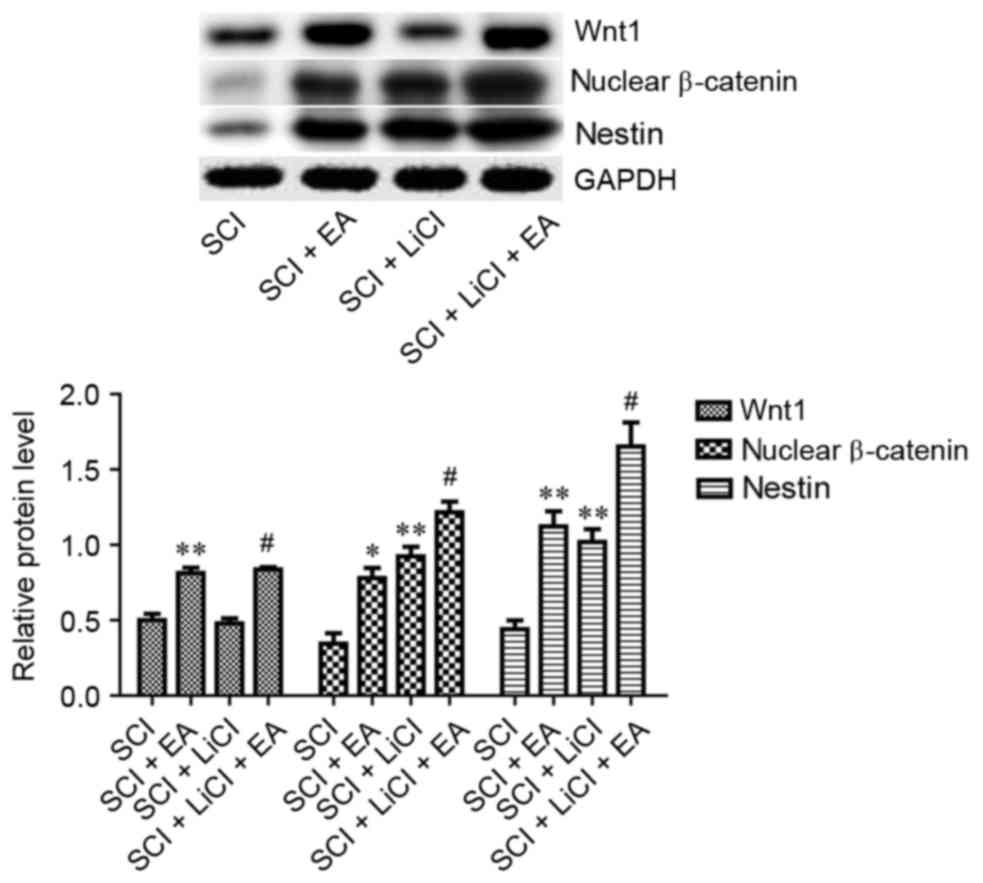 | Figure 5.Relative protein expression levels of
Wnt1, nuclear β-catenin and Nestin in spinal tissue from rats in
the SCI, SCI + EA, SCI + LiCl and SCI + LiCl + EA groups, as
assessed on day 3 following SCI. Data are expressed as the mean ±
standard error of the mean; n=9 rats/group. *P<0.05, **P<0.01
vs. the SCI group; #P<0.05 vs. the SCI + LiCl group.
SCI group, rats that received SCI and no treatment; SCI + EA group,
rats that received SCI and EA treatment; SCI + LiCl group, rats
that received SCI and LiCl treatment; SCI + LiCl + EA rats that
received SCI and a combination of EA and LiCl treatment. SCI,
spinal cord injury; EA, electroacupuncture; LiCl, lithium
chloride. |
Nestin expression in spinal tissue was significantly
increased on day 3 following SCI in rats receiving EA treatment
(Fig. 5). Furthermore, when
compared with in LiCl-treated rats, Nestin levels were
significantly higher in SCI rats that received a combination of
LiCl and EA treatment. These results suggested that EA may promote
neural recovery following SCI via regulating the expression of
Nestin.
Effects of EA on BBB score and NeuN
expression on day 21 following SCI
NeuN is a neuron-specific biomarker, and
fluctuations in its levels correspond to alterations in neuronal
numbers (25). NeuN expression in
EA-treated rats was significantly upregulated compared with in SCI
untreated rats (Fig. 6A and B).
Similarly, rats treated with a combination of LiCl and EA exhibited
significantly higher NeuN levels compared with rats treated with
LiCl alone following SCI (Fig. 6A and
B). These results suggested that the observed NeuN upregulation
was induced by EA therapy.
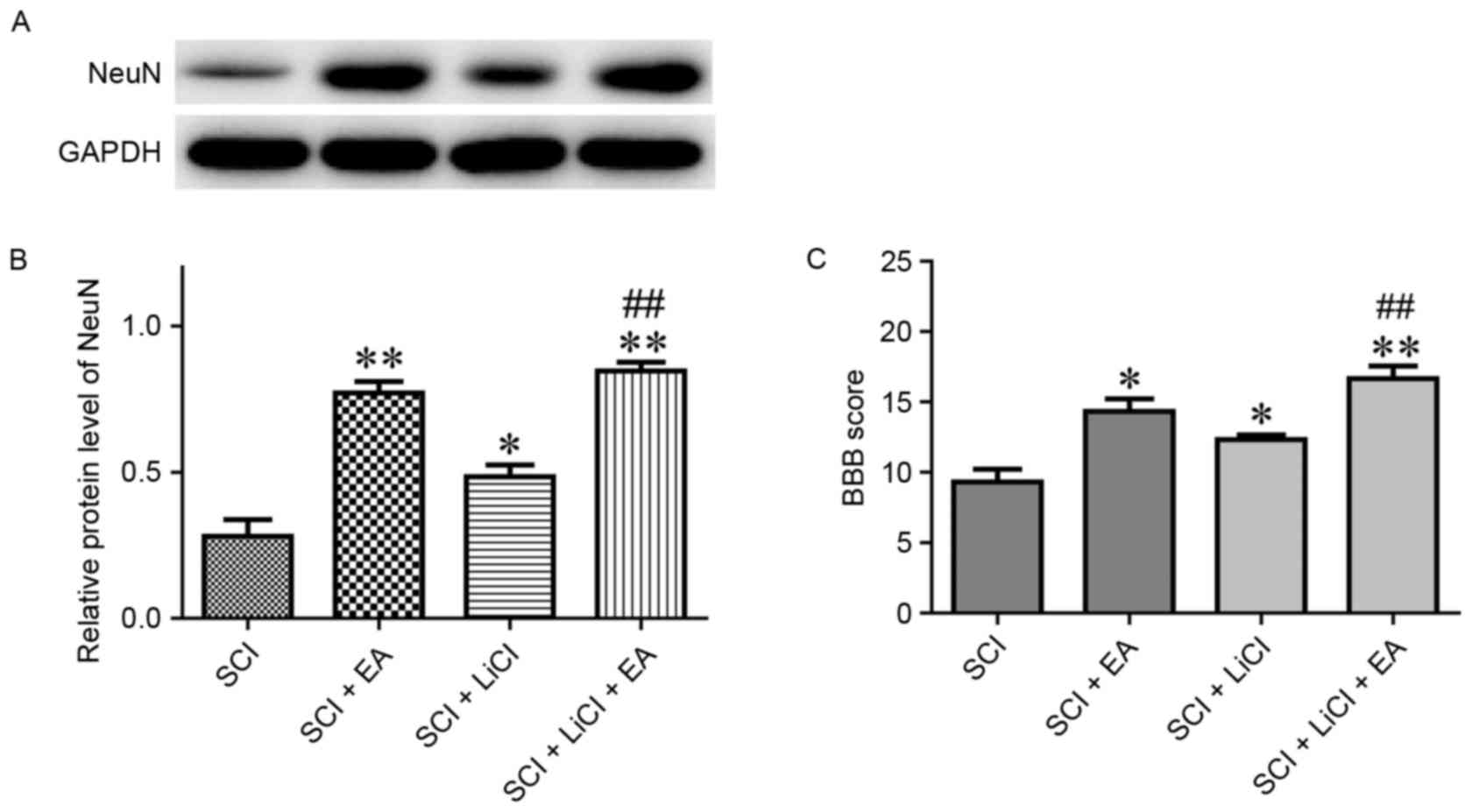 | Figure 6.(A) Relative protein expression levels
(B) quantifaction of NeuN in spinal tissue from rats in the SCI,
SCI + EA, SCI + LiCl and SCI + LiCl + EA groups, as assessed on day
21 following SCI. Data are expressed as the mean ± standard error
of the mean; n=9 rats/group. (C) BBB scores of SCI in rats in the
SCI, SCI + EA, SCI + LiCl and SCI + LiCl + EA groups, as assessed
on day 28 following SCI. Data are expressed as the mean ± standard
error of the mean; n=6 rats/group. *P<0.05, **P<0.01 vs. the
SCI group; ##P<0.01 vs. the SCI + LiCl group. SCI
group, rats that received SCI and no treatment; SCI + EA group,
rats that received SCI and EA treatment; SCI + LiCl group, rats
that received SCI and LiCl treatment; SCI + LiCl + EA rats that
received SCI and a combination of EA and LiCl treatment. NeuN,
Neuronal Nuclei; SCI, spinal cord injury; EA, electroacupuncture;
LiCl, lithium chloride; BBB, Basso, Beattie and Bresnahan. |
On day 28 following SCI, the BBB score was evaluated
across all groups. Rats that received EA treatment exhibited
significantly higher BBB scores compared with untreated rats
following SCI (Fig. 6C). Notably,
the effect of EA treatment on the BBB score appeared significantly
greater than the effect of LiCl therapy (Fig. 6C). These results suggested that EA
applied at Dazhui and Mingmen points may promote spinal recovery
following SCI in rats.
Discussion
The signaling pathways involved in neuronal recovery
and regeneration following SCI have garnered much attention. The
Wnt/β-catenin signaling pathway, which is highly conserved among
multicellular eukaryotic organisms, has been reported to
participate in the regulation of cellular proliferation (26), differentiation (27,28),
migration (29) and apoptosis
(30,31). Several Wnt genes have been
identified, including Wnt1, 2, 2b, 3 and 3a. The Wnt family has
been reported to serve an important role in the development of the
CNS, since it has previously been demonstrated that the
Wnt/β-catenin signaling pathway promoted the differentiation of
neural stem cells into neuronal cells, whereas it inhibited their
differentiation into astrocytes (32,33).
In the present study, EA therapy was revealed to
upregulate the expression of Wnt1 and nuclear β-catenin, thus
suggesting that EA treatment may enhance Wnt/β-catenin signal
transduction pathways. The nuclear accumulation of β-catenin can
activate the transcription of genes related to, among others,
cellular proliferation and terminal differentiation (34). Nestin is an intermediate filament
protein expressed in dividing cells during the early stages of
development in the CNS. Upon differentiation, Nestin expression
decreases and it is replaced by tissue-specific intermediate
filament proteins. In mature organisms, Nestin expression has been
revealed to be upregulated under pathological conditions, including
glial scar formation following CNS injury (24). In the present study, the expression
of Nestin appeared to be upregulated in EA-treated rats compared
with in untreated rats following SCI, thus suggesting that EA may
promote the proliferation of neural stem cells. In order to further
examine whether EA may promote neuronal proliferation, the
expression of the neuron-specific marker NeuN (35) was investigated. The present results
revealed that EA-treated rats exhibited significantly higher NeuN
levels compared with untreated rats following SCI. The increase in
Nestin and NeuN expression reported in the present study suggested
that EA treatment may promote the differentiation of neural stem
cells into neuronal cells in spinal tissue following SCI.
Therefore, it may be hypothesized that EA can promote neuronal
recovery following SCI, via enhancing Wnt/β-catenin signaling and
promoting the differentiation of neural stem cells into spinal
neurons.
The increased expression of Nestin and NeuN in
spinal tissue reported in the present study following EA therapy
did not directly reflect alterations in neural stem cell and
neuronal cell numbers. In conclusion, in the present study, EA
treatment appeared to promote spinal recovery following SCI, via
interfering with the Wnt1/β-catenin signaling pathway. Furthermore,
EA may promote the differentiation of neural stem cells into spinal
neurons, via enhancing Wnt1/β-catenin signaling; however, further
studies are required to investigate this hypothesis.
Acknowledgements
The present study was supported by the Shanghai
Cultivation Plan of New Stars in Xinglin (grant no.
ZYSNXD011-RC-XLXX-20130046), Lu's Acupuncture Inheritance Study of
Shanghai Schools of Traditional Chinese Medicine (grant no.
ZYSNXD-CC-HPGC-JD-004) and the Scientific Research Project of
Chinese Medicine of Shanghai Health and Family Planning Committee
(grant no. 2014LP026B).
References
|
1
|
McDonald JW and Sadowsky C: Spinal-cord
injury. Lancet. 359:417–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tator CH: Update on the pathophysiology
and pathology of acute spinal cord injury. Brain Pathol. 5:407–413.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sekhon LH and Fehlings MG: Epidemiology,
demographics, and pathophysiology of acute spinal cord injury.
Spine (Phila Pa 1976). 26 24 Suppl:S2–S12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crowe MJ, Bresnahan JC, Shuman SL, Masters
JN and Crowe MS: Apoptosis and delayed degeneration after spinal
cord injury in rats and monkeys. Nat Med. 3:73–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou J, Lenke LG, Ludwig FJ and O'Brien MF:
Apoptosis as a mechanism of neuronal cell death following acute
experimental spinal cord injury. Spinal Cord. 36:683–690. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osterholm JL and Mathews GJ: Altered
norepinephrine metabolism following experimental spinal cord
injury. 1. Relationship to hemorrhagic necrosis and post-wounding
neurological deficits. J Neurosurg. 36:386–394. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Donnelly DJ and Popovich PG: Inflammation
and its role in neuroprotection, axonal regeneration and functional
recovery after spinal cord injury. Exp Neurol. 209:378–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tysseling-Mattiace VM, Sahni V, Niece KL,
Birch D, Czeisler C, Fehlings MG, Stupp SI and Kessler JA:
Self-assembling nanofibers inhibit glial scar formation and promote
axon elongation after spinal cord injury. J Neurosci. 28:3814–3823.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gajos-Michniewicz A and Czyz M: Modulation
of WNT/β-catenin pathway in melanoma by biologically active
components derived from plants. Fitoterapia. 109:283–292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YB, Wang XF, Lu CC, Sherman R, Steward
O, Xu XM and Zou Y: Repulsive Wnt signaling inhibits axon
regeneration after CNS injury. J Neurosci. 28:8376–8382. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zechner D, Fujita Y, Hülsken J, Müller T,
Walther I, Taketo MM, Crenshaw EB III, Birchmeier W and Birchmeier
C: beta-Catenin signals regulate cell growth and the balance
between progenitor cell expansion and differentiation in the
nervous system. Dev Biol. 258:406–418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lavezzi AM, Corna MF and Matturri L:
Neuronal nuclear antigen (NeuN): A useful marker of neuronal
immaturity in sudden unexplained perinatal death. J Neurol Sci.
329:45–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Obermair FJ, Schröter A and Thallmair M:
Endogenous neural progenitor cells as therapeutic target after
spinal cord injury. Physiology (Bethesda). 23:296–304. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okano H, Ogawa Y, Nakamura M, Kaneko S,
Iwanami A and Toyama Y: Transplantation of neural stem cells into
the spinal cord after injury. Semin Cell Dev Biol. 14:191–198.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yan Q, Ruan JW, Ding Y, Li WJ, Li Y and
Zeng YS: Electro-acupuncture promotes differentiation of
mesenchymal stem cells, regeneration of nerve fibers and partial
functional recovery after spinal cord injury. Exp Toxicol Pathol.
63:151–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang SH, Tu WZ, Zou EM, Hu J, Wang S, Li
JR, Wang WS, He R, Cheng RD and Liao WJ: Neuroprotective effects of
different modalities of acupuncture on traumatic spinal cord injury
in rats. Evid Based Complement Alternat Med. 2014:4315802014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YY, Zhang W, Chen YL, Chen SJ, Dong H
and Zeng YS: Electro-acupuncture improves survival and migration of
transplanted neural stem cells in injured spinal cord in rats.
Acupunct Electrother Res. 33:19–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi DC, Lee JY, Moon YJ, Kim SW, Oh TH
and Yune TY: Acupuncture-mediated inhibition of inflammation
facilitates significant functional recovery after spinal cord
injury. Neurobiol Dis. 39:272–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faden AI and Simon RP: A potential role
for excitotoxins in the pathophysiology of spinal cord injury. Ann
Neurol. 23:623–626. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SM, Yune TY, Kim SJ, Park DW, Lee YK,
Kim YC, Oh YJ, Markelonis GJ and Oh TH: Minocycline reduces cell
death and improves functional recovery after traumatic spinal cord
injury in the rat. J Neurotrauma. 20:1017–1027. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Wang X, Lu CC, Kerman R, Steward O,
Xu XM and Zou Y: Repulsive Wnt signaling inhibits axon regeneration
after CNS injury. J Neurosci. 28:8376–8382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park D, Xiang AP, Mao FF, Zhang L, Di CG,
Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, et al: Nestin is required for
the proper self-renewal of neural stem cells. Stem Cells.
28:2162–2171. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mullen RJ, Buck CR and Smith AM: NeuN, a
neuronal specific nuclear protein in vertebrates. Development.
116:201–211. 1992.PubMed/NCBI
|
|
26
|
Dravid G, Ye Z, Hammond H, Chen G, Pyle A,
Donovan P, Yu X and Cheng L: Defining the role of Wnt/beta-catenin
signaling in the survival, proliferation, and self-renewal of human
embryonic stem cells. Stem Cells. 23:1489–1501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirabayashi Y, Itoh Y, Tabata H, Nakajima
K, Akiyama T, Masuyama N and Gotoh Y: The Wnt/beta-catenin pathway
directs neuronal differentiation of cortical neural precursor
cells. Development. 131:2791–2801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/beta-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aman A and Piotrowski T: Wnt/beta-catenin
and Fgf signaling control collective cell migration by restricting
chemokine receptor expression. Dev Cell. 15:749–761. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen S, Guttridge DC, You Z, Zhang Z,
Fribley A, Mayo MW, Kitajewski J and Wang C: Wnt-1 signaling
inhibits apoptosis by activating beta-catenin/T cell
factor-mediated transcription. J Cell Biol. 152:87–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Almeida M, Han L, Bellido T, Manolagas SC
and Kousteni S: Wnt proteins prevent apoptosis of both uncommitted
osteoblast progenitors and differentiated osteoblasts by
beta-catenin-dependent and-independent signaling cascades involving
Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem.
280:41342–41351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang F, Huang Q, Lan Q and Li ST:
Expression of Wnt-1 gene in the course of th human embrynoic neural
stem cells differentiating into neurons. Chinese Journal of
Neurosurgery. 20:409–412. 2005.
|
|
33
|
Davidson KC, Adams AM, Goodson JM,
McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ and Moon
RT: Wnt/β-catenin signaling promotes differentiation, not
self-renewal, of human embryonic stem cells and is repressed by
Oct4. Proc Natl Acad Sci USA. 109:4485–4490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wolf HK, Buslei R, Schmidt-Kastner R,
Schmidt-Kastner PK, Pietsch T, Wiestler OD and Blümcke I: NeuN: A
useful neuronal marker for diagnostic histopathology. J Histochem
Cytochem. 44:1167–1171. 1996. View Article : Google Scholar : PubMed/NCBI
|