Introduction
Soybean is one of the most important sources of
dietary protein and lipid worldwide; however, soybean is also one
of the commonest food allergens. An immunological study using the
sera of patients with soybean allergies identified 16
immunoglobulin (Ig)E-binding components (1). Among them, Gly m Bd 68K, Gly m Bd 30K
and Gly m Bd 28K proteins in the 7S globulin (β-conglycinin)
fraction are considered to be the major soybean allergens (1). Gly m Bd 68K was identified as the
α-subunit of β-conglycinin (2–4),
which is main storage protein. Gly m Bd 30K was demonstrated to be
the previously identified 34 kDa soybean seed vacuolar
oil-body-associated glycoprotein P34, and a thiol protease family
of the papain superfamily (1,5). Gly
m Bd 28K is a glycoprotein with an asparagine-linked sugar moiety
(1,6,7). In
addition, Gly m 4 was reported that is related to cross reaction
with pollenosis (8), and oleosin
which is oil body membrane protein is reported as major allergen of
sesame (9–12) and peanuts (9,12–14).
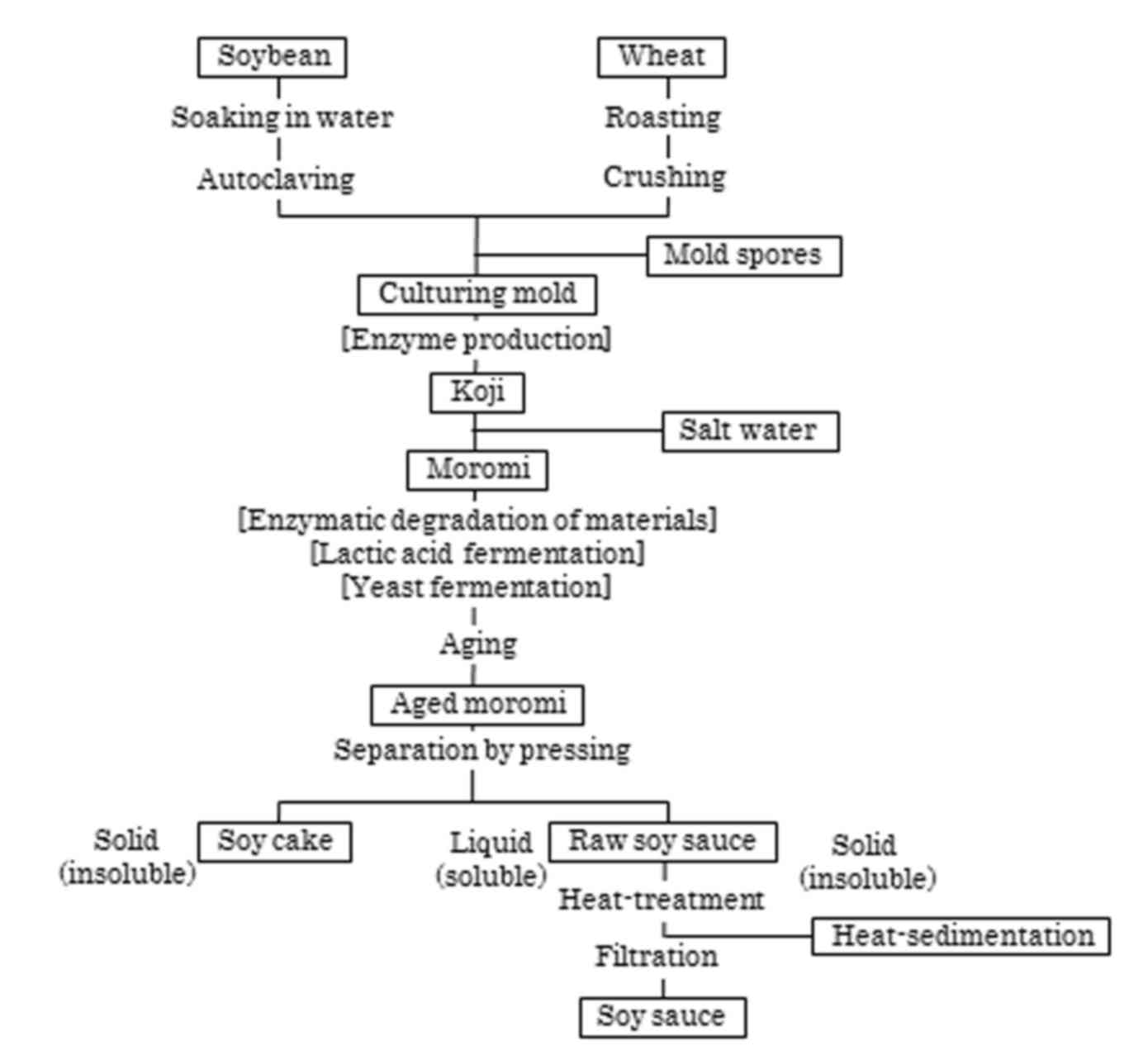
Soy sauce, or shoyu, is a traditional Japanese
fermented seasoning that is available worldwide (15). In Japanese soy sauce, the two
primary raw materials, soybeans and wheat, are used in roughly
equal amounts. Naturally brewed soy sauce is prepared as presented
in Fig. 1 (16–19).
Briefly, the soybeans are steamed under conditions of high pressure
and temperature, and wheat undergoes roasting and crushing. The
soybeans and wheat are combined and a small amount of mold spores
(Aspergillus oryzae or A. sojae) is added, and the
mixture is subsequently placed on a large, porous plate. To provide
optimal conditions for the growth of the mold and enzyme
production, temperature- and moisture-controlled air is passed
through the plate. Koji is the term used to describe the
mold-cultured mixture, and the koji is subsequently mixed
with water containing a high concentration of NaCl to produce a
mash, which is termed moromi. The moromi is stored
for several months in large tanks at room or an elevated
temperature. During storage, the moromi is fermented with
lactobacilli and yeasts, and is well aged. Following aging, the
moromi is pressed and the liquid portion, which is referred
to as raw soy sauce, is pasteurized by heat treatment. The
heat-treatment process functions to deactivate microbial enzymes
and to insolubilize the residual proteins in the raw soy sauce. The
insoluble proteins are completely removed by filtration to obtain
the final soy sauce product. During fermentation by microbial
proteolytic enzymes, allergens from the raw materials are also
degraded into peptides and amino acids; therefore, wheat and major
soybean allergens (Gly m Bd 30K and Gly m Bd 28K) are not detected
in soy sauce (19–22). However, soy sauce contains certain
bioactive components, in addition to taste and aroma compounds; for
example, certain polysaccharides, termed shoyu polysaccharides
(SPS), originating from the cell wall of soybeans are resistant to
enzymatic hydrolyses and remain even after fermentation (23). These SPS exhibit potent
antiallergic activities (23–25),
enhancing macrophage and lymphocyte functions (26), iron absorption promotion (27) and hypolipidemic effects (28) in vitro and in
vivo.
The present study investigated the degradation of
soybean proteins during fermentation and the presence of soybean
allergens in commercial raw soy sauce in Japan by immunoblot
analysis using anti-soybean protein antibody from rabbit and sera
from 2 children with soybean allergy. The results demonstrated that
soybean allergens remained in the raw soy sauce and were not
completely degraded; soybean allergens were detected in 3 out of 7
commercial raw soy sauces in Japan. The soluble soybean allergens
in raw soy sauce are denatured to insoluble allergens by heat
treatment, and then removed by filtration. Therefore, in addition
to the degradation of raw materials during fermentation,
heat-treatment and filtration are very important processes for the
low allergenicity of soy sauce.
Materials and methods
Soy sauce
Soy sauce was prepared by Higashimaru Shoyu Co. Ltd.
(Tastuno, Japan) as previously described (19). To examine soybean proteins during
the brewing of soy sauce, raw soy sauce (salt-soluble fractions of
soy sauce) was obtained from each moromi at 2 weeks, 2 months and 6
months after fermentation has begun. The raw soy sauce liquid
fraction was pressed from each moromi, and then raw soy sauce was
pasteurized by heat-treatment at >85°C. The heat-treated soy
sauce was centrifuged at 21,500 × g for 10 min to obtain a
supernatant (the heat-treated soy sauce fraction). The solid
portion remaining after centrifugation (the heat-denatured
sedimentation product) was washed twice with 18% NaCl, and
extracted with 0.1 M Tris-HCl buffer (pH 8.6) containing 4 M urea
for 30 min (19), followed by
centrifugation at 21,500 × g for 10 min to obtain the
heat-denatured sedimentation fraction.
In addition, 7 items of commercial raw soy sauce in
Japan (including 4 typical kinds of soy sauce: ‘Koikuchi’, dark soy
sauce; ‘usukuchi’, light soy sauce; ‘tamari’, very dark soy sauce;
and, ‘saishikomi’, refermented soy sauce) were also examined.
Koikuchi is dark in color and made from equal amounts of soybeans
and wheat. Usukuchi is also made from a mixture containing
approximately equal amounts of soybeans and wheat; however, rice
and gluten may also be added. The principles of the preparation of
usukuchi and koikuchi are similar, but all the procedures are
directed towards producing a lighter color in the final usukuchi
product. Tamari is very dark in color and is made primarily from
soybeans with a small quantity of wheat. Saishikomi is made from
the moromi mash mixed with both the koji and raw soy
sauce instead of more commonly used salt water.
Sera
The present study was approved by the institutional
ethics committee of Higashimaru Shoyu Co., Ltd., and informed
consent was obtained from each patient or her parents. Sera were
obtained from 2 female patients (aged 11 and 9 years) with soybean
allergy at the Department of Pediatrics, Kansai Medical University
(Hirakata, Japan). The soybean-specific IgE titers of the patients
were 1.01 and 10.9 IU/ml, respectively, as estimated with a
radioallergosorbent test (RAST; CAP System FEIA, Pharmacia
Diagnostics, Thermo Fisher Scientific, Inc., Uppsala, Sweden), the
RAST score was 2–3 for soybean allergy according to the
manufacturer's protocol. Sera were used for immunoblotting analysis
following 50 fold dilution with distilled water.
Protein electrophoresis and
immunoblotting
Proteins were separated by 14% SDS-PAGE according to
the method described by Laemmli (29). To prevent thermal denaturation of
samples, 20 µl of sample buffer [62.5 mM Tris-HCl (pH 6.8), 1% SDS,
5% 2-mercaptoethanol, 10% glycerol and 0.01% bromophenol blue] was
added to 80 µl of soy sauce samples and incubated at 37°C
overnight. The denatured sample was applied in 10 µl aliquots and
the molecular weight marker used was Protein Molecular Weight
Marker (Low; 3450; Takara Bio, Inc., Shiga, Japan). The gel was
divided into 2 parts. Proteins separated on one part of the gel
were stained with Coomassie Brilliant Blue R-250 dye (Quick-CBB for
electrophoresis; Wako Pure Chemical Industries, Ltd., Osaka, Japan)
and those separated on the other part of the gel were transferred
electrophoretically onto a polyvinylidene difluoride membrane
(AE-6666; Atto Corporation, Tokyo, Japan) for 1 h at a fixed
current of 100 mA. For immunoblotting of soybean protein, the
membrane was blocked overnight with 1% bovine serum albumin (BSA;
FIA/RIA grade, essentially globulin free; Nacalai Tesque, Inc.,
Kyoto, Japan) in PBS-Tween-20 (0.1%; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 4°C. The proteins on the membrane were
probed with primary rabbit anti-soybean protein polyclonal antibody
(S2519; 1:5,000; Sigma-Aldrich; Merck KGaA) diluted with
PBS-Tween-20 at 25°C overnight. After washing for 30 min with 1%
BSA in PBS-Tween-20 (0.1%), the membrane was incubated with
secondary biotinylated goat anti-rabbit IgG (PK-4001; Vectastain®
ABC HRP Rabbit IgG kit; Vector Laboratories, Inc., Burlingame, CA,
USA) following 5,000 fold dilution with PBS-Tween-20 (0.1%) at 25°C
for 1 h, according to the manufacturer's protocol. After washing
for 30 min with 1% BSA in PBS-Tween-20 (0.1%), the membrane was
incubated with an avidin-biotinylated enzyme complex-peroxidase
(PK-4001; Vectastain® ABC HRP Rabbit IgG kit; Vector Laboratories,
Inc.) at 25°C for 30 min. After washing for 30 min with 1% BSA in
PBS-Tween-20 (0.1%), detection was performed by the addition of
3,3′-diaminobenzidine tetrahydrochloride (DAB; SK-4100; DAB
Peroxidase HRP Substrate kit; Vector Laboratories, Inc.), according
to the manufacturer's protocol.
For immunoblotting of soybean allergen, the membrane
(AE-6666; Atto Corporation) was blocked for 3 h with 1% BSA in
PBS-Tween-20 (0.1%) at 4°C. The proteins on the membrane were
probed with the sera of patients at 25°C for 3 h. After washing for
30 min with 1% BSA in PBS-Tween-20 (0.1%), the membrane was
incubated with biotinylated goat anti-human IgE (BA-3040; Vector
Laboratories, Inc.) at 25°C for 1 h, according to the protocol of
this kit. After washing for 30 min with PBS-Tween-20 (0.1%), the
membrane was incubated with the avidin-biotinylated enzyme
complex-peroxidase at 25°C for 30 min, and subsequently washed for
30 min with 1% BSA in PBS-Tween-20 (0.1%). Detection was performed
by the addition of DAB. All experiments were repeated ≥2 times.
Results and Discussion
Soybean protein degradation during soy
sauce fermentation
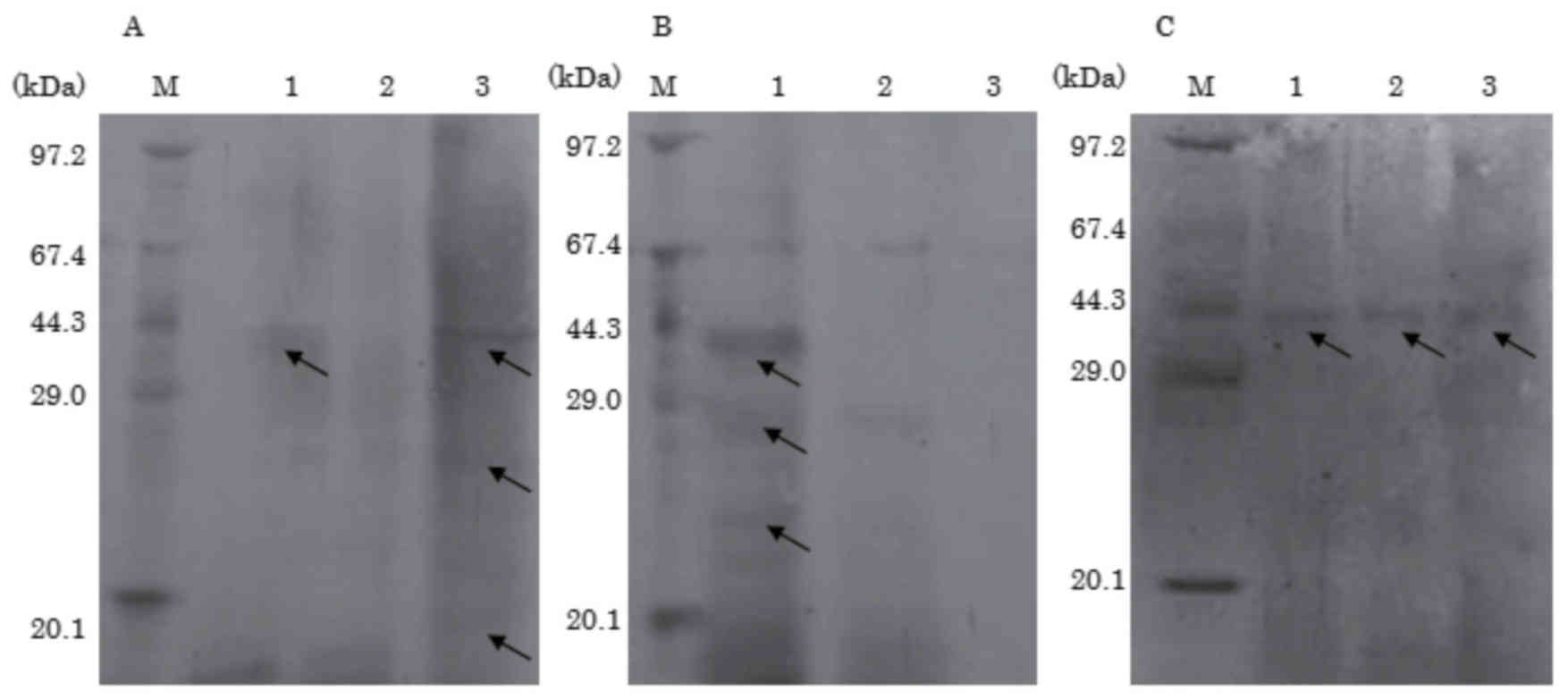
To examine the degradation of soybean proteins
during soy sauce fermentation, immunoblotting analyses of raw soy
sauce from each moromi at 2 weeks, 2 months and 6 months of
fermentation were performed using rabbit anti-soybean protein
antibody. Soybean proteins from the raw materials are gradually
degraded and solubilized during fermentation, but Gly m Bd 30K and
partially degraded soybean proteins remained in raw soy sauce of
the 6 months moromi and soybean proteins were not completely
degraded even after fermentation (Fig.
2A). Following heat treatment (85°C) and filtration to separate
it into the heat-treated soy sauce and the heat-denatured
sedimentation fraction, no soybean protein was detected in the
heat-treated soy sauce of the 6 months moromi, although soybean
proteins (e.g., Gly m Bd 30K and partly degraded proteins) were
detected in the heat-treated soy sauce of the 2 weeks and 2 months
moromi (Fig. 2B). It was regarded
as an effect of thermal denaturation that more soybean protein was
detected in raw soy sauce compared with heat-treated soy sauce. In
the heat-sedimentation fraction, soybean proteins were detected in
the moromi at 2 weeks, 2 months and 6 months (Fig. 2C), indicating that the soluble
soybean proteins were denatured to insoluble soybean proteins by
heat-treatment and then removed completely by filtration.
Soybean allergens in commercial raw
soy sauce
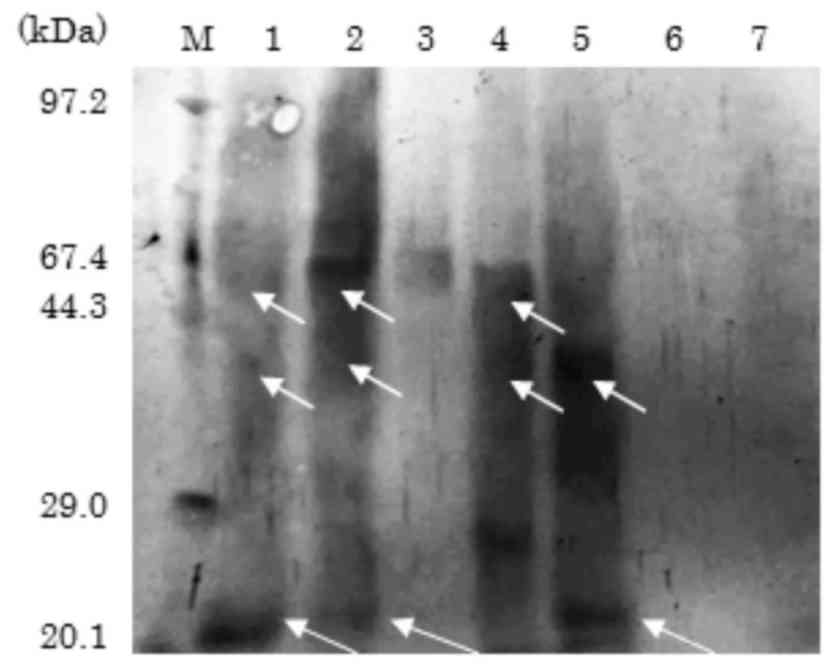
The majority of soy sauce produced in Japan is
heat-treated; however, a certain amount of raw soy sauce is also
produced. The present study investigated 7 commercially available
raw soy sauce in Japan. Immunoblot analysis with the rabbit
anti-soybean protein antibody detected soybean proteins in 4 of the
7 sauces, including 3 types of koikuchi (lanes 1, 2 and 4) and the
saishikomi (lane 5; Fig. 3).
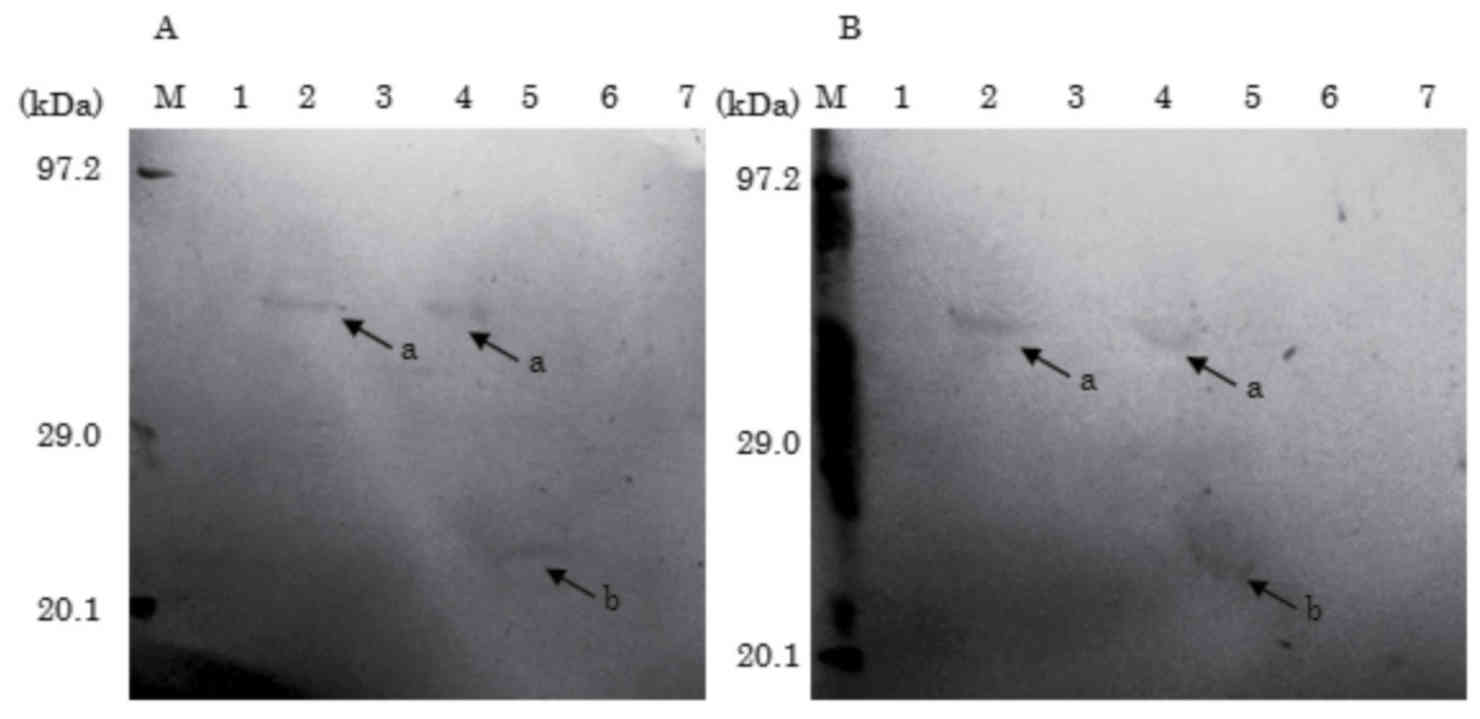
Immunoblotting with the sera from two children with soybean
allergy, detected soybean allergens in 3 of the 7 soy sauces,
including 2 of the koikuchi (lanes 2 and 4) and the saishikomi
(lane 5; Fig. 4). The molecular
weight of soybean allergen detected in koikuchi of lane 2 and 4 was
~50 kDa and it was predicted to be the β-subunit of β-conglycinin.
The soybean allergen detected in saishikomi of lane 5 was ~23 kDa
and predicted to be oleosin. Gly m Bd 30K and Gly m Bd 28K were not
detected, consistent with the results of previous studies (20–22).
In the current study, by using immunoblotting with
the anti-soybean antibody and the sera from 2 children with soybean
allergy, it was clearly demonstrated that soybean protein was not
completely degraded in moromi and remained in the raw soy
sauce. Furthermore, the soluble soybean proteins that remained in
the raw soy sauce were denatured to insoluble allergens by
heat-treatment and were completely removed from 6 month
moromi by subsequent filtration. Soybean proteins were
detected in certain commercial raw soy sauces (Fig. 3), and these soybean proteins were
identified to be soybean allergens as they were immunostained by
the sera from 2 children with soybean allergy. Notably, soybean
allergens detected in commercial raw soy sauce were predicted to be
the β-subunit of β-conglycinin and oleosin, which are not known as
major soybean allergens. The β-subunit of β-conglycinin is highly
homologous with the α-subunit, which is a major soybean allergen
(3). Oleosin is a hydrophobic
protein that is located in the outer layer of the oil body of plant
cells, and is reported as an allergen in sesame (9–12)
and peanuts (9,12–14).
In conclusion, for soy sauce to have low allergenicity,
heat-treatment and filtration are important processes for the
removal soybean allergens, in addition to the enzymatic degradation
in the moromi fermentation.
References
|
1
|
Ogawa T, Bando N, Tsuji H and Sasaoka K,
Nishikawa K and Sasaoka K: Investigation of the IgE-binding
proteins in soybeans by immunoblotting with the sera of the
soybean-sensitive patients with atopic dermatitis. J Nutr Sci
Vitaminol (Tokyo). 37:555–565. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogawa T, Bando N, Tsuji H, Nishikawa K and
Kitamura K: Alpha-subunit of beta-conglycinin, an allergenic
protein recognized by IgE antibodies of soybean-sensitive patients
with atopic dermatitis. Biosci Biotechnol Biochem. 59:831–833.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krishnan HB, Kim WS, Jang S and Kerley MS:
All three subunits of soybean beta-conglycinin are potential food
allergens. J Agri Food Chem. 57:938–943. 2009. View Article : Google Scholar
|
|
4
|
Adachi A, Horikawa T, Shimizu H, Sarayama
Y, Ogawa T, Sjolander S, Tanaka A and Moriyama T: Soybean
beta-conglycinin as the main allergen in a patient with
food-dependent exercise-induced anaphylaxis by tofu: Food
processing alter pepsin resistance. Clin Exp Allergy. 39:167–173.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogawa T, Tsuji H, Bando N, Kitamura K, Zhu
YI, Hirano H and Nishikawa K: Identification of the soybean
allergenic protein, Gly m Bd 30K, with the soybean seed 34-kDa
oil-body-associated protein. Biosci Biotechnol Biochem.
57:1030–1033. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsuji H, Bando N, Hiemori M, Yamanishi R,
Kimoto M, Nishikawa K and Ogawa T: Purification of characterization
of soybean allergen Gly m Bd 28K. Biosci Biotechnol Biochem.
61:942–947. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuji H, Hiemori M, Kimoto M, Yamashita H,
Kobatake R, Adachi M, Fukuda T, Bando N, Okita M and Utsumi S:
Cloning of cDNA encoding a soybean allergen, Gly m Bd 28K. Biochim
Biophys Acta. 1518:178–182. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kleine-Tebbe J, Vogel L, Crowell DN,
Haustein UF and Vieths S: Severe oral allergy syndrome and
anaphylactic reactions caused by a Bet v 1- related PR-10 protein
in soybean, SAM2. J Allergy Clin Immunol. 110:797–804. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morisset M, Moneret-Vautrin DA, Kanny G,
Guénard L, Beaudouin E, Flabbée J and Hatahet R: Thresholds of
clinical reactivity to milk, egg, peanut and sesame in
immunoglobulin E-dependent allergies: Evaluation by double-blind or
single-blind placebo-controlled oral challenges. Clin Exp Allergy.
33:1046–1051. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JC, Lin RH, Huang HC and Tzen JT:
Cloning, expression and isoform classification of a minor oleosin
in sesame oil bodies. J Biochem. 122:819–824. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leduc V, Moneret-Vautrin DA, Tzen JT,
Morisset M, Guerin L and Kanny G: Identification of oleosins as
major allergens in sesame seed allergic patients. Allergy.
61:349–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pons L, Olszewski A and Guéant JL:
Characterization of the oligomeric behavior of a 16.5 kDa peanut
oleosin by chromatography and electrophoresis of the iodinated
form. J Chromatogr B Biomed Sci Appl. 706:131–140. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang AHC: Oil bodies and oleosins in
seeds. Ann Rev Plant Physiol Plant Mol Biol. 43:177–200. 1992.
View Article : Google Scholar
|
|
14
|
Pons L, Chery C, Romano A, Namour F,
Artesani MC and Guéant JL: The 18 kDa peanut oleosin is a candidate
allergen for IgE-mediated reactions to peanuts. Allergy. 57 Suppl
72:S88–S93. 2002. View Article : Google Scholar
|
|
15
|
Yokotsuka T: Soy sauce biochemistry. Adv
Food Res. 30:195–329. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi M and Hayashi S: Modeling
combined effects of temperature and pH on growth of
Zygosaccharomyces rouxii in soy sauce mash. J Ferment Bioeng.
85:638–641. 1998. View Article : Google Scholar
|
|
17
|
Kobayashi M and Hayashi S: Supplementation
of NaCl to starter culture of the soy yeast Zygosaccharomyces
rouxii. J Ferment Bioeng. 85:642–644. 1998. View Article : Google Scholar
|
|
18
|
Nagai H, Kobayashi M, Tsuji Y, Nakashimada
Y, Kakizono T and Nishio N: Biological and chemical treatment of
solid waste from soy sauce manufacture. Water Sci Technol.
45:335–338. 2002.PubMed/NCBI
|
|
19
|
Kobayashi M, Hashimoto Y, Taniuchi S and
Tanabe S: Degradation of wheat allergen in Japanese soy sauce. Int
J Mol Med. 13:821–827. 2004.PubMed/NCBI
|
|
20
|
Tsuji H, Okada N, Yamanishi R, Bando N,
Kimoto M and Ogawa T: Measurement of Gly m Bd 30K, a major soybean
allergen, in soybean products by a sandwich enzyme-linked
immunosorbent assay. Biosci Biotechnol Biochem. 59:150–151. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogawa T, Samoto M and Takahashi K: Soybean
allergens and hypoallergenic soybean products. J Nutr Sci Vitaminol
(Tokyo). 46:271–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bando N, Tsuji H, Hiemori M, Yoshimizu K,
Yamanishi R, Kimoto M and Ogawa T: Quantitative analysis of Gly m
Bd 28K in soybean products by a sandwich enzyme-linked
immunosorbent assay. J Nutr Sci Vitaminol (Tokyo). 44:655–664.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi M, Matsushita H, Yoshida K,
Tsukiyama R, Sugimura T and Yamamoto K: In vitro and in vivo
anti-allergic activity of soy sauce. Int J Mol Med. 14:879–884.
2004.PubMed/NCBI
|
|
24
|
Kobayashi M, Matsushita H, Shioya I, Nagai
M, Tsukiyama R, Saito M, Sugita T, Sugimura T and Yamamoto K:
Quality of life improvement with soy sauce ingredients, shoyu
polysaccharides, in perennial allergic rhinitis: A double-blind
placebo-controlled clinical study. Int J Mol Med. 14:885–889.
2004.PubMed/NCBI
|
|
25
|
Kobayashi M, Matsushita H, Tsukiyama R,
Saito M and Sugita T: Shoyu polysaccharides from soy sauce improve
quality of life for patients with seasonal allergic rhinitis: A
double-blind placebo-controlled clinical study. Int J Mol Med.
15:463–467. 2005.PubMed/NCBI
|
|
26
|
Matsushita H, Kobayashi M, Tsukiyama R and
Yamamoto K: In vitro and in vivo immunomodulating activities of
Shoyu polysaccharides from soy sauce. Int J Mol Med. 17:905–909.
2006.PubMed/NCBI
|
|
27
|
Kobayashi M, Nagatani Y, Magishi N,
Tokuriki N, Nakata Y, Tsukiyama R, Imai H, Suzuki M, Saito M and
Tsuji K: Promotive effect of Shoyu polysaccharides from soy sauce
on iron absorption in animals and humans. Int J Mol Med.
18:1159–1163. 2006.PubMed/NCBI
|
|
28
|
Kobayashi M, Magishi N, Matsushita H,
Hashimoto T, Fujimoto M, Suzuki M, Tsuji K, Saito M, Inoue E,
Yoshikawa Y and Matsuura T: Hypolimidemic effect of Shoyu
polysaccharides from soy sauce in animals and humans. Int J Mol
Med. 22:565–570. 2008.PubMed/NCBI
|
|
29
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|