Introduction
Endocrine-disrupting chemicals (EDCs) are exogenous
compounds, which are present in the environment and food, and have
an effect on the normal endocrine system (1,2).
EDCs have effects on male and female reproduction, developmental
disorders, the development and progression of cancer, metabolism
and obesity, and cardiovascular endocrinology (1,3,4).
EDCs can induce estrogen-like or androgen-like effects by binding
to hormone receptors, including the estrogen receptor (ER) and
androgen receptor (AR), therefore, they may interfere with the
actions of endogenous steroid hormones or induce hormone-mediated
responses. The abnormal activation of estrogen signaling by EDCs
leads to altered gene expression in target tissues and
carcinogenesis (5).
Nonylphenol (NP) is a well-known EDC. It is a
degradation product of alkylphenol polyethoxylate (APE). The use of
pesticides, polystyrene plastics and paints, including APE, can
lead to the bioaccumulation of NP in the food chain. The
accumulation of NP in the body can result in endocrine disruption,
and immunological and reproductive disorders (6). It has been reported that NP has an
estrogenic effect in humans (6).
Previous studies have demonstrated that NP can enhance the
progression of cancer by acting on the cell cycle, apoptosis and
metastasis in breast, ovarian and prostate cancer (7–10).
Colorectal cancer (CRC) is the third most common
type of malignancy with high mortality rates in men and women
worldwide (11). In disease
progression, the processes of the DNA repair system, inflammation
and apoptosis are altered, and metastases are the primary cause of
poor prognosis and cancer-associated mortality in patients with CRC
(12). The risks of human CRC are
associated with smoking, alcohol intake, dietary factors and
obesity. A number of studies have suggested that estrogen has a
potential role in the development of CRC (13,14).
Men are more susceptible to colon cancer than women, and the use of
hormone-replacement therapy reduces the risk of CRC in
postmenopausal women (15).
However, the evidence that high concentrations of circulating
estrogen confer an increased risk for CRC in men and women is
inconsistent (16–18). Estrogen concentrations are higher
in CRC tissues, compared with concentrations in nonneoplastic
tissues in patients with CRC, and patient prognosis is poorer when
intratumoral estrogen concentrations are higher (19). Therefore, xenoestrogens, including
NP, may also be associated with the risk of CRC.
In the present study, the in vitro effects of
NP at different concentrations on COLO205 CRC cell cycle and
apoptosis were examined, and the mechanism of action was
investigated by analyzing alterations in the expression of genes in
the ERK pathway and TGFβ pathway.
Materials and methods
Cell culture and treatment
Human COLO205 CRC cells were obtained from the
American Type Culture Collection (ATCC CCL-222; Manassas, VA, USA).
The cells were cultured in Roswell Park Memorial Institute-1640
medium (Hyclone Laboratories; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% fetal bovine serum (Zhejiang
Tianhang Biotechnology Co., Ltd., Huzhou, China), 100 IU/ml
penicillin and 100 mg/ml streptomycin at 37°C in a humidified 5%
CO2 atmosphere. NP with analytical standard purity was
purchased from Aladdin Industrial Corporation (Shanghai, China),
and was dissolved in absolute ethyl alcohol to 50 mmol/l.
Flow cytometric analysis of cell cycle
and cell apoptosis
The effects of NP and estradiol (E2) on cell cycle
progression were determined using flow cytometry. Following
fixation, the cells were stained with propidium iodide (PI)
solution (50 µg/ml PI and 100 µg/ml RNase A in PBS) and then
subjected to cell cycle analysis. The extent of cell apoptosis was
measured using Annexin V/PI double staining. Binding buffer (300
µl) was used for cell resuspension (6×104 cells), and 5
µl of Annexin V-FITC was added to the cell suspension for 10 min
incubation in the dark. Subsequently, 5 µl of PI was added to the
cell suspension for 5 min in the dark. The samples were analyzed
with a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The COLO205 cells were seeded at a density of
0.5×106 cells/well in 6-well plates at 37°C in a
humidified atmosphere of 5% CO2 until >70% confluent
growth. The cells were treated with medium containing E2
(10−7 M) or NP (10−7, 10−5 and
10−4 M) and cultured for 48 h at 37°C in a humidified 5%
CO2 atmosphere. Total RNA was extracted using TRIzol
reagent (Invitrogen Life Technologies; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Total RNA was reverse transcribed using a First Strand cDNA
Synthesis kit (Toboyo Co., Ltd., Dalian, China) according to the
manufacturer's protocol. The cDNA was stored at −20°C. The qPCR
analysis was performed on a StepOne™ system (Thermo Fisher
Scientific, Inc.) in a 10 µl volume containing 5 µl of 2X qPCR mix
of SYBR® Premix Ex Taq™ (Takara Biotechnology Co., Ltd., Dalian,
China), 1.0 µl of each forward and reverse primer (Table I; 2.5 µM), 1.0 µl of cDNA (~100 ng)
and 3 µl ddH2O. the reaction consisted of 5 min at 95°C,
followed by 40 cycles of 95°C (5 sec), 72°C (40 sec) and then
melting curves from 60 to 95°C (0.1°C/sec), ending with a step at
15°C. Melting curves were used to confirm the specificity of each
primer, and no primer-dimer were identified. The relative gene
expression levels were calculated using the 2−ΔΔCq
method (20) and β-actin was used
as an internal control. The sample containing six biological
replicates was amplified in triplicate.
 | Table I.Primer sequences and product sizes of
products for reverse transcription-quantitative polymerase chain
reaction analysis. |
Table I.
Primer sequences and product sizes of
products for reverse transcription-quantitative polymerase chain
reaction analysis.
| Target gene | Sequence | Product size
(bp) |
|---|
| Actin |
| 110 |
|
Forward |
5′-CGTTGACATCCGTAAAGACCTC-3′ |
|
|
Reverse |
5′-TAGGAGCCAGGGCAGTAATCT-3′ |
|
| PI3K |
| 262 |
|
Forward |
5′-CTTCACAATGCCATCCTACTCC-3′ |
|
|
Reverse |
5′-ATTCAGCCATTCATTCCACCT-3′ |
|
| ERK1 |
| 219 |
|
Forward |
5′-CTGGCTTTCTGACCGAGTATGT-3′ |
|
|
Reverse |
5′-AATTTAGGTCCTCTTGGGATGG-3′ |
|
| ERK2 |
| 173 |
|
Forward |
5′-GCACCAACCATTGAGCAGAT-3′ |
|
|
Reverse |
5′-TCACGGTGCAGAACATTAGCT-3′ |
|
Western blot analysis
To measure the protein expression levels of ERK,
PI3Kp85, c-Fos, SnoN and β-actin, the COLO205 cells were cultured
to a density of 1×106 cells and then incubated with E2
(10−7 M) or NP (10−7-10-4 M) for
48 h at 37°C in a humidified 5% CO2 atmosphere.
Following treatment, whole cell lysates of the COLO205 cells were
prepared in 1X RIPA buffer (Beyotime Institute of Biotechnology)
for 30 min on ice. The total protein concentrations were determined
using bicinchoninic acid (Thermo Fisher Scientific, Inc.). The
total protein (40 µg) was separated by SDS-polyacrylamide gel
electrophoresis (5% stacking gel and 10% separating gel), following
which the proteins were transferred onto polyvinylidenedifluoride
membranes (EMD Millipore, Billerica, MA, USA), and these membranes
were blocked with 5% skim milk powder (BD Biosciences) for 60 min
at room temperature. The membranes were then incubated with primary
antibodies overnight at 4°C: Rabbit anti-β-actin (cat no. TDY051;
1:10,000; TDY Biotech Co., Ltd., Beijing, China) and anti-PI3K (cat
no. ab40755; 1:500; Abcam, Cambridge, MA, USA) diluted in 5%
skimmed milk, and anti-ERK1/2 (cat no. 4695; 1:1,000),
anti-p-ERK1/2 (cat no. 4370; 1:1,000) (both from Cell Signaling
Technology, Inc., Danvers, MA, USA) diluted in 5% BSA. The
membranes were subsequently probed with secondary antibody
(HRP-goat anti rabbit; cat no. ab6721; 1:10,000; Abcam) for 30 min
at room temperature. Target proteins were detected using Clarity™
Western ECL Substrate (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The optical density was analyzed using AlphaEaseFC software
version 6.0. All experiments were performed at least three
times.
Statistical analysis
Each experiment was repeated three times and
analyzed using Excel 2013 (Microsoft Corporation, Redmond, WA,
USA). Data are presented as the mean ± standard deviation.
Statistical analyses were performed using a one-tail t-test,
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of NP on cell cycle and
apoptosis
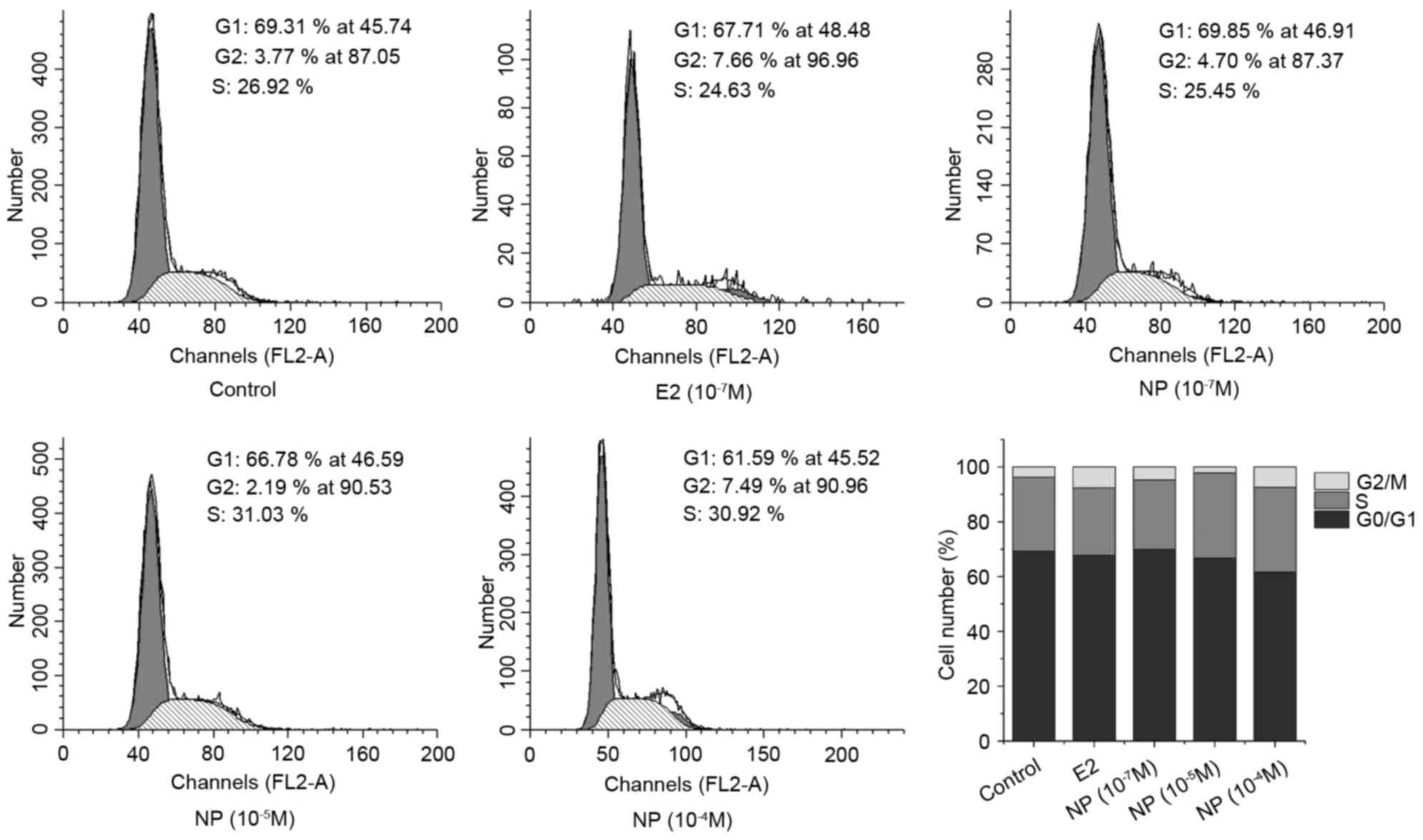
To evaluate the effect of NP on cell growth, COLO205
cells were cultured with E2 (10−7 M) or NP
(10−7-10−4 M) for 48 h, and cell cycle was
examined using flow cytometry. Compared with the control group, E2
significantly increased the proportion of cells in the S phase. NP
caused a significant decrease in the proportion of cells in the
G0/G1 phase in a dose-dependent manner, which was accompanied by
marginal increase in the proportions of cells in the S and G2/M
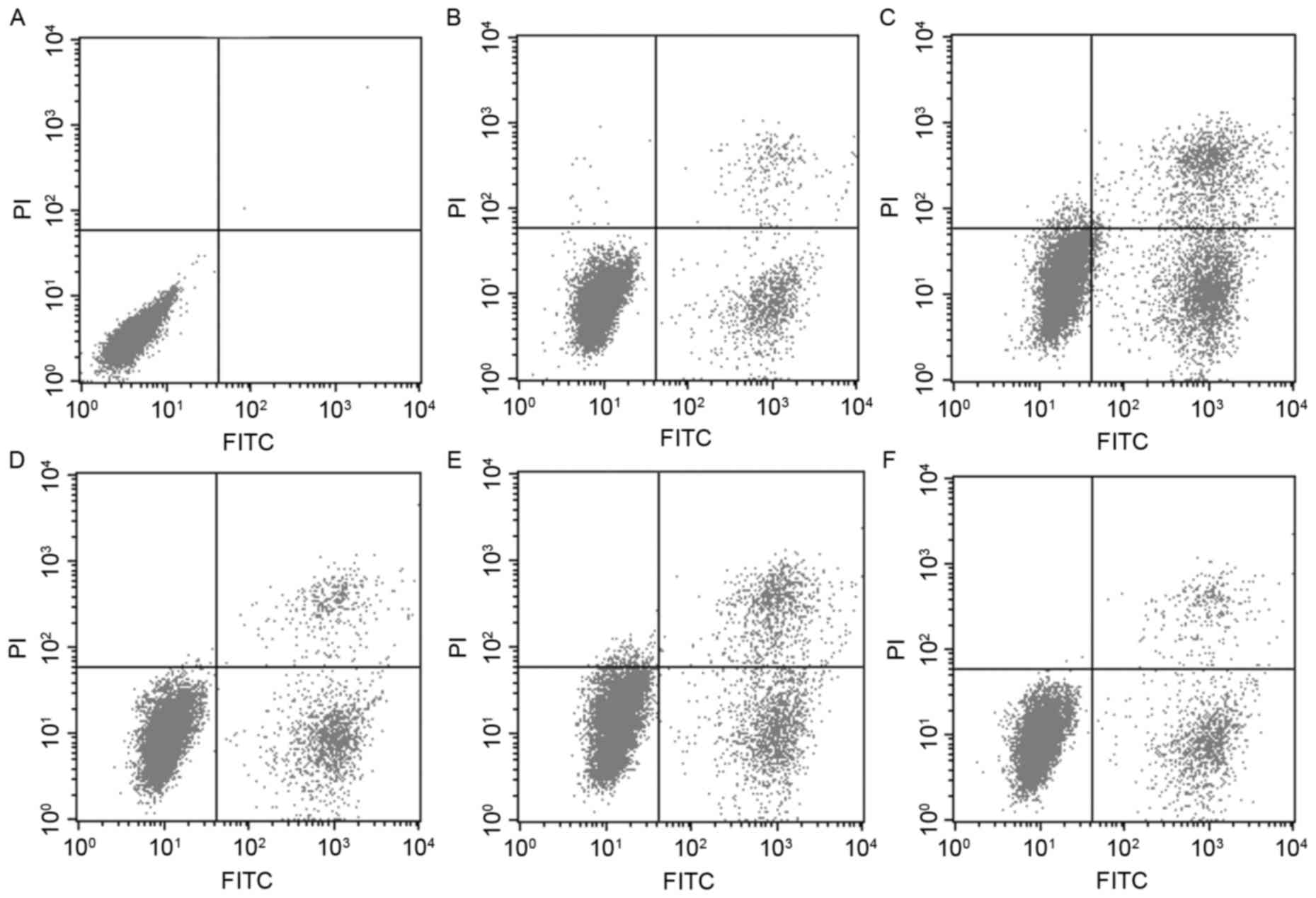
phases (Fig. 1). Flow cytometry
was also used to investigate whether NP affects cell apoptosis
(Fig. 2A-F). The results showed
that the apoptotic rate increased in the E2 treatment group and in
the middle dose NP (10−5 M) treatment group, compared
with the control group, however, no significant changes were
observed in the low and high dose NP (10−7 M and
10−4 M) treatment groups.
Alterations in expression of the ERK
pathway by NP
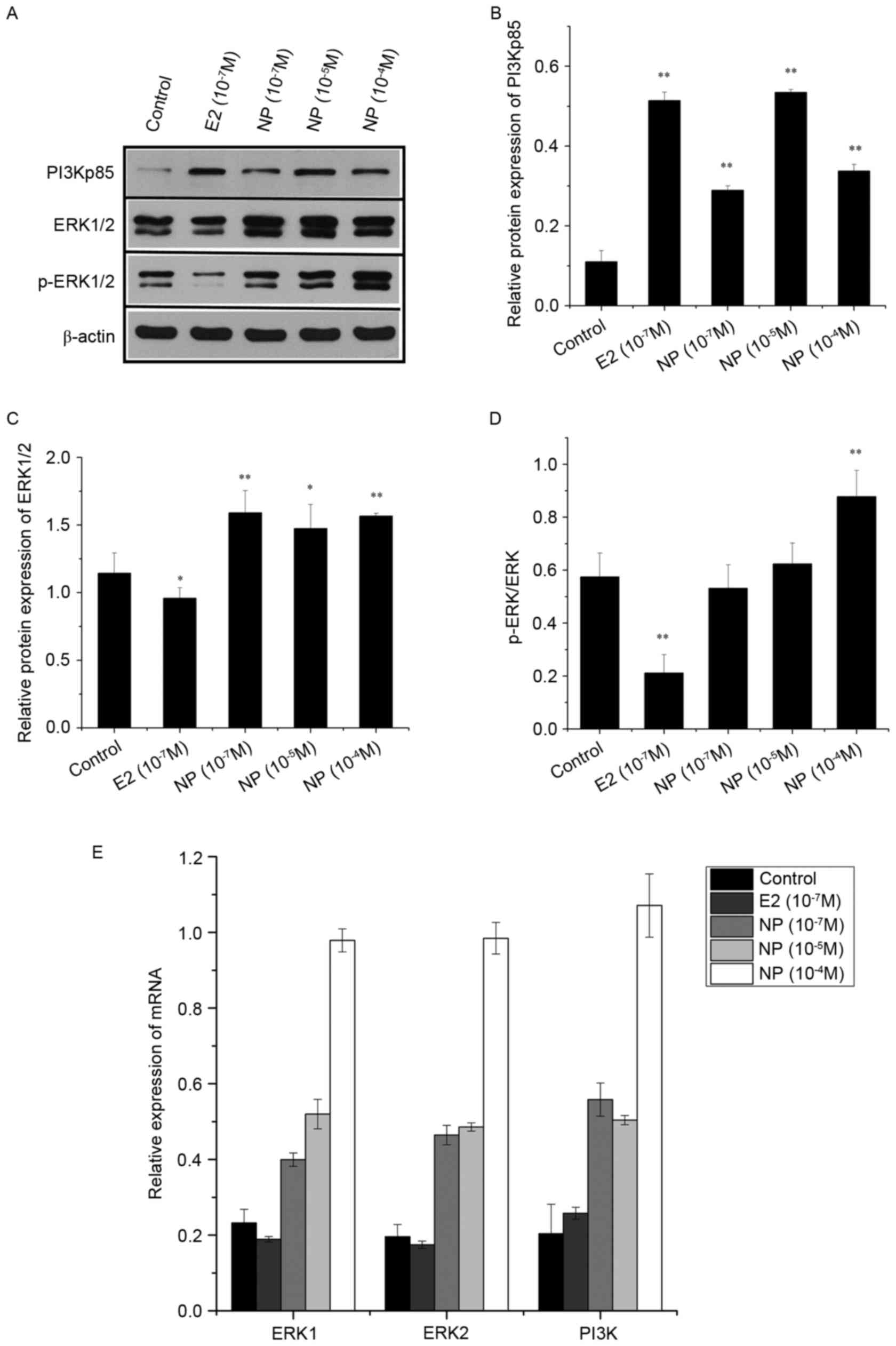
The effects of E2 and NP on expression of the ERK
pathway in the COLO205 cells were determined using RT-qPCR and
western blot analyses (Fig. 3).
The protein expression levels of PI3Kp85 and ERK1/2 were
significantly increased by NP (P<0.01; Fig. 3A-C). Phosphorylation of the ERK
protein was significantly increased by treatment with NP at a high
concentration (10−4 M; P<0.01; Fig. 3A and D), however it was
significantly decreased by E2 (P<0.01). The results of the
RT-qPCR analysis showed the same expression patterns in the mRNA
expression levels of PI3K, ERK1 and ERK2 (Fig. 3E).
Alterations in expression of the TGF
pathway in response to NP
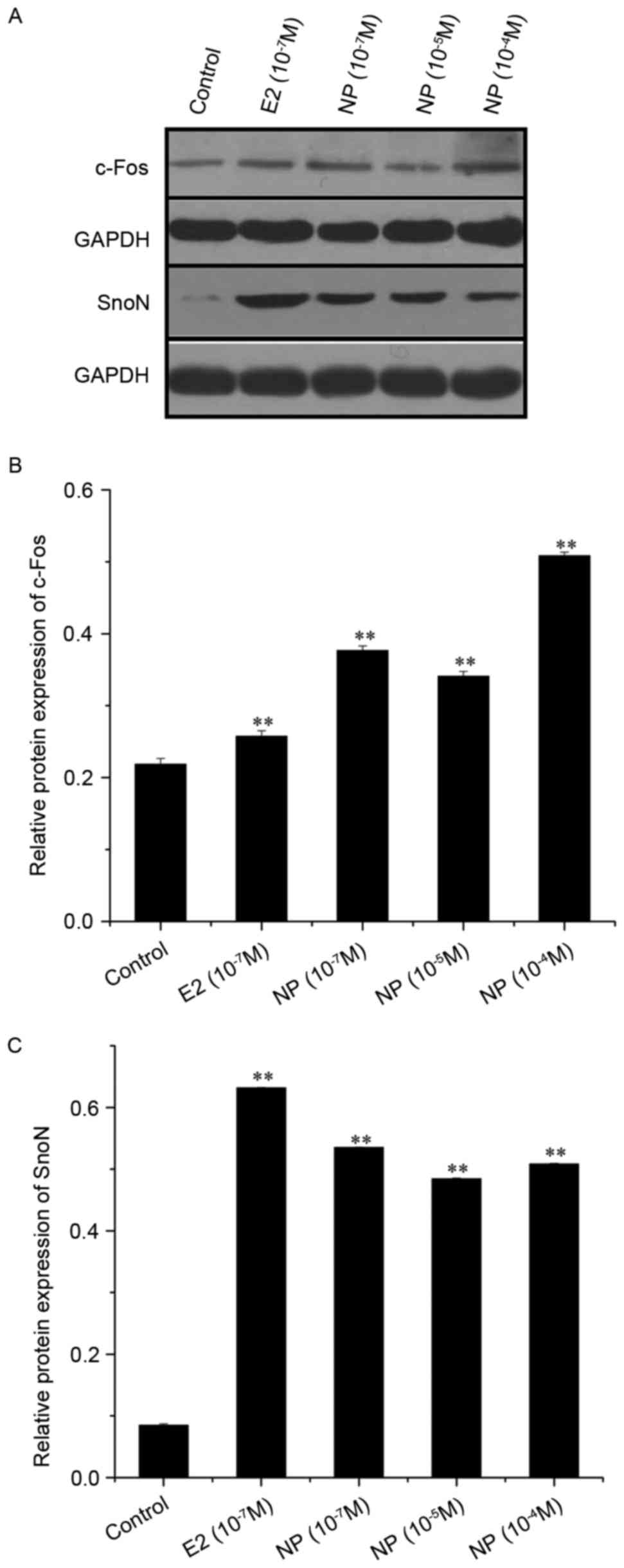
Two key proteins in the TGFβ pathway (c-Fos and
SnoN) were selected for examination using western blot analysis in
COLO205 cells treated by NP and E2 (Fig. 4A). The expression levels of c-Fos
and SnoN were significantly enhanced by treatment with E2
(10−7 M; P<0.01) and NP
(10−7-10−4 M; P<0.01) as shown in Fig. 4B and C.
Discussion
EDCs can interfere with hormone systems and produce
adverse developmental, reproductive, neurological and immunological
effects in mammals (21). Previous
studies have shown that these substances also adversely affect
human health, resulting in reduced fertility and increased
progression of certain diseases, including obesity, diabetes,
endometriosis and certain types of cancer (9,22–24).
EDCs can interrupt the normal functions of reproductive organs by
aberrantly binding to hormone receptors and further triggering
hormone-responsive cancer initiation and progression, including in
breast, ovarian and prostatic cancer (8–10).
The present study examined whether one of the EDCs, NP, enhances
the progression CRC.
The results of the present study revealed that the
proportion of COLO205 CRC cells in the G0/G1 stage was
significantly reduced by NP in a dose-dependent manner, which
indicated that NP promoted CRC cell growth, as reported in previous
cancer studies (8,25). However, despite the alterations
observed in cell viability, the apoptotic rates of the cells were
unaffected by NP. These results suggested that NP promoted cell
proliferation, but had no effect on apoptosis.
Mutation activation of the ERK signaling pathway is
frequently observed in human cancer, including CRC (26), and is important in the regulation
of malignant cellular proliferation, migration and invasion.
Previous studies have demonstrated that estrogens and xenoestrogens
can activate the phosphorylation of ERK1/2 in rat pituitary tumor
cells, and that responses are inhibited by ERK inhibitors (27,28).
The present study found that the phosphorylation of ERK1/2 was
significantly increased in CRC cells treated with NP in a
dose-dependent manner; however, this phosphorylation was decreased
by E2. This difference may be due to the two types of cell being
derived from different genetic backgrounds and undergoing several
other genomic hits by treatment with E2. These results indicated
that NP promoted the proliferation of CRC cells through activating
the ERK signaling pathway, however, the mechanism of activation and
the reason underlying the different effects induced by E2 and NP
require further investigation.
The TGFβ signaling pathway is also a key determinant
of carcinoma cell behavior, acting as a tumor suppressor pathway,
and a promoter of tumor progression and invasion (29). In ovarian cancer models, EDCs have
been shown to inhibit the TGFβ signaling pathway by preventing the
degradation of SnoN protein and increasing the protein expression
of c-Fos (8). Members of the Fos
family, including c-Fos, FosB, and its smaller splice variants,
Fra-1 and Fra-2, dimerize with Jun proteins to form the activating
protein 1 transcription factor complex, which is central during
malignant transformation and progression (30). In addition, SnoN is a negative
regulator of the TGFβ signaling pathway and is directly linked to
its ability to repress the transcription of TGFβ-inducible genes
(31). In the present study, it
was also found that NP induced the expression of c-Fos and SnoN, as
did E2. Therefore, it was hypothesized that NP may inhibit the TGFβ
signaling pathway in CRC cells as it does in ovarian cancer
cells.
In conclusion, the results of the present study
suggested that NP may affect the growth of CRC cells by activating
the ERK signaling pathway via increasing the phosphorylation of
ERK1/2 and inhibiting the TGFβ signaling pathway via the
upregulation of c-Fos and SnoN. This is the first report, to the
best of our knowledge, to demonstrate the potential mechanism
between NP and CRC. The results of the present study demonstrated
the effect of NP on the progression of CRC. Further investigations
are required to establish the mechanism underlying the effects of
NP on the ERK and TGFβ signaling pathways.
Acknowledgements
This study was supported by Natural Scientific
Foundation in the Science and Technology Department of Guizhou
Province, China (grant no. J20142185).
References
|
1
|
Diamanti-Kandarakis E, Bourguignon JP,
Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT and Gore AC:
Endocrine-disrupting chemicals: An endocrine society scientific
statement. Endocr Rev. 30:293–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diamanti-Kandarakis E, Palioura E,
Kandarakis SA and Koutsilieris M: The impact of endocrine
disruptors on endocrine targets. Horm Metab Res. 42:543–552. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoon K, Kwack SJ, Kim HS and Lee BM:
Estrogenic endocrine-disrupting chemicals: Molecular mechanisms of
actions on putative human diseases. J Toxicol Environ Health B Crit
Rev. 17:127–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Safe SH, Pallaroni L, Yoon K, Gaido K,
Ross S, Saville B and McDonnellc D: Toxicology of environmental
estrogens. Reprod Fertil Dev. 13:307–315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi SM, Yoo SD and Lee BM: Toxicological
characteristics of endocrine-disrupting chemicals: Developmental
toxicity, carcinogenicity, and mutagenicity. J Toxicol Environ
Health B Crit Rev. 7:1–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonefeld-Jørgensen EC, Long M, Hofmeister
MV and Vinggaard AM: Endocrine-disrupting potential of bisphenol A,
bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in
vitro: New data and a brief review. Environ Health Perspect. 115
Suppl 1:S69–S76. 2007. View
Article : Google Scholar
|
|
7
|
Kim YS, Hwang KA, Hyun SH, Nam KH, Lee CK
and Choi KC: Bisphenol A and nonylphenol have the potential to
stimulate the migration of ovarian cancer cells by inducing
epithelial-mesenchymal transition via an estrogen receptor
dependent pathway. Chem Res Toxicol. 28:662–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park MA and Choi KC: Effects of
4-nonylphenol and bisphenol A on stimulation of cell growth via
disruption of the transforming growth factor-β signaling pathway in
ovarian cancer models. Chem Res Toxicol. 27:119–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH, Nam KH, Hwang KA and Choi KC:
Influence of hexabromocyclododecane and 4-nonylphenol on the
regulation of cell growth, apoptosis and migration in prostatic
cancer cells. Toxicol In Vitro. 32:240–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
In SJ, Kim SH, Go RE, Hwang KA and Choi
KC: Benzophenone-1 and nonylphenol stimulated MCF-7 breast cancer
growth by regulating cell cycle and metastasis-related genes via an
estrogen receptor α-dependent pathway. J Toxicol Environ Health A.
78:492–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Cutsem E and Oliveira J; ESMO
Guidelines Working Group, : Advanced colorectal cancer: ESMO
clinical recommendations for diagnosis, treatment and follow-up.
Ann Oncol. 20 Suppl 4:S61–S63. 2009. View Article : Google Scholar
|
|
13
|
Kennelly R, Kavanagh DO, Hogan AM and
Winter DC: Oestrogen and the colon: Potential mechanisms for cancer
prevention. Lancet Oncol. 9:385–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin JH and Giovannucci E: Sex hormones and
colorectal cancer: What have we learned so far? J Natl Cancer Inst.
102:1746–1747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grodstein F, Newcomb PA and Stampfer MJ:
Postmenopausal hormone therapy and the risk of colorectal cancer: A
review and meta-analysis. Am J Med. 106:574–582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gunter MJ, Hoover DR, Yu H,
Wassertheil-Smoller S, Rohan TE, Manson JE, Howard BV, Wylie-Rosett
J, Anderson GL, Ho GY, et al: Insulin, insulin-like growth
factor-I, endogenous estradiol and risk of colorectal cancer in
postmenopausal women. Cancer Res. 68:329–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clendenen TV, Koenig KL, Shore RE, Levitz
M, Arslan AA and Zeleniuch-Jacquotte A: Postmenopausal levels of
endogenous sex hormones and risk of colorectal cancer. Cancer
Epidemiol Biomarkers Prev. 18:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu H, Xu L, Chen J, Hu J, Yu S, Hu G,
Huang L, Chen X, Yuan X and Li G: Association of estrogen receptor
beta variants and serum levels of estradiol with risk of colorectal
cancer: A case control study. BMC Cancer. 12:2762012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sato R, Suzuki T, Katayose Y, Miura K,
Shiiba K, Tateno H, Miki Y, Akahira J, Kamogawa Y, Nagasaki S,
Yamamoto K, et al: Steroid sulfatase and estrogen sulfotransferase
in colon carcinoma: Regulators of intratumoral estrogen
concentrations and potent prognostic factors. Cancer Res.
69:914–922. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang O, Kim HL, Weon JI and Seo YR:
Endocrine-disrupting chemicals: Review of toxicological mechanisms
using molecular pathway analysis. J Cancer Prev. 20:12–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ariemma F, D'Esposito V, Liguoro D,
Oriente F, Cabaro S, Liotti A, Cimmino I, Longo M, Beguinot F,
Formisano P and Valentino R: Low-dose bisphenol-A impairs
adipogenesis and generates dysfunctional 3T3-L1 adipocytes. PloS
One. 11:e01507622016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jambor T, Lukáčová J, Tvrdá E, Knážická Z,
Forgács Z and Lukáč N: The impact of 4-nonylphenol on the viability
and hormone production of mouse leydig cells. Folia Biol (Praha).
62:34–39. 2016.PubMed/NCBI
|
|
24
|
Lee HR, Hwang KA, Park MA, Yi BR, Jeung EB
and Choi KC: Treatment with bisphenol A and methoxychlor results in
the growth of human breast cancer cells and alteration of the
expression of cell cycle-related genes, cyclin D1 and p21, via an
estrogen receptor-dependent signaling pathway. Int J Mol Med.
29:883–890. 2012.PubMed/NCBI
|
|
25
|
Dong Y, Araki M, Hirane M, Tanabe E,
Fukushima N and Tsujiuchi T: Effects of bisphenol A and
4-nonylphenol on cellular responses through the different induction
of LPA receptors in liver epithelial WB-F344 cells. J Recept Signal
Transduct Res. 34:201–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Z, Ma W, Qi X, Zhu X, Wang Y, Xu Z,
Luo J, Wang D, Guo W, Li X, et al: Pinin facilitated proliferation
and metastasis of colorectal cancer through activating EGFR/ERK
signaling pathway. Oncotarget. 7:29429–29439. 2016.PubMed/NCBI
|
|
27
|
Jeng YJ and Watson CS: Combinations of
physiologic estrogens with xenoestrogens alter ERK phosphorylation
profiles in rat pituitary cells. Environ Health Perspect.
119:104–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watson CS, Jeng YJ, Hu G, Wozniak A,
Bulayeva N and Guptarak J: Estrogen- and xenoestrogen-induced ERK
signaling in pituitary tumor cells involves estrogen receptor-α
interactions with G protein-αi and caveolin I. Steroids.
77:424–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Milde-Langosch K: The Fos family of
transcription factors and their role in tumourigenesis. Eur J
Cancer. 41:2449–2461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deheuninck J and Luo K: Ski and SnoN,
potent negative regulators of TGF-beta signaling. Cell Res.
19:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|