Introduction
Prenatal alcohol exposure may result in fetal
alcohol syndrome (FAS), a severe form of fetal alcohol spectrum
disorder (FASD); symptoms of FAS include craniofacial malformation,
structural abnormalities of the nervous systems and long-term
neurobehavioral disorders (1,2).
Neurobehavioral disorders are the primary manifestation of FAS and
affect up to 2–5% of young children in the United States and
Western Europe (3,4). The precise mechanisms of FASD
development remain largely unknown, and there are no effective
clinical treatments to prevent FASD except for the avoidance of
alcohol consumption.
Previous studies in both animals and humans have
reported that alcohol exposure during early prenatal development
was associated with the loss of neuronal mass or a reduction in the
volume of specific brain regions (2,5,6).
Other studies using rodents or non-human primates have demonstrated
that a single exposure to alcohol during a period equivalent to the
human third trimester was able to induce widespread apoptosis of
neurons and glia (5,7). Once neuronal and glial apoptosis
occur, secondary mechanisms begin to compensate for the
alcohol-induced apoptotic injuries and may result in permanent
damage. Therefore, apoptosis is considered to be the primary
mechanism that results in the occurrence of FASD, and modulating
apoptosis may be key to preventing and treating FASD (8).
Dopamine is a neurotransmitter that is widely
expressed in the brain and retina, and is often used as a
vasoactive drug. Dopamine activates two types of dopamine receptor,
the D1-like (such as D1 and D5) and D2-like (such as D2, D3 and D4)
families, and modulates intracellular cyclic adenosine
monophosphate (cAMP) levels (9,10). A
recent study demonstrated that alcohol exposure increased
neuroapoptosis through the downregulation of D1 dopamine receptor
(D1R) expression in prenatal rat brains (11), which indicated that the
dopaminergic system may be involved in alcohol-induced neuronal
apoptosis. In addition, dopamine has been revealed to protect
neurons against glutamate-induced neuronal death through both D1-
and D2-like receptors (12), and
activation of the D2 dopamine receptor (D2R) inhibited neonatal
cardiomyocyte apoptosis induced by ischemia/reperfusion injury
(13). However, whether dopamine,
or the activation of dopamine receptor subtypes, protects against
alcohol-induced neuroapoptosis in the developing retina remains
unclear. In addition, D2R and AA2AR may form heterodimers and
activation by dopamine may activate AC, resulting in upregulation
of cAMP (10,14). Whether activation of the
heteromeric complexes protects against alcohol-induced
neuroapoptosis remains unknown.
The cAMP/protein kinase A (PKA) signal transduction
pathway has been reported to serve an important role in the
survival of neuronal populations in the neonatal nervous system,
including retinal ganglion cells (15–17).
However, the role of intracellular cAMP or PKA levels in
alcohol-induced neuroapoptosis remains unclear, as both up- and
downregulation of intracellular cAMP or PKA levels have been
reported to induce neuronal apoptosis (18–22).
The present study used immunohistochemistry and
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling (TUNEL) to explore the effects and mechanisms of dopamine
on alcohol-induced neuronal apoptosis in the developing rat
retina.
Materials and methods
Animals
A total of 55 postnatal day 7 (P7) Sprague-Dawley
rat pups weighing 15–17 g were obtained from the Experimental
Animal Center at the Shanghai General Hospital (Shanghai, China).
Male and female rat pups were included and were kept with their
mother under a 12-h light/dark cycle, at 35–37°C, with food and
water available. All experimental procedures were reviewed and
approved by the Animal Care Committee at the Shanghai General
Hospital, Shanghai Jiao Tong University School of Medicine
(Shanghai, China) and were conducted following the guidelines of
the Care and Use of Laboratory Animals published by The US National
Institutes of Health and The Association of Research in Vision and
Ophthalmology Statement for the Use of Animals in Ophthalmic and
Vision Research. Every effort was made to minimize the number and
discomfort of animals during all experimental procedures.
Experimental procedures
The basic experimental protocol was slightly
modified from a previously published study (23). Briefly, all experimental rat pups
were sacrificed by decapitation and their eyes were rapidly
dissected using fine scissors and transferred to an ice-cold
(0–4°C) bath of artificial cerebrospinal fluid for ~2 min prior to
being cut open [ACSF; NaCl (119 mM), KCl (2.5 mM),
K2HPO4 (1.0 mM), CaCl2 (2.5 mM),
MgCl2 (1.3 mM), NaHCO3 (26.2 mM) and
D-Glucose (11 mM)]. The bath was continuously bubbled with a 95%
O2/5% CO2 gas mixture. Approximately
one-fifth of the eyeball circumference at the edge of the cornea
and sclera was cut using ophthalmology scissors to facilitate the
perfusion of ACSF and interventional drugs into the retina.
Following 1 h recovery in normal ACSF at 37°C bubbled with a 95%
O2/5% CO2 gas mixture, the recovered eyeballs
were incubated with different concentrations of ethanol, dopamine
or various antagonists, either in combination or separately, in
ACSF at 37°C with a 95% O2/5% CO2 gas mixture
for 5 h, according to a previous study (24). In order to reduce ethanol
evaporation from the ACSF and to keep the ethanol concentration of
the ACSF at a stable level, cell culture dishes, in which eyeballs
were cultured, were placed in a larger cell culture dish containing
ACSF. Following the various drug treatments, the retinas were
dissected from the eyeballs and the flatter part of the retina
between the central and peripheral areas was selected for
immunohistochemistry and TUNEL experiments.
Drugs
Ethanol was purchased from Sinopharm Chemical
Reagent Co., Ltd. (Shanghai, China) and dopamine was purchased from
Fujian GuTian Pharmaceutical Company (Fujian, China). All
inhibitors and antagonists were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany), including the following: SCH23390, a D1R
antagonist; raclopride, a D2R antagonist; SCH58261, an adenosine
A2A receptor (AA2AR); SQ22536, an adenylyl cyclase (AC) inhibitor;
and H-89, a PKA inhibitor (24).
All drugs were dissolved in ACSF except SCH58261; SCH58261 was
first dissolved in DMSO to form a stock solution, and the
concentration of DMSO in the working solution was <0.1% as in a
previous study (24).
Caspase-3 immunohistochemistry
Following drug treatments, eyeballs were quickly
transferred to an ice-cold (0–4°C) bath of ACSF and the retina was
immediately detached and fixed in 4% paraformaldehyde at 4°C for 24
h. Subsequent to the retinas being embedded in paraffin, retinal
sections (4–6 µm-thick) were obtained with a Leica RM2135 Rotary
Microtome (Leica Microsystems GmbH, Wetzlar, Germany) and mounted
onto slides. Subsequently, the retinal sections were deparaffinized
and rehydrated (with 100% xylene, 100% ethanol, 95% ethanol, 85%
ethanol, 75% ethanol and double distilled water), endogenous
peroxidases were inactivated with 3% hydrogen peroxide at room
temperature for 10 min, and 0.1 M EDTA (pH 9.0) was used for
heat-induced (95–97°C) antigen retrieval for 8–10 min. Retinal
sections were rinsed in PBS, blocked with 10% donkey serum (cat.
no. D9663; Merck KGaA) in PBS at room temperature for 10 min and
incubated with rabbit anti-cleaved caspase-3 antibody (1:300; cat.
no. 9661; Cell Signaling Technology, Danvers, MA, USA) overnight at
4°C. Following rinsing in PBS, retinal sections were incubated with
a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin
G secondary antibody (cat. no. PV-9001; ZSGB-BIO, Beijing, China)
at 37°C for 1 h and stained by chromogenic reaction via
3,3′-diaminobenzidine (DAB; cat. no. ZLI-9017; ZSGB-BIO, Beijing,
China). All sections were then counterstained with hematoxylin to
stain the nuclei blue. Finally, sections were dehydrated (with 75%
ethanol, 85% ethanol, 95% ethanol, 100% ethanol and 100% xylene)
and mounted using neutral balsam (cat. no. 10004160, Sinopharm
Chemical Reagent Co., Ltd.) for microscopic examination (Leica
DM5500B; Leica Microsystems GmbH).
TUNEL assay
A TUNEL kit (Roche Applied Science, Penzberg,
Germany) was used for the TUNEL assay. Retinal sections from the
various treatment groups were deparaffinized and rehydrated as
mentioned above. The sections were washed with PBS, treated with
proteinase K at 37°C for 30 min and quenched with 3% hydrogen
peroxide at room temperature for 10 min. Following washing with
PBS, the sections were incubated in a TUNEL Label and Enzyme
solution mix at 37°C for 1 h, with two sections incubated in Label
solution only to exclude false positive results. Sections were
subsequently incubated for 5 min with DAPI at room temperature. The
TUNEL-positive cells were counted in a double-blinded manner from
five randomly selected and discontinuous sampling areas under high
magnification using a Leica TCS SP8 microscope (Leica Microsystems
GmbH).
Quantitative cell counts
Five discontinuous images in each retina were
randomly captured under high magnification (x200). A total of 25
randomly selected and discontinuous views from 5 retinas per group
were analyzed for quantitative cell count (n=5 retinas/group). The
number of apoptotic cells, either caspase-3 positive (visible
cellular structures stained brown in color) or TUNEL-positive
(indicated by red fluorescence), were counted in the retinal
ganglion cell layer (GCL) using Image-Pro Plus 6.0 (Media
Cybernetics Inc., Rockville, MD, USA) and the percentage of
apoptotic cells in the rat GCL was calculated.
Statistical analysis
Data in the figures are presented as the mean ±
standard error of the mean and analyzed with GraphPad Prism 5
software (GraphPad Software Inc., La Jolla, CA, USA). One-way
analysis of variance (ANOVA) or the non-parametric Kruskal-Wallis
test was used for comparisons among groups with different
concentrations of ethanol, dopamine or drug treatments, either
together or separately, followed by Tukey's or Fisher's least
significant difference post hoc test. Two-way ANOVA was used for
comparisons among groups with or without ethanol and dopamine. The
Mann-Whitney test or Kruskal-Wallis test was used to compare data
without normality or equal variances between groups, and a P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Ethanol induces neuroapoptosis in
developing rat retina in a dose-dependent manner
Caspase-3 immunohistochemistry and TUNEL staining
were performed on whole-mount retinas to evaluate the effects of
alcohol on developing rats in vitro. The results
demonstrated that ethanol exposure increased the number of
apoptotic cells in P7 rat GCL in a dose-dependent manner (Fig. 1). In particular, 100 mM ethanol
treatment for 5 h did not appear to affect the percentage of
caspase-3-positive or TUNEL-positive cells in the retinal GCL of P7
rats. However, retinas cultured with 200 or 500 mM ethanol
exhibited a significant increase in apoptotic cells: The number of
caspase-3-positive cells increased from 2.3±0.4 to 4.3±0.5%
(P<0.05) and 5.3±0.8% (P<0.01), respectively (Fig. 1A and C); and the TUNEL-positive
cells increased from 12.4±1.4 to 22.1±1.7% (P<0.01) and
37.3±2.7% (P<0.01), respectively (Fig. 1B and D). As 200 mM ethanol is
closer to the blood alcohol concentration in patients with severe
alcohol intoxication (25) and may
better-simulate clinical scenarios, 200 mM ethanol was used in the
following experiments, rather than 500 mM ethanol.
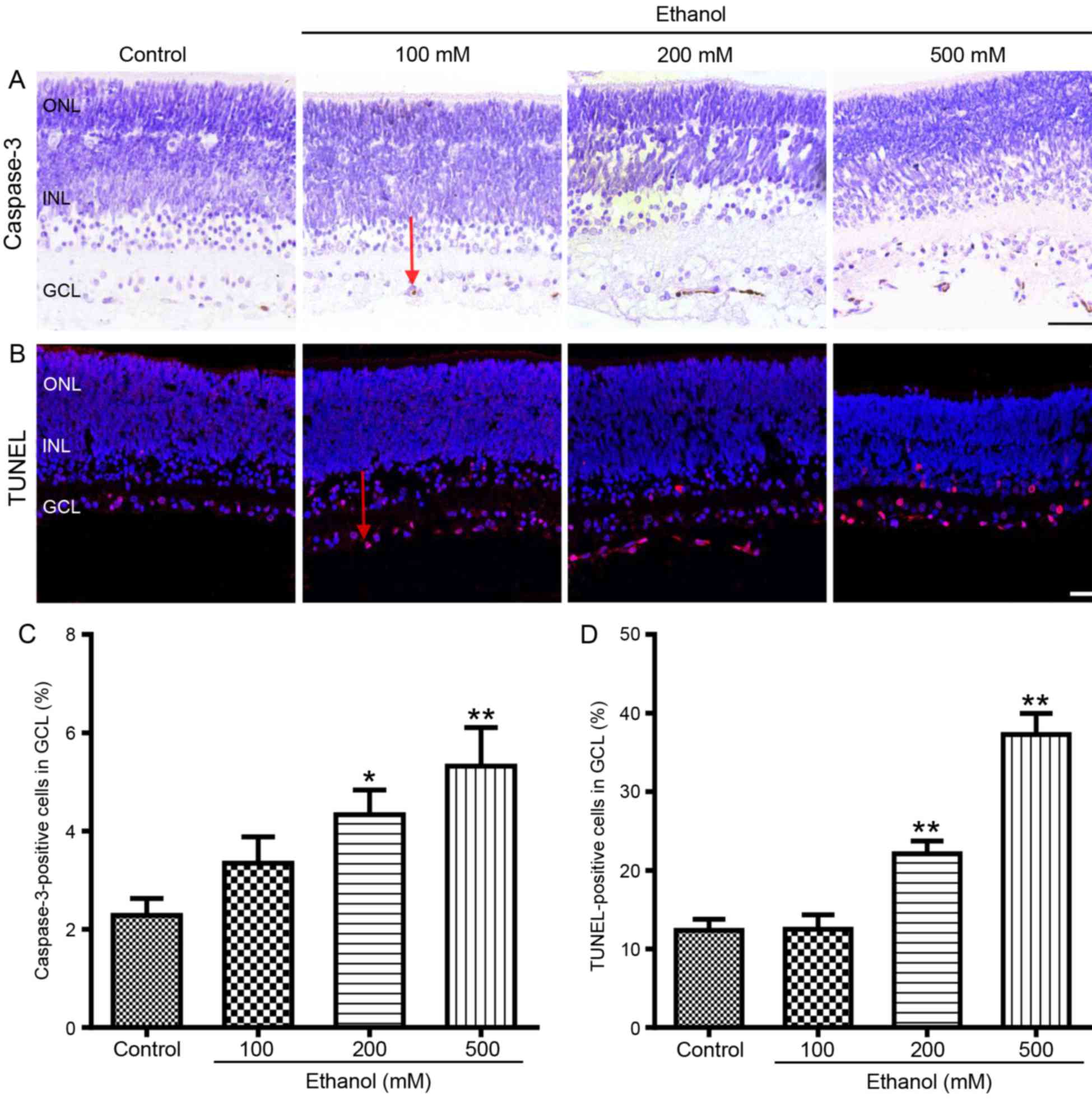 | Figure 1.Ethanol exposure induces
neuroapoptosis in P7 rat retinal GCL. Rat retinas were treated with
ethanol (0, 100, 200 or 500 mM; saline was used for the negative
control) for 5 h and apoptotic cells were detected by caspase-3
immunohistochemistry and by TUNEL assays. (A) Representative
photomicrograph of caspase-3-positive cells (brown color; indicted
with a red arrow) in rat retinal GCL. Scale bar, 50 µm. (B)
Representative photomicrograph of TUNEL-positive cells (red
fluorescence indicated with a red arrow) in rat retinal GCL. Scale
bar, 25 µm. (C) The mean percentages of caspase-3-positive cells
from the various treatment groups. (D) The mean percentages of
TUNEL-positive cells in retinal GCL following the various
treatments. Comparisons were made by one-way analysis of variance
followed by Tukey's post hoc test, and results are presented as the
mean ± standard error of the mean; n=5 retinas/group. *P<0.05
and **P<0.01 vs. control. GCL, ganglion cell layer; INL, inner
nuclear layer; ONL, outer nuclear layer; P7, postnatal day 7;
TUNEL, terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling. |
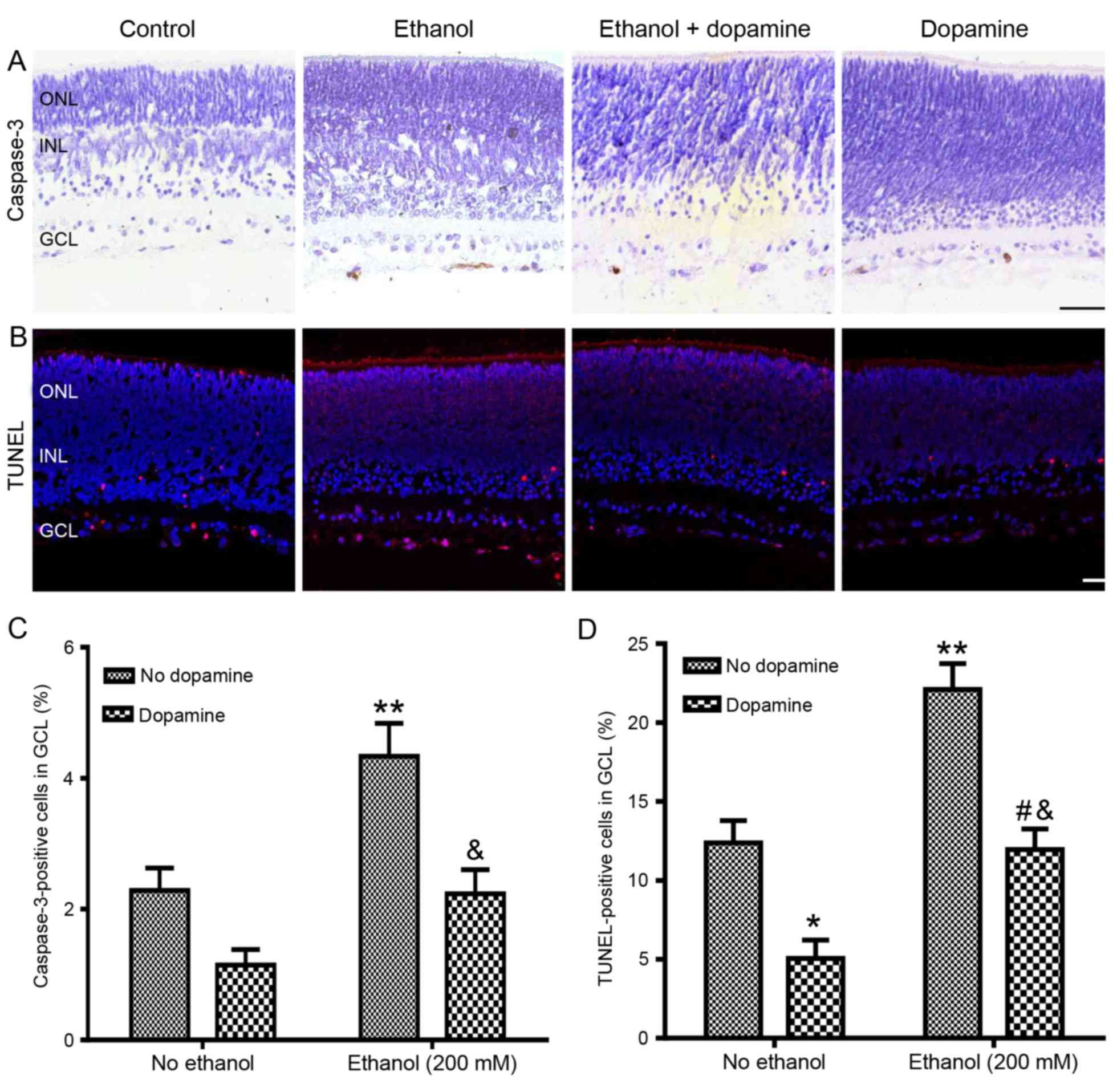
Effects of dopamine on alcohol-induced
neuroapoptosis in rat GCL
P7 rat retinas were treated with or without ethanol
(200 mM) and with or without exogenous dopamine (10 µM) (Fig. 2). Retinas treated with dopamine
alone exhibited a reduction in neuronal apoptosis compared with
untreated control retinas, with the number of caspase-3-positive
cells reduced from 2.3±0.4 to 1.1±0.2%, although this reduction was
determined to be non-significant (P=0.147; Fig. 2A and C), and the number of
TUNEL-positive cells was significantly reduced from 12.4±1.4 to
5.1±1.2% (P<0.05; Fig. 2B and
D). Retinas co-treated with ethanol and dopamine exhibited a
significant reduction in ethanol-induced neuroapoptosis, with the
caspase-3-positive cells reduced from 4.3±0.5 to 2.2±0.37%
(P<0.01 vs. ethanol alone; Fig. 2A
and C), and the TUNEL-positive cells were reduced from
22.1±1.65 to 12.0±1.32% (P<0.01 vs. ethanol alone; Fig. 2B and D). Exogenous dopamine
treatment also significantly reduced 500 mM ethanol-induced
neuroapoptosis in the retinal GCL of P7 rats (data not shown).
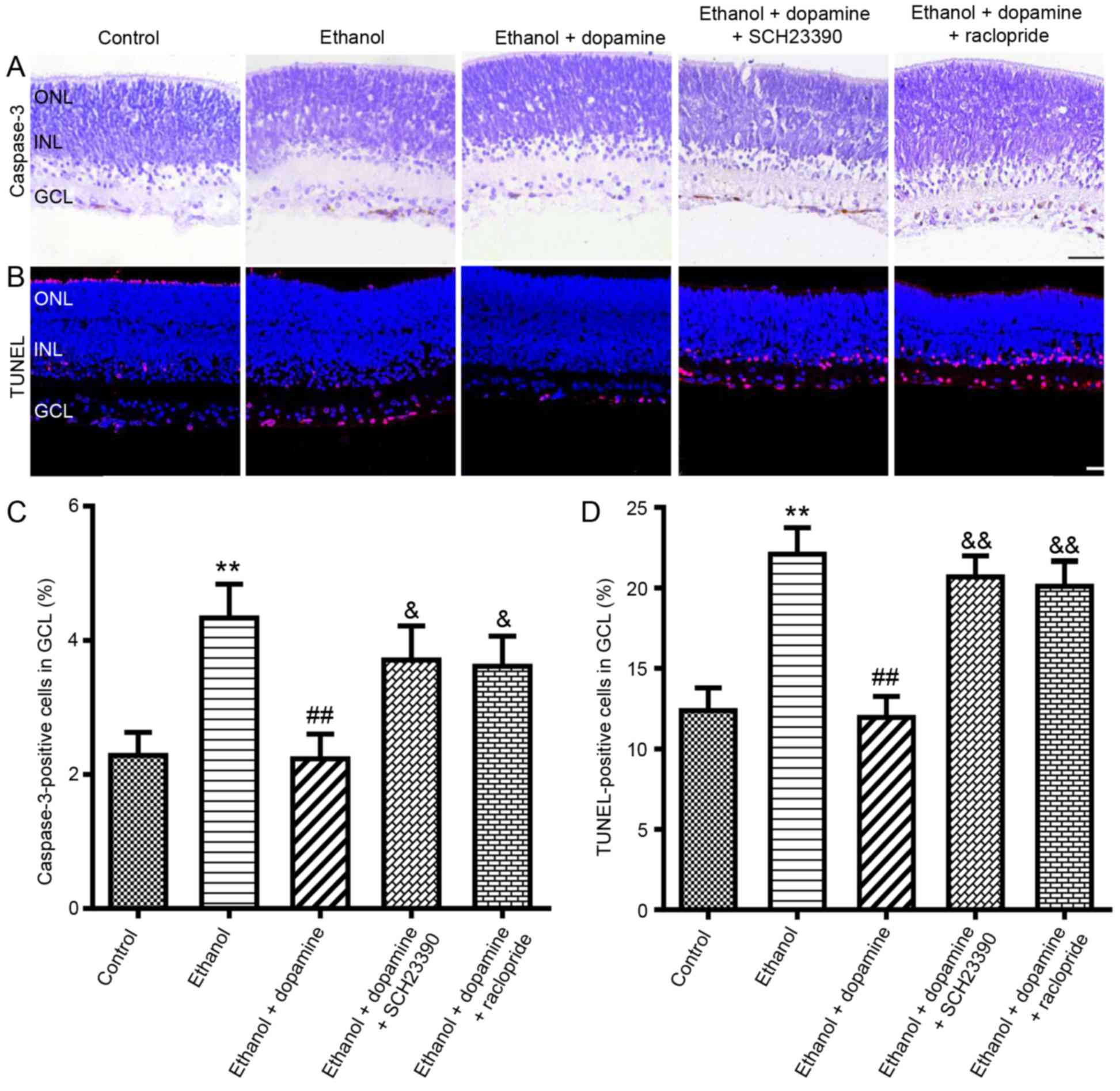
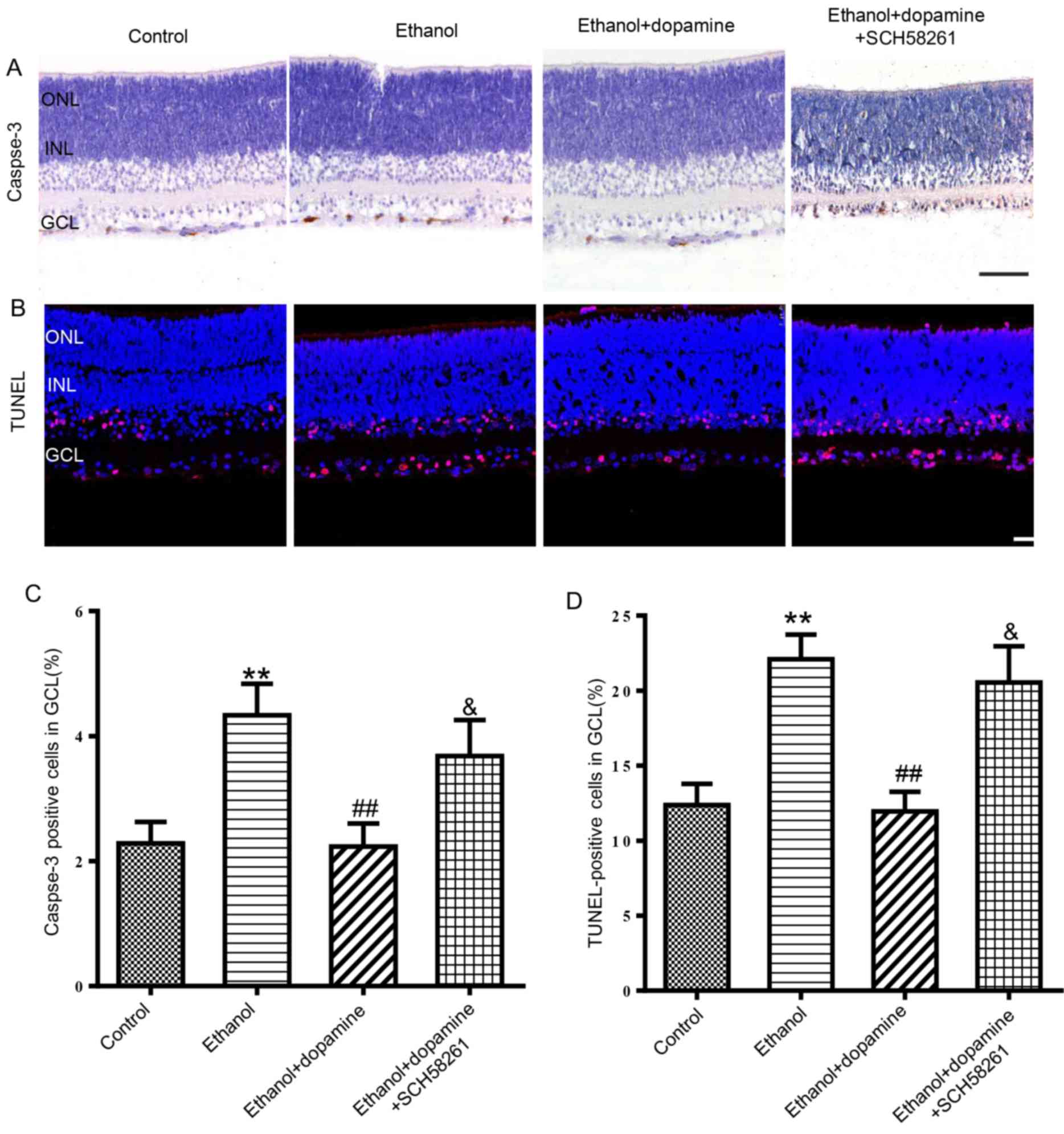
D1R, D2R and AA2AR are involved in the
neuroprotective effects of dopamine against alcohol-induced
neuroapoptosis
To further explore the mechanisms of dopamine
against alcohol-induced neuronal apoptosis, the roles of D1R and
D2R activation against alcohol-induced retinal apoptosis were
examined (Fig. 3), as well as the
role of AA2AR (Fig. 4), as the
dopamine receptors form heteromeric complexes that are comprised of
D1R, D2R and AA2AR. The D1-like receptor antagonist SCH23390 (10
µM) significantly reduced the protective effects of dopamine
against ethanol-induced neuroapoptosis, with the mean number of
caspase-3-positive cells increased from 2.2±0.4 to 3.7±0.5%
(P<0.05; Fig. 3A and C), and
the mean number of TUNEL-positive cells increased from 12.0±1.3 to
20.7±1.3% (P<0.01; Fig. 3B and
D). Co-treatment with the D2-like receptor antagonist
raclopride (40 µM) significantly increased the number of
caspase-3-positive cells from 2.2±0.4 to 3.6±0.5% (P<0.05) and
the number of TUNEL-positive cells from 12.0±1.3 to 20.1±1.5%,
compared with retinas treated with ethanol and dopamine (P<0.01;
Fig. 3). Similar results were
observed in retinas co-treated with the AA2AR antagonist SCH58261
(100 nM), in which the mean number of caspase-3-positive cells
increased from 2.2±0.4 to 3.7±0.6% (P<0.05; Fig. 4A and C) and the number of
TUNEL-positive cells increased from 12.0±1.3 to 20.6±2.4%, compared
with retinas treated with ethanol and dopamine (P<0.05; Fig. 4B and D). The results of a pilot
study (24) demonstrated that
treatment with antagonists alone did not induce neuroapoptosis;
therefore, in the present study, incubations with antagonists alone
were not performed.
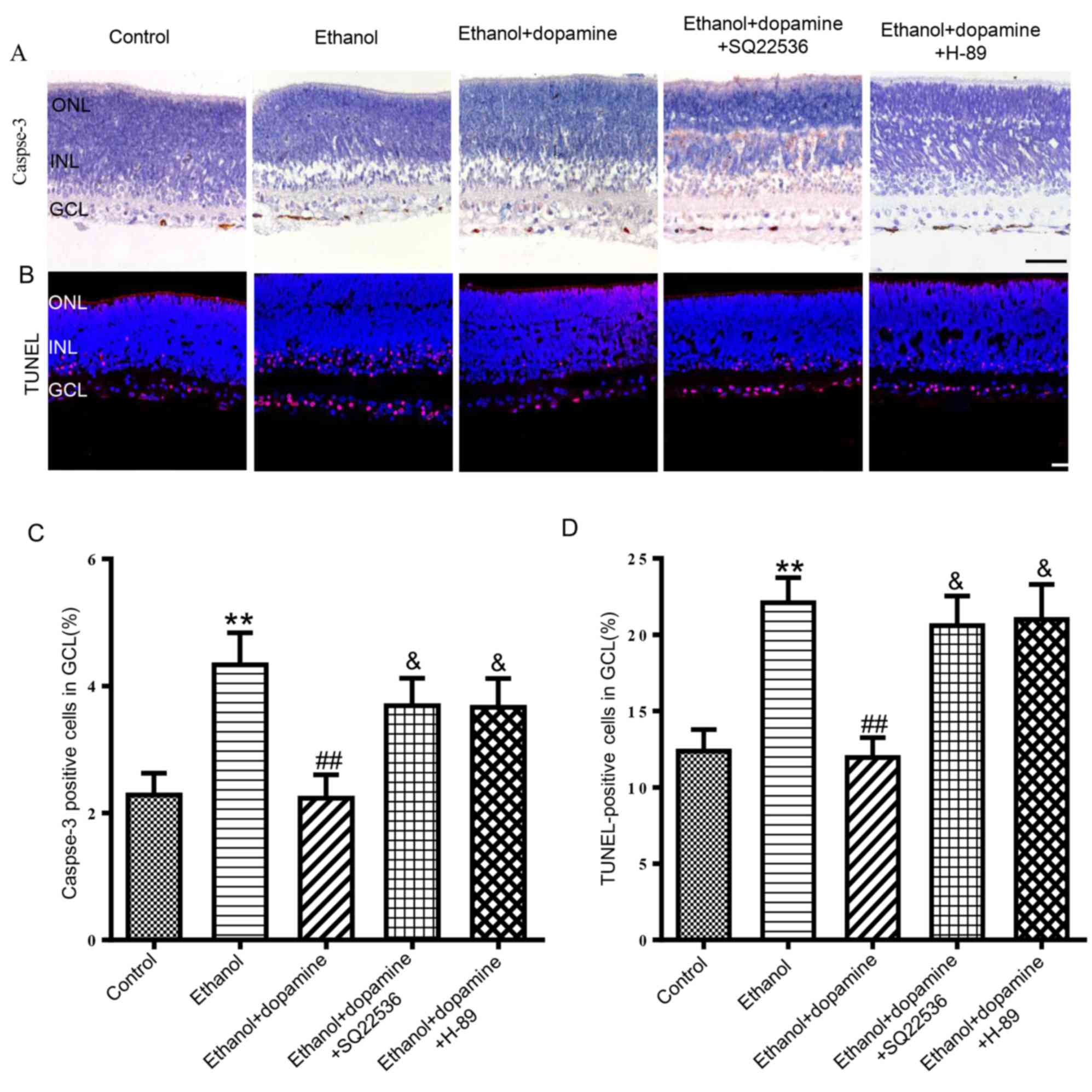
Role of cAMP/PKA signaling in the
dopamine-induced neuroprotective action against alcohol-induced
neuroapoptosis
The activation of AA2AR and D1R has been previously
reported to initiate a cascade of biochemical events, including the
activation of AC and the stimulation of cAMP-dependent protein
kinase (26,27). Therefore, the present study
explored the relationship between the cAMP/PKA signaling pathway
and the protective action of dopamine against alcohol-induced
neuronal apoptosis. Treatment with either an AC inhibitor, SQ22536
(100 µM), or a PKA inhibitor, H-89 (1 µM), significantly attenuated
the protective effects of dopamine against ethanol-induced neuronal
apoptosis (Fig. 5). In particular,
ethanol + dopamine treated retinas that were co-treated with either
SQ22536 or H-89 exhibited an increase in the number of
caspase-3-positive cells from 2.2±0.4 to 3.7±0.4% (P<0.05) and
3.7±0.5% (P<0.05), respectively (Fig. 5A and C). In addition, SQ22536 and
H-89 treatments increased the number of TUNEL-positive cells from
12.0±1.3 to 20.6±1.9% (P<0.05) and 21.0±2.2% (P<0.05),
respectively (Fig. 5B and D).
Discussion
Results from the present study demonstrated that
ethanol treatment was able to induce neuronal apoptosis in
developing P7 rat retinal GCL in a dose-dependent manner.
Administration of exogenous dopamine was revealed to alleviate the
alcohol-induced neuroapoptosis; whereas the inhibition of AA2AR,
D1R or D2R partially reversed the protective effects of dopamine.
In addition, it was determined that the cAMP/PKA signaling pathway
may also be partially involved in the protective action of dopamine
against alcohol-induced neuronal apoptosis in developing rat
GCL.
As previously reported from studies in animals
(28), children with FASD exhibit
retinal developmental defects (29); in rodents, postnatal days 4–10 were
reported to be equivalent to the third trimester of pregnancy in
humans. Anesthesia- and alcohol-induced neuroapoptosis in the
rodent brain is age dependent, and developing rodent brains are
most sensitive to anesthetics and alcohol at the peak of
synaptogenesis (P7) and least sensitive at the end of
synaptogenesis (P14) (30,31). In the developing rodent retina, P7
corresponds to a time point just before birth in humans (32). The present study was able to
consistently induce widespread neuronal apoptosis by treating P7
retinas with 200 mM ethanol for 5 h and, similar to previous
reports on the effects of alcohol on the developing brain,
demonstrated that alcohol exposure caused widespread neuroapoptosis
in the retinas in a dose-dependent manner (5,33).
In contrast to brain slices and cell cultures, whole-mount retinal
cultures possess similar organizational structures and
physiological characteristics with the brain (34). In addition, whole-mount retinal
cultures ensure structural and connective integrity of the neural
network. Retinal cultures also exclude other factors that might
confound results such as those factors associated with general
anesthesia and noxious stimulations in intact animal models,
including hypoxia, CO2 accumulation and stress (34). Therefore, the in vitro
whole-mount retinal culture method used in the present study may be
useful for studying the functions and mechanisms of the central
nervous system.
Although ethanol concentrations in the fetal brain
and retina may be hard to determine, the ethanol concentrations in
the fetal brain and retina should at least be close to maternal
blood ethanol concentration since ethanol easily passes through
blood-brain barrier and blood-placenta barrier (35). According to previous reports, a
single incident of alcohol intoxication during the early postnatal
period was demonstrated to trigger apoptosis in GCL and in neurons
at higher levels of the central nervous system (6). The average blood alcohol
concentration (BAC) of patients with alcohol intoxication in an
adult emergency room is reported to be ~467 mg/dl (100 mM), and
some reported to be >600 mg/dl (25). A previous study demonstrated that
ethanol induced neuroapoptosis in a time- and dose-dependent manner
(36). In addition, a previous
study demonstrated that ketamine induced rat retinal neuroapoptosis
following incubation of the eyeballs for 5 h (24); therefore the eyeballs were
incubated with ethanol for 5 h in the present study. Although 100
mM ethanol did not significantly increase apoptosis in the present
study, retinas treated with 200 or 500 mM ethanol exhibited a
significant increase in apoptosis, which was similar to a previous
in vivo and in vitro study (36). Previous in vivo studies
revealed that the optimal time for visualizing caspase-3 activation
was at 8 h following the first dose of subcutaneous ethanol
administration, and the blood ethanol concentration reaches peak
levels (500 mg/dl; 108.7 mM) at 3 h following the first dose
(37). Previous in vitro
studies demonstrated that the concentration-dependent increase in
caspase-3 activity induced by ethanol (100–500 mM) reached maximal
levels at ~12 h post-ethanol exposure (36). Therefore, the 100 mM ethanol
treatment used in the present study did not significantly increase
apoptosis, which may be due to the short incubation time (5 h) or
the incubation of the eyeball with ethanol in vitro rather
than injecting the ethanol subcutaneously in vivo. In
addition, ethanol evaporation cannot be completely ruled out in the
present study, even though compensatory strategies were used.
The different percentages of neuroapoptosis detected
by caspase-3 immunohistochemistry and the TUNEL assay in the
present study may be due to the ephemeral phenomenon of the
caspase-3 assay or caspase-3 independent neuronal apoptosis
(6,36). Although necrosis cannot be
completely ruled out, the present study demonstrated that the
percent of neuroapoptosis detected by the caspase-3 assay and the
TUNEL assay increased as the concentration of ethanol increased
from 200 to 500 mM, confirming that lower ethanol (<500 mM)
exposure caused neuronal death primarily in the form of apoptosis,
as demonstrated in a previous study (36).
As a second messenger, cAMP modulates numerous
physiological functions and pathophysiological changes; for
example, cAMP has been reported to be involved in alcohol-induced
neuroapoptosis as either a pro- or an anti-apoptotic messenger
(19,38). The present study demonstrated that
inhibition of AC and PKA significantly reduced the protective
effects of dopamine against alcohol-induced neuronal apoptosis.
This result suggested that dopamine may be able to attenuate
ethanol-induced neuroapoptosis partially through the activation of
the cAMP/PKA signaling pathway, which is consistent with previous
studies that reported a downregulation of intracellular cAMP and
PKA following alcohol exposure (19,39).
Data from the present study are consistent with a previous report
that suggested that cAMP attenuates apoptosis in developing
hypothalamic cells (39). It
should be noted that the present results differ from certain
previous studies that evaluated the effects of alcohol on
relatively mature neurons (>4–6 weeks old), which demonstrated
that alcohol exposure induced an increase in intracellular levels
of cAMP and PKA type II regulatory subunits in the rat brain
(20), as well as brain PKA
activation in mice (40).
Dopamine is associated with a spectrum of
neurophysiological processes, including the regulation of neuronal
differentiation, axonal and/or dendritic growth in the developing
brain and retina (41,42). D1R and D2R were previously
demonstrated to be widely distributed in the inner plexiform layer,
the ganglion cell layer, the outer plexiform layer and the
photoreceptors, where they are activated by dopamine released from
dopaminergic amacrine cell and/or interplexiform dopaminergic cells
in the retinal inner plexiform layer (43,44).
Although the activation of D1R is generally considered to be
related to the increase in intracellular cAMP and enhancement of
neuronal apoptosis and activation of D2 receptor is opposite, an
increasing number of studies have reported that D1R and/or D2R may
form different heteromeric complexes with AA2AR to produce
different effects (9,10). Furthermore, it has been postulated
that D2R may be able to synergize with AA2AR to stimulate AC
expression (45). In addition, to
activate cAMP-dependent processes through the co-activation of D1R
and D2R (46), dopamine may also
activate a heterodimer of D1R and D2R to generate a
calcium-dependent signaling pathway (47), indicating that dopamine
heteroreceptor complexes, or the synergy between D2R and AA2AR, may
be involved in the protective effects of dopamine. Therefore, it
may be possible that the inhibition of D1R, D2R or AA2AR was able
to bring the dopamine-induced reduction in apoptosis back to
similar levels in retinas treated with ethanol alone. Furthermore,
inhibition of the cAMP/PKA signaling pathway reduced the protective
effects of dopamine on alcohol-induced neuroapoptosis in developing
rat retina, indicating that cAMP/PKA signaling pathway may be
involved in this process.
A few limitations to the present study should be
noted. First, it is unclear whether dopamine heteroreceptor
complexes or the synergy between D2R and AA2AR are involved in the
protective action of dopamine on the alcohol-induced apoptosis that
was observed in developing rat GCL. In addition, the intracellular
levels of cAMP/PKA and the downstream targets of the cAMP/PKA
signaling pathway, such as extracellular signal-regulated kinase
1/2, proto-oncogene c-Akt and cAMP-responsive element-binding
protein also need to be investigated. As neuroapoptosis in response
to ethanol treatment was most marked at P7 in the rat GCL, the
retinal GCL was the focus of the present study; future studies are
required to analyze the effect of ethanol on other retinal
layers.
In conclusion, the present study demonstrated that
dopamine treatment was able to attenuate ethanol-induced
neuroapoptosis in developing P7 rat retinas, possibly through the
activation of D1R, D2R and AA2AR, as well as by upregulating the
cAMP/PKA signaling pathway.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (Beijing, China; grant no. 81271263 to
Jijian Zheng and grant no. 81270414 to Mazhong Zhang) and The
Shanghai Municipal Commission of Health and Family Planning, Key
Developing Disciplines (grant no. 2015ZB0106).
References
|
1
|
Clarren SK and Smith DW: The fetal alcohol
syndrome. N Engl J Med. 298:1063–1067. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sulik KK, Johnston MC and Webb MA: Fetal
alcohol syndrome: Embryogenesis in a mouse model. Science.
214:936–938. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
May PA, Gossage JP, Kalberg WO, Robinson
LK, Buckley D, Manning M and Hoyme HE: Prevalence and epidemiologic
characteristics of FASD from various research methods with an
emphasis on recent in-school studies. Dev Disabil Res Rev.
15:176–192. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
May PA, Baete A, Russo J, Elliott AJ,
Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J,
Abdul-Rahman O, et al: Prevalence and characteristics of fetal
alcohol spectrum disorders. Pediatrics. 134:855–866. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikonomidou C, Bittigau P, Ishimaru MJ,
Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F,
Tenkova T, et al: Ethanol-induced apoptotic neurodegeneration and
fetal alcohol syndrome. Science. 287:1056–1060. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tenkova T, Young C, Dikranian K, Labruyere
J and Olney JW: Ethanol-induced apoptosis in the developing visual
system during synaptogenesis. Invest Ophthalmol Vis Sci.
44:2809–2817. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Creeley CE, Dikranian KT, Johnson SA,
Farber NB and Olney JW: Alcohol-induced apoptosis of
oligodendrocytes in the fetal macaque brain. Acta Neuropathol
Commun. 1:232013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olney JW: Focus on apoptosis to decipher
how alcohol and many other drugs disrupt brain development. Front
Pediatr. 2:812014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beaulieu JM and Gainetdinov RR: The
physiology, signaling, and pharmacology of dopamine receptors.
Pharmacol Rev. 63:182–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beaulieu JM, Espinoza S and Gainetdinov
RR: Dopamine receptors-IUPHAR Review 13. Br J Pharmacol. 172:1–23.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naseer MI, Ullah I, Rasool M, Ansari SA,
Sheikh IA, Bibi F, Chaudhary AG, Al-Qahtani MH and Kim MO:
Downregulation of dopamine D1 receptors and increased neuronal
apoptosis upon ethanol and PTZ exposure in prenatal rat cortical
and hippocampal neurons. Neurol Sci. 35:1681–1688. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaarmann A, Kovac S, Holmström KM, Gandhi
S and Abramov AY: Dopamine protects neurons against
glutamate-induced excitotoxicity. Cell Death Dis. 4:e4552013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li HZ, Guo J, Gao J, Han LP, Jiang CM, Li
HX, Bai SZ, Zhang WH, Li GW, Wang LN, et al: Role of dopamine D2
receptors in ischemia/reperfusion induced apoptosis of cultured
neonatal rat cardiomyocytes. J Biomed Sci. 18:182011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Canals M, Marcellino D, Fanelli F, Ciruela
F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods
AS, et al: Adenosine A2A-dopamine D2 receptor-receptor
heteromerization: Qualitative and quantitative assessment by
fluorescence and bioluminescence energy transfer. J Biol Chem.
278:46741–46749. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D'Mello SR, Galli C, Ciotti T and
Calissano P: Induction of apoptosis in cerebellar granule neurons
by low potassium: Inhibition of death by insulin-like growth factor
I and cAMP. Proc Natl Acad Sci USA. 90:10989–10993. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meyer-Franke A, Kaplan MR, Pfrieger FW and
Barres BA: Characterization of the signaling interactions that
promote the survival and growth of developing retinal ganglion
cells in culture. Neuron. 15:805–819. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanson MG Jr, Shen S, Wiemelt AP, McMorris
FA and Barres BA: Cyclic AMP elevation is sufficient to promote the
survival of spinal motor neurons in vitro. J Neurosci.
18:7361–7371. 1998.PubMed/NCBI
|
|
18
|
Sapru MK, Diamond I and Gordon AS:
Adenosine receptors mediate cellular adaptation to ethanol in
NG108-15 cells. J Pharmacol Exp Ther. 271:542–548. 1994.PubMed/NCBI
|
|
19
|
Han JY, Jeong JY, Lee YK, Roh GS, Kim HJ,
Kang SS, Cho GJ and Choi WS: Suppression of survival kinases and
activation of JNK mediate ethanol-induced cell death in the
developing rat brain. Neurosci Lett. 398:113–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gigante ED, Santerre JL, Carter JM and
Werner DF: Adolescent and adult rat cortical protein kinase A
display divergent responses to acute ethanol exposure. Alcohol.
48:463–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Z, Liu Y, Gao R, Li H, Dunn T, Wu P,
Smith RG, Sarkar PS and Fang X: Ethanol suppresses PGC-1α
expression by interfering with the cAMP-CREB pathway in neuronal
cells. PLoS One. 9:e1042472014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Yang Z, Sun Y, Zhou H, Chu G,
Zhang J and Meng X: Ethanol activation of PKA mediates
single-minded 2 expression in neuronal cells. Mol Neurobiol.
52:1234–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kadam RS, Williams J, Tyagi P, Edelhauser
HF and Kompella UB: Suprachoroidal delivery in a rabbit ex vivo eye
model: Influence of drug properties, regional differences in
delivery, and comparison with intravitreal and intracameral routes.
Mol Vis. 19:1198–1210. 2013.PubMed/NCBI
|
|
24
|
Dong J, Gao L, Han J, Zhang J and Zheng J:
Dopamine attenuates ketamine-induced neuronal apoptosis in the
developing rat retina independent of early synchronized spontaneous
network activity. Mol Neurobiol. 54:3407–3417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minion GE, Slovis CM and Boutiette L:
Severe alcohol intoxication: A study of 204 consecutive patients. J
Toxicol Clin Toxicol. 27:375–384. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fredholm BB, Arslan G, Halldner L, Kull B,
Schulte G and Wasserman W: Structure and function of adenosine
receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol.
362:364–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song ZM, Undie AS, Koh PO, Fang YY, Zhang
L, Dracheva S, Sealfon SC and Lidow MS: D1 dopamine receptor
regulation of microtubule-associated protein-2 phosphorylation in
developing cerebral cortical neurons. J Neurosci. 22:6092–6105.
2002.PubMed/NCBI
|
|
28
|
Dursun I, Jakubowska-Doğru E, van der List
D, Liets LC, Coombs JL and Berman RF: Effects of early postnatal
exposure to ethanol on retinal ganglion cell morphology and numbers
of neurons in the dorsolateral geniculate in mice. Alcohol Clin Exp
Res. 35:2063–2074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ribeiro IM, Vale PJ, Tenedorio PA,
Rodrigues PA, Bilhoto MA and Pereira HC: Ocular manifestations in
fetal alcohol syndrome. Eur J Ophthalmol. 17:104–109.
2007.PubMed/NCBI
|
|
30
|
Bayer SA, Altman J, Russo RJ and Zhang X:
Timetables of neurogenesis in the human brain based on
experimentally determined patterns in the rat. Neurotoxicology.
14:83–144. 1993.PubMed/NCBI
|
|
31
|
Yon JH, Daniel-Johnson J, Carter LB and
Jevtovic-Todorovic V: Anesthesia induces neuronal cell death in the
developing rat brain via the intrinsic and extrinsic apoptotic
pathways. Neuroscience. 135:815–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng Y, He L, Prasad V, Wang S and Levy
RJ: Anesthesia-induced neuronal apoptosis in the developing retina:
A window of opportunity. Anesth Analg. 121:1325–1335. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikonomidou C, Bosch F, Miksa M, Bittigau
P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L and
Olney JW: Blockade of NMDA receptors and apoptotic
neurodegeneration in the developing brain. Science. 283:70–74.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ogilvie JM, Speck JD, Lett JM and Fleming
TT: A reliable method for organ culture of neonatal mouse retina
with long-term survival. J Neurosci Methods. 87:57–65. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cammer W, Tansey F, Abramovitz M, Ishigaki
S and Listowsky I: Differential localization of
glutathione-S-transferase Yp and Yb subunits in oligodendrocytes
and astrocytes of rat brain. J Neurochem. 52:876–883. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nowoslawski L, Klocke BJ and Roth KA:
Molecular regulation of acute ethanol-induced neuron apoptosis. J
Neuropathol Exp Neurol. 64:490–497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Young C, Klocke BJ, Tenkova T, Choi J,
Labruyere J, Qin YQ, Holtzman DM, Roth KA and Olney JW:
Ethanol-induced neuronal apoptosis in vivo requires BAX in the
developing mouse brain. Cell Death Differ. 10:1148–1155. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Insel PA, Zhang L, Murray F, Yokouchi H
and Zambon AC: Cyclic AMP is both a pro-apoptotic and
anti-apoptotic second messenger. Acta Physiol (Oxf). 204:277–287.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boyadjieva NI and Sarkar DK: Cyclic
adenosine monophosphate and brain-derived neurotrophic factor
decreased oxidative stress and apoptosis in developing hypothalamic
neuronal cells: Role of microglia. Alcohol Clin Exp Res.
37:1370–1379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balino P, Ledesma JC and Aragon CM: In
vivo study of ethanol-activated brain protein kinase A:
Manipulations of Ca2+ distribution and flux. Alcohol
Clin Exp Res. 38:629–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Girault JA and Greengard P: The
neurobiology of dopamine signaling. Arch Neurol. 61:641–644. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Witkovsky P: Dopamine and retinal
function. Doc Ophthalmol. 108:17–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bjelke B, Goldstein M, Tinner B, Andersson
C, Sesack SR, Steinbusch HW, Lew JY, He X, Watson S, Tengroth B and
Fuxe K: Dopaminergic transmission in the rat retina: Evidence for
volume transmission. J Chem Neuroanat. 12:37–50. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ogata G, Stradleigh TW, Partida GJ and
Ishida AT: Dopamine and full-field illumination activate D1 and
D2-D5-type receptors in adult rat retinal ganglion cells. J Comp
Neurol. 520:4032–4049. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kudlacek O, Just H, Korkhov VM, Vartian N,
Klinger M, Pankevych H, Yang Q, Nanoff C, Freissmuth M and Boehm S:
The human D2 dopamine receptor synergizes with the A2A adenosine
receptor to stimulate adenylyl cyclase in PC12 cells.
Neuropsychopharmacology. 28:1317–1327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hopf FW, Cascini MG, Gordon AS, Diamond I
and Bonci A: Cooperative activation of dopamine D1 and D2 receptors
increases spike firing of nucleus accumbens neurons via G-protein
betagamma subunits. J Neurosci. 23:5079–5087. 2003.PubMed/NCBI
|
|
47
|
Ng J, Rashid AJ, So CH, O'Dowd BF and
George SR: Activation of calcium/calmodulin-dependent protein
kinase IIalpha in the striatum by the heteromeric D1-D2 dopamine
receptor complex. Neuroscience. 165:535–541. 2010. View Article : Google Scholar : PubMed/NCBI
|