Introduction
Non-small cell lung cancer (NSCLC) is one of the
most common types of cancer, owing to poor air quality and
environmental pollution in the world (1). A previous study reported that tumor
growth, migration and invasion in NSCLC are the most important
features in tumor metastasis, development and reoccurrence
(2). The occurrence of NSCLC has
increased due to industrial pollution and the degradation of the
ecological environment in the last century (3,4).
NSCLC comprises adenocarcinoma, large cell carcinoma and squamous
cell carcinoma, and is the most frequent type of lung cancer, which
accounts for ~80% of all lung cancer cases (5–7).
Despite increasing therapeutic improvements for NSCLC, the survival
rates of patients with NSCLC are poor, with overall 5-year survival
rates of <15%, indicating it a critical clinical problem
(6,8,9). In
addition, the migration and invasion of NSCLC are the primary
reasons for the poor survival rates in treatment and recurrence for
patients with NSCLC (10,11). Therefore, inhibiting the migration
and invasion of NSCLC may offer potential as an efficient
therapeutic schedule for patients with cancer (12,13).
Li et al reported that an increase in a
pre-mRNA splicing regulator for serine-arginine protein kinase-1
(SRPK-1) increased cancer development and metastasis (14). Clinical investigations of the
expression of SRPK-1 in NSCLC have also been performed, the results
of which indicated that SRPK-1 exerted an oncogenic function in
NSCLC and suggested that SRPK-1 may serve as a therapeutic target
for patients with NSCLC (15). The
increased expression of SRPK-1 is correlated with disease grade in
several types of tumor, including retinoblastoma, breast cancer,
pancreatic cancer and colon cancer (15–18).
In the present study, a chimeric antibody target for SRPK-1
(ChanSRPK-1) was constructed and used to examine whether ChanSRPK-1
showed beneficial efficacy on the increased expression of SRPK1 in
an NSCLC mouse model. The present study also evaluated the possible
long-term survival of NSCLC-bearing mice, and investigated the
therapeutic effects on expression of the β-catenin/T-cell factor
(TCF) complex and the phosphorylation levels of glycogen synthase
kinase 3-β (GSK3-β) in cancer-associated processes.
The development of effective drugs to inhibit tumor
cell growth, migration and invasion in patients with NSCLC has
already focused on public and personalized medication. A previous
study indicated that SRPK-1 was specifically expressed in
epithelial cells in normal breast and colon cells (18). Of note, ChanSRPK-1-mediated
neutralization has been demonstrated as a novel and beneficial
antibody in the progress and metastasis of NSCLC (19).
Although suppressing the expression of SRPK-1 in
tumor cells has demonstrated beneficial outcomes, the use of
anti-SRPK-1 antibody for NSCLC therapy has not been investigated.
The present study aimed to investigate the expression of SRPK-1,
and the inhibitory effects of ChanSRPK-1 on migration and invasion
in an NSCLC mouse model. ChanSRPK-1 inhibited the mRNA expression
levels of TCF, matrix metalloproteinase-9 (MMP), collagen type I
(CT1) and fibronectin (FBC) in NSCLC-derived vascular endothelial
cells (NSCLCDVECs). The present study also investigated the
therapeutic outcomes and molecular mechanism via long-term
treatment with ChanSRPK-1, which indicated that the migration and
invasion of NSCLC were inhibited following treatment with
ChanSRPK-1.
Materials and methods
Cell culture
The MRC-5 cells were purchased from American Type
Culture Collection (Manassas, VA, USA). The NSCLCDVECs were a
provided by the University of Toronto (Toronto, Canada). The MRC-5
cells and NSCLCDVECs were cultured in EMEM supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C, 5% CO2 and reasonable
humidity.
MTT assay
The NSCLCDVECs (5×103) were grown in 12-well plates
to ~95% monolayer cells. Subsequently, either ChanSRPK-1 (400
mg/ml) or PBS was added to the plates for 24, 48, 72 and 96 h at
37°C, respectively. MTT, at concentration of 5 mg/ml (50 µl;
Amresco, LLC, Solon, OH, USA), was added to the cells and incubated
for 4 h. DMSO was added for incubation for 30 min to dissolve the
precipitate following removal of the supernatant. The results were
determined using a spectrophotometer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 570 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total mRNA was isolated from the MRC-5 cells and
NSCLCDVECs pre- and post-treatment with ChanSRPK-1 using an RNAeasy
Mini kit (Qiagen Sciences, Inc., Gaithersburg, MD). The total mRNA
(1 µg) was reverse transcribed into cDNA using a reverse
transcription kit (Qiagen Sciences, Inc.). The cDNA (10 ng) was
used for qPCR (Bio-Rad Laboratories, Inc. Hercules, CA, USA) with
the SYBR Green Master Mix system (50 ng of genomic DNA, 200 µM
dNTP, 2.5 units of Taq DNA polymerase, and 200 µM primers)
according to manufacturers' protocols, followed by preliminary
denaturation at 94°C for 2 min, 35 cycles at 94°C for 30 sec,
annealing temperature reduced to 64°C for 30 sec and 72°C for 10
min. All forward and reverse primers (Table I) were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.). Relative mRNA expression changes
were calculated using the 2−ΔΔCq method (20). The results are expressed as the
fold change, compared with the control.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Target gene | Forward primer | Reverse primer |
|---|
| SPRK-1 |
5′-GGACCGTCTTCGGACATCA-3′ |
5′-ATCTTTTGGGGTCCGTCAACT-3′ |
| TCF |
5′-TGACCCATCTCAGAAGCAG-3′ |
5′-GCAGAGAGGAGGTTGACTTTC-3′ |
| MMP |
5′-TGCCCACCGTCCTTTCTTGTT-3′ |
5′-GCTGACTTGACTCATGGCT-3′ |
| CT1 |
5′-CACAAAGCAGCCTTGCAGAA-3′ |
5′-AGAGCAGGCAGCATAGCAGTG-3′ |
| FBC |
5′-AACCCGAGGTATGCTTATCT-3′ |
5′-CCAGTTCTTCATTGCATTGC-3′ |
| β-actin |
5′-GTGGGCGCCCAGGCACCA-3′ |
5′-CTCCTTAATGTCACGCACGATTT-3′ |
Cell viability assay
The MRC-5 cells and NSCLCDVECs were grown in a
24-well plate at a concentration of 2.5×105/ml (total column of
1,000 µl). The MRC-5 cells and NSCLCDVECs were treated with
ChanSRPK-1 (400 mg/ml) or PBS (control) for 48 h at 37°C. The
viabilities of the MRC-5 cells and NSCLCDVECs were examined using a
Cell Counting kit (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) in eight view windows.
Cell invasion and migration
assays
The MRC-5 cells and NSCLCDVECs were treated with
ChanSRPK-1 or PBS (control). For the invasion assay, the cells were
suspended as a density of 1×105 in 500 µl serum-free EMEM.
Following treatment of the MRC-5 cells and NSCLCDVECs with
ChanSRPK-1 or PBS, the cells were added to the upper chamber of BD
BioCoat Matrigel Invasion chambers (BD Biosciences, Franklin Lakes,
NJ, USA) according to the manufacturer's protocol. For the
migration assay, the cells were inoculated with ChanSRPK-1 or PBS
for 48 h using a control insert (BD Biosciences) rather than a
Matrigel invasion chamber. The tumor cells invasion and migration
were counted in at least three randomly selected stained MRC-5
cells or NSCLCDVECs fields from every membrane under a light
microscope (N-STORM, Nikon Corporation, Tokyo, Japan).
ELISA
The levels of SRPK-1 were determined using a
commercial ELISA kit (CSB-EL022682MO, Cusabio Biotech Co., Ltd,
Wuhan, China) for SRPK-1, and the procedure was performed according
to the manufacturer's protocol. Briefly, ChanSRPK-1 (20–420 µg/ml,
50 µl) was added to ELISA plates wells for 30 min at 37°C. These
were then washed three times with PBS and followed by blocking for
2 h with 300 µl 5% non-fat dried milk in PBS. Then, 100 µl
horseradish peroxidase (HRP)-conjugated SRPK-1 (0.2 µg/ml) was
added to the wells and incubated for 1 h at 37°C. The plates were
washed three times with PBS and absorbance was measured at 450 nm
in an ELISA reader.
Western blot analysis
The NSCLCDVECs were treated with ChanSRPK-1 (400
ng/ml) or PBS for 48 h. The NSCLCDVECs were then harvested by
scraping and were lysed in RIPA buffer, followed by homogenization
at 4°C for 10 min. A total of 20 µg protein extracts was
electrophoresed on 12.5% polyacrylamide gradient gels and then
transferred to nitrocellulose membranes. The membranes were
incubated in blocking buffer (5% milk) prior to incubation with
primary antibodies at 4°C overnight. The ChanSRPK-1-treated and
PBS-treated NSCLCDVECs were inoculated with the correlating mouse
anti-human primary antibody: SRPK-1 (ab50927; 1:500),
Cytochalasin-D (ab143484; 1:500), G-actin (ab123034; 1:500) and
β-actin (ab5694; 1:500; all from Abcam, China) for 12 h at 4°C. All
samples were washed with Tris-buffered saline and incubated with
HRP-conjugated bovine anti-mouse IgG antibodies (sc-2371; 1:10,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 h at 37°C.
The results were visualized by using a chemiluminescence detection
system (BD Biosciences).
Animal experiments
A total of 100 specific pathogen-free male BABL/C
(six-week old, body weight: 32–36 g) mice were purchased from
Shanghai Slack Experimental Animals Co., Ltd. (Shanghai, China).
All mice were housed in a temperature-controlled facility at 23±1°C
and treated in accordance with the Guide for the Care and Use of
Laboratory Animals of Tianjin Chest Hospital (Tianjin, China). Mice
were maintained at a 12-h light/dark cycle with free access to food
and water. The mice were implanted with NSCLCDVECs and were divided
into two groups, which received ChanSRPK-1 or PBS treatment (n=50
in each group). Treatment was started 7 days post-tumor
implantation, when the tumor diameter reached 6–8 mm. The
tumor-bearing mice were intravenously injected with ChanSRPK-1 (400
ng), with the same volume PBS injected in the control group mice.
The treatment was continued for 14 days (one injection per day).
The tumor volumes were calculated according to a previous study
(21).
Statistical analysis
All data are presented as the means ± standard error
of the mean of triplicate experiments. Unpaired data was analyzed
using Student's t-test and comparisons of data between multiple
groups were analyzed by one-way analysis of variance (GraphPad
version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). The
Kaplan-Meier test was used to estimate survival during the 120-day
observation period. P<0.05 was considered to indicate a
statistically significant difference.
Results
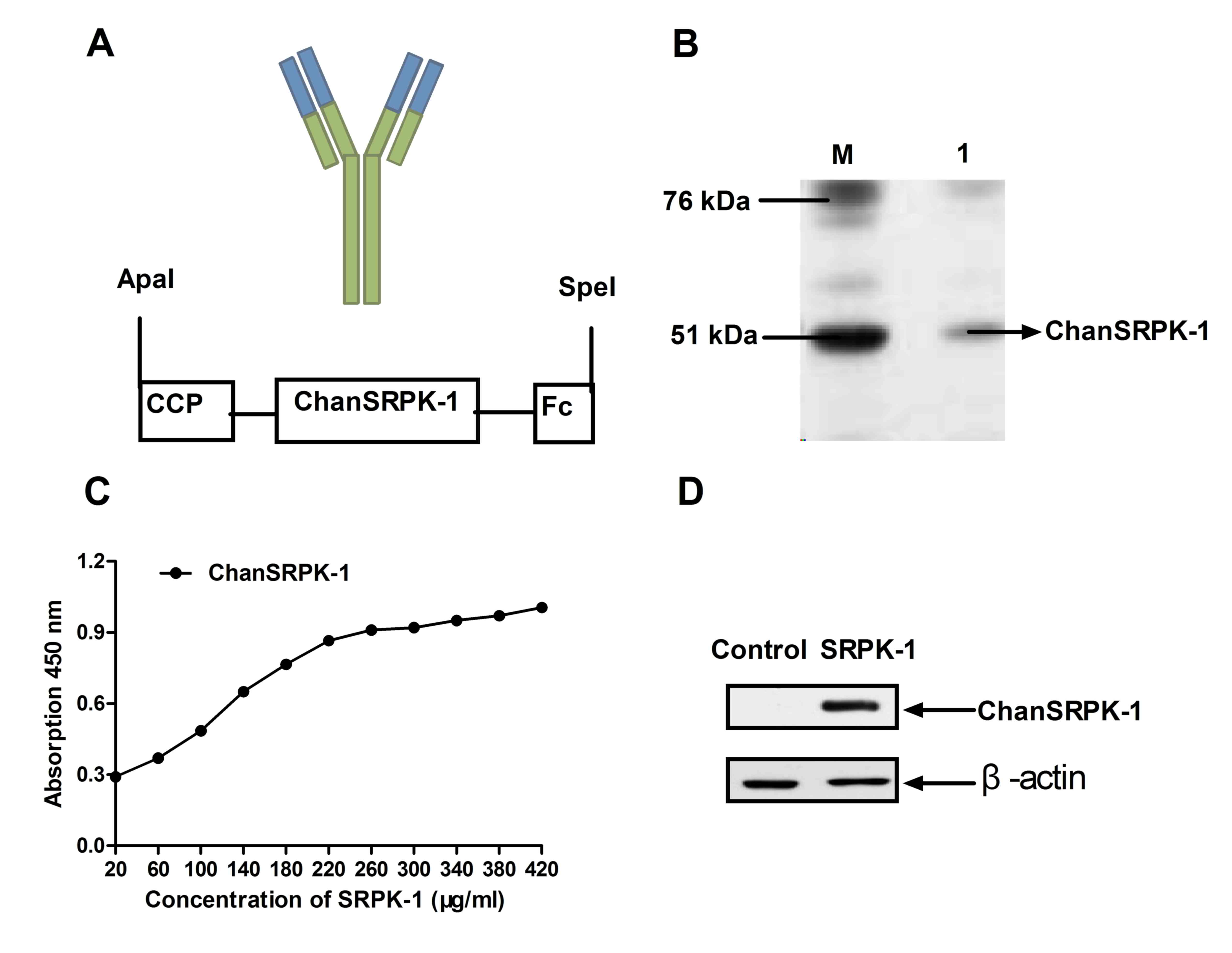
Construction of a chimeric antibody
targeting SRPK-1 and determination of in vitro activity
The antibody targeting of SRPK1 was screened using a
conventional approach and the constant region of antibody was
replaced with a constant region from human antibody. To enhance the
stability and half-life of the antibody targeting SRPK-1, the
crystalline fragment was linked to the antibody targeting SRPK-1,
and ChanSRPK-1 was analyzed. The structure of ChanSRPK-1,
containing the crystalline fragment and cell-penetrating peptide to
enhance stability and transmembrane ability, is shown in Fig. 1A. ChanSRPK-1 was expressed by
pET-27b in Escherichia coli. As shown in Fig. 2B, the molecular weight of
ChanSRPK-1 was ~67.5 kDa, determined using SDS-PAGE gel
electrophoresis. In addition, the affinity of ChanSRPK-1 for SRPK-1
was determined using ELISA and western blot analysis. The results
(Fig. 1C) demonstrated that
ChanSRPK-1 maintained a high affinity with SRPK-1 in the ELISA
assay. A band at ~67.5 kDa was confirmed by SDS-PAGE gel
electrophoresis, and the specific binding to SRPK-1 was confirmed
using western blot analysis (Fig.
1D). These results indicated that ChanSRPK-1 was expressed at a
high level and had the capacity to bind with SRPK-1.
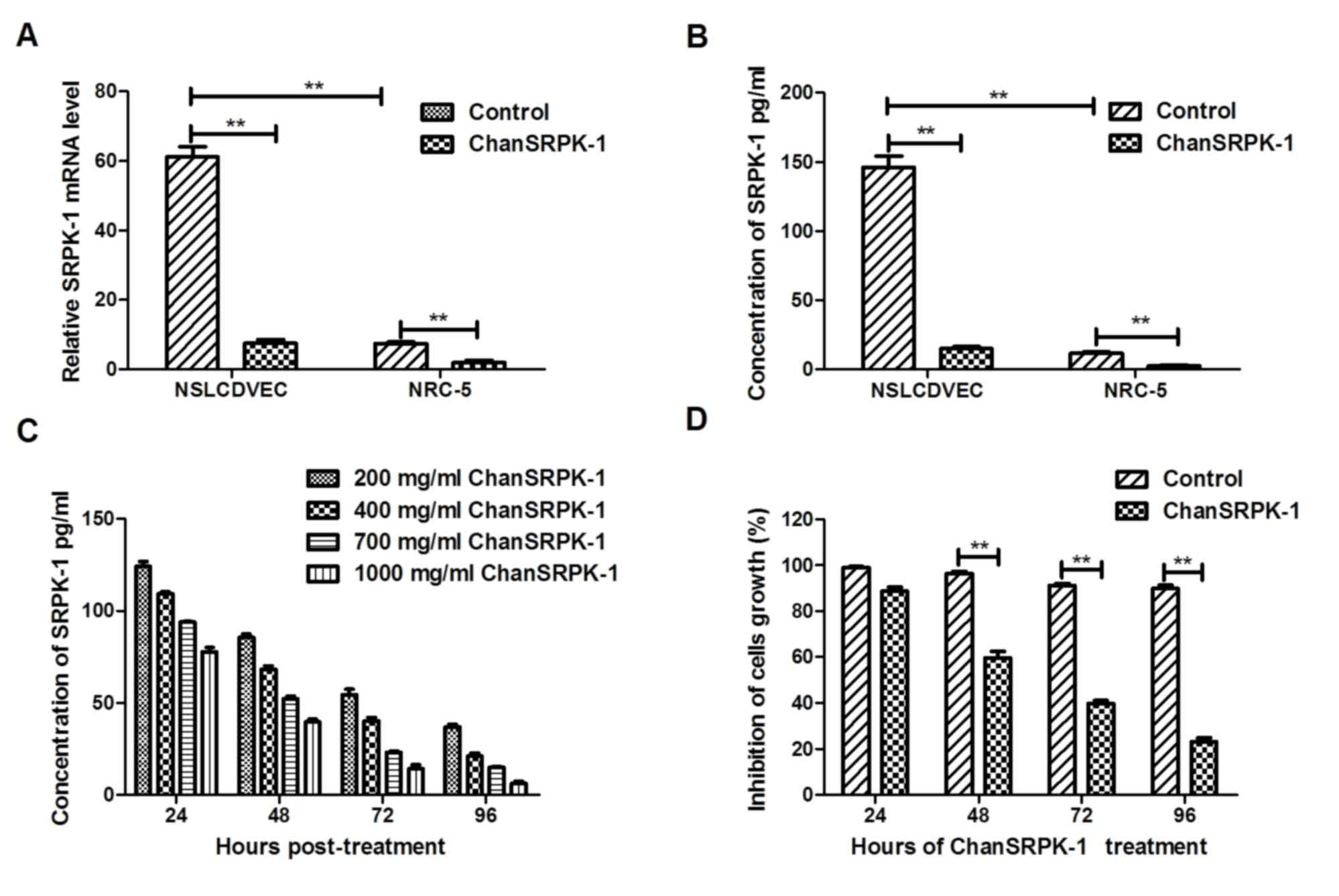
In vitro effects of ChanSRPK-1 on the
expression of SRPK-1 in NSCLC cells
In order to confirm the in vitro effects of
ChanSRPK-1 (400 mg/ml) on the expression of SRPK-1 in NSCLC cells,
NSCLCDVECs and MRC-5 cells were analyzed using RT-qPCR analysis and
ELISA. The results (Fig. 2A and B)
showed that the expression of SRPK-1 was upregulated in the
NSCLCDVECs, compared with that in the MRC-5 human lung cells. In
addition, the data (Fig. 2C)
showed that ChanSRPK-1 treatment neutralized the expression of
SRPK-1 in a dose-dependent manner (200–2,000 ng/ml). ChanSRPK-1
treatment also neutralized the expression of SRPK-1 in a
time-dependent manner (24, 48, 72 and 96 h; Fig. 2C). The in vitro effect of
ChanSRPK-1 on NSCLCDVEC growth was also examined. The results
(Fig. 2D) showed that ChanSRPK-1
(400 mg/ml) suppressed NSCLCDVEC growth from 48 h. These data
suggested that the expression of SRPK-1 was increased in
NSCLCDVECs, and that ChanSRPK-1 decreased the expression of SRPK-1
in NSCLCDVECs and inhibited growth.
In vitro inhibitory effects of
ChanSRPK-1 on migration and invasion in NSCLC
The expression of SRPK-1 has been associated with
the prognosis of human cancer, as TCF has been shown to promote the
migration and invasion of NSCLC cells (15). Although the role of SRPK-1 in NSCLC
cells has been investigated, the role of ChanSRPK-1 in the
inhibition of migration and invasion in NSCLC cells has not been
reported previously (21). To
investigate the inhibitory effects of ChanSRPK-1 on NSCLCDVECs,
cell viability, migration and invasion were analyzed. The results
(Fig. 3A) showed that the
viability of NSCLCDVECs was significantly decreased in the
ChanSRPK-1-treated groups (200, 400 and 1,000 mg/ml for 48 h),
compared with that of the MRC-5 cells. In addition, the results
(Fig. 3B and C) indicated that the
presence of SRPK-1 markedly promoted NSCLCDVEC migration and
invasion at doses of 200, 400 and 1,000 mg/ml for 24 h, compared
with the untreated group. By contrast, it was demonstrated that
200–1,000 ng/ml of ChanSRPK-1 markedly inhibited the migration,
invasion and apoptosis of NSCLCDVECs (Fig. 3D-F). Of note, 400 mg/ml of
ChanSRPK-1 was sufficient to inhibit the migration and invasion of
NSCLCDVECs. These results suggested that ChanSRPK-1 not only
affected cell viability, but also inhibited the migration and
invasion of NSCLCDVECs.
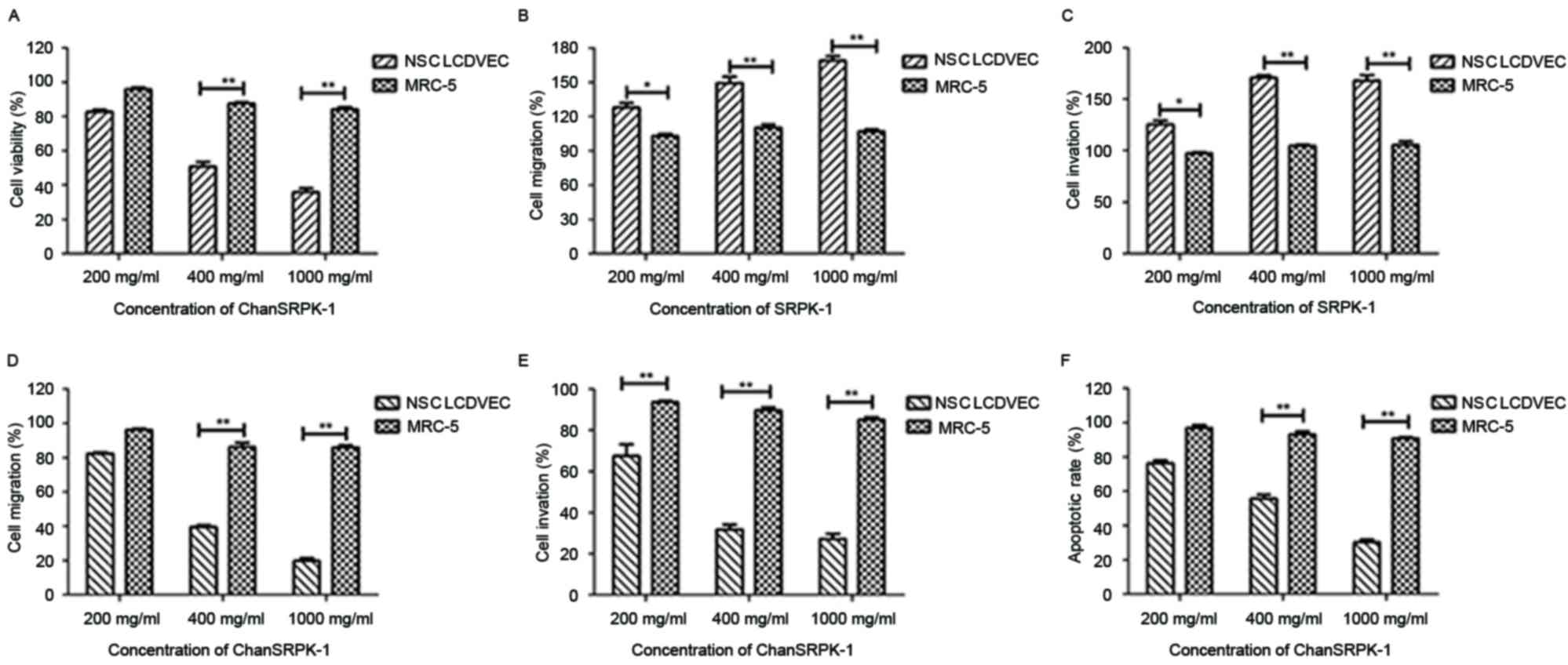 | Figure 3.Effects of ChanSRPK-1 on the
viability, migration, invasion and apoptosis of NSCLCDVECs. (A)
Cell viabilities were significantly altered in ChanSRPK-1-treated
NCSLCDVECs at different doses, compared with MRC-5 cells. (B)
SRPK-1 promoted the migration of NSCLCDVECs following exposed to
200 mg/ml, compared with control cells. (C) SRPK-1 promoted the
invasion of NSCLCDVECs exposed to 200 mg/ml, compared with control
cells. (D) ChanSRPK-1 inhibited migration of NSCLCDVEC after
exposure 200 mg/ml, compared with control cells. (E) ChanSRPK-1
inhibited the invasion of NSCLCDVECs following exposure to 200
mg/ml, compared with control cells. (F) ChanSRPK-1 induced the
apoptosis of NSCLCDVECs following exposure to 400 mg/ml, compared
with control cells. *P<0.05 and **P<0.01. SEPK-1,
serine-arginine protein kinase-1; ChanSRPK-1, chimeric antibody
target for SRPK-1; NSCLCDVES, non-small cell lung cancer-derived
vascular endothelial cells. |
Effects of ChanSRPK-1 on NSCLCDVECs by
targeting TCF and in vivo efficacy in NSCLC-bearing mice
To determine the efficacy of ChanSRPK-1 for NSCLC
treatment, the present study analyzed the expression of TCF, MMP,
CT1 and FBC. As shown in Fig. 4A,
the mRNA expression levels of TCF, MMP, CT1 and FBC in the
NSCLCDVECs were significantly higher, compared with those in the
ChanSRPK-1-treated cells. The difference in the expression of
migration-promoting proteins between the ChanSRPK-1 and control
groups was also determined to be statistically significant using a
paired t-test (P<0.01). A previous study showed that
upregulation in the phosphorylation level of GSK3-β by SRPK-1 was
correlated with tumor cell migration (15). In the present study (Fig. 4B), it was found that the
downregulation in the expression of Cytochalasin-D and G-actin, and
the phosphorylation of GSK3-β were significantly different in the
ChanSRPK-1-treated tumor cells, compared with those in the control.
In addition, NSCLC-bearing mice were used to determine the in vivo
antitumor efficacy of ChanSRPK-1. As shown in Fig. 4C, tumor size was significantly
suppressed in xenograph mice in the ChanSRPK-1-treated group,
compared with those in the control group. In addition, long-term
(120 day) survival was preceded following treatment with
ChanSRPK-1. The data (Fig. 4D)
revealed that treatment with ChanSRPK-1 (n=10 in each group)
prolonged the survival of NSCLC-bearing mice, compared with control
mice. Furthermore, it was demonstrated (Fig. 4E) that treatment with ChanSRPK-1
significantly protected tumor-bearing mice via eradicating tumors.
As demonstrated in Fig. 4F, it was
found that ChanSRPK-1 inhibited NSCLC tumor metastasis compared
with PBS-treated mice. The data showed that the inhibitory effect
of ChanSRPK-1 on tumor metastasis in NSCLC-bearing mice was
enhanced, compared with that in the control. These results
suggested that ChanSRPK-1 suppressed the migration-promoting
protein and contributed to beneficial efficacy in NSCLC-bearing
mice.
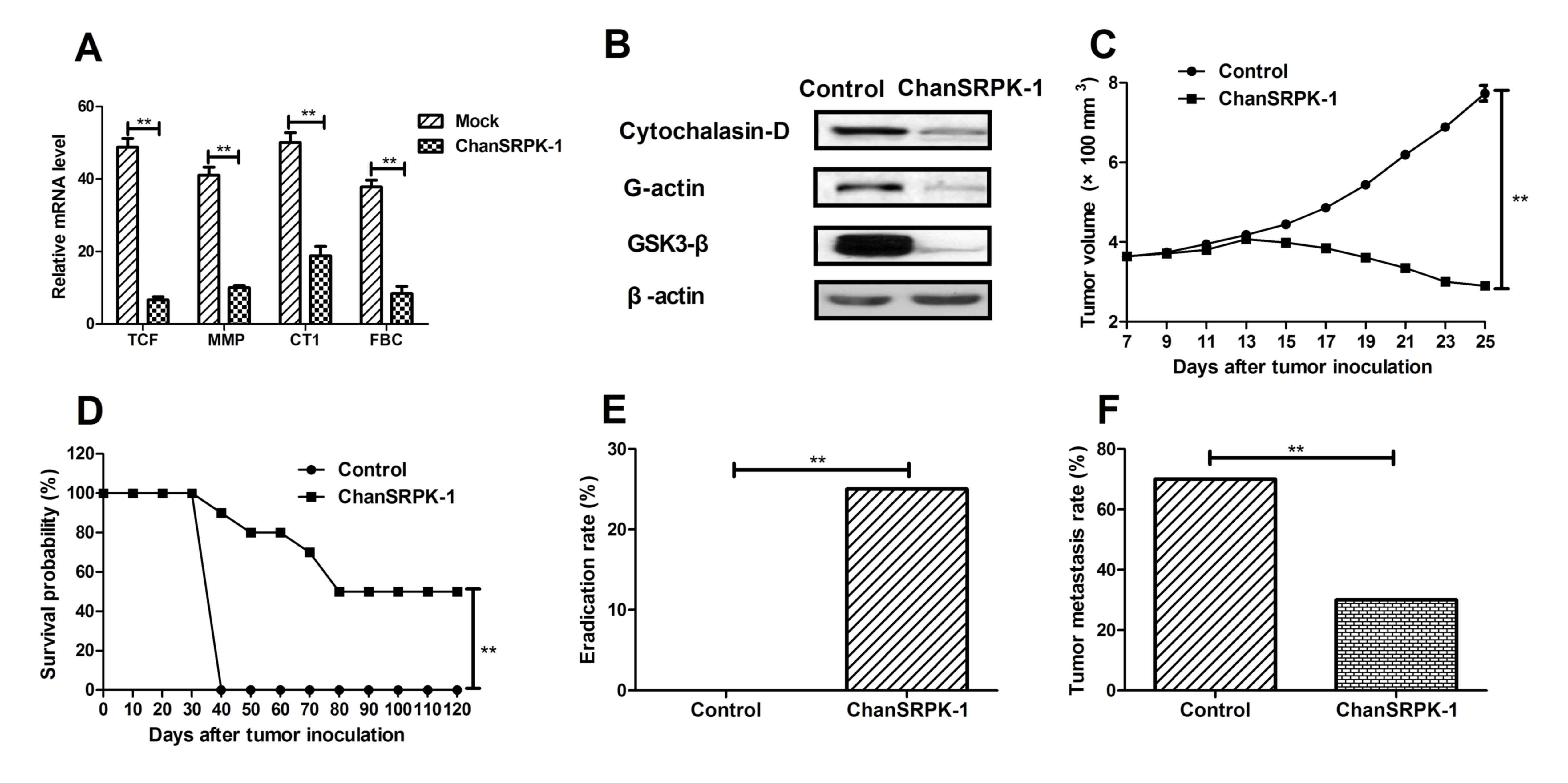 | Figure 4.Beneficial therapeutic effects and
inhibitory migration effects of ChanSRPK-1 in NSCLC-bearing mice.
(A) ChanSRPK-1 had a beneficial effect on protein expression of
tumor metastasis-associated TCF, MMP, CT1 and FBC in tumor-bearing
mice, compared with control treatment. (B) ChanSRPK-1 decreased
expression levels of Cytochalasin-D and G-actin, and the
phosphorylation of GSK3-β in tumors, compared with the untreated
group. (C) ChanSRPK-1 treatment significantly inhibited tumor cell
growth in NSCLC-bearing mice. (D) Survival was prolonged following
treatment with ChanSRPK-1 over a 120-day observation period,
compared with survival in the control group. (E) Eradication rate
of NSCLC was enhanced following treatment with ChanSRPK-1. (F)
Metastatic rate of NSCLC was decreased following treatment with
ChanSRPK-1. The Kaplan-Meier test was used to estimate survival
during the 120-day treatment period. *P<0.05 and **P<0.01.
SEPK-1, serine-arginine protein kinase-1; ChanSRPK-1, chimeric
antibody target for SRPK-1; NSCLCDVES, non-small cell lung
cancer-derived vascular endothelial cells; TCF, β-catenin/T-cell
factor; MMP, matrix metalloproteinase; CT1, collagen type I; FBC,
fibronectin; GSK3-β, glycogen synthase kinase 3-β. |
Discussion
Lung cancer is a respiratory disease leading to the
majority of cancer-associated mortality in the world owing to air
contamination (22). At present,
NSCLC accounts for ~80% of lung cancer cases and its incidence is
increasing. It includes adenocarcinoma, which comprises large cell
carcinoma and squamous cell carcinoma (5–7). The
high mortality rates of NSCLC present a challenge, and a rapidly
increasing trend has been observed, which has gradually become a
focus of public opinion and a significant burden on human health
(23). The difficulty in the early
detection of NSCLC is the primary reason for lower survival rates
in advanced NSCLC (24). The
majority of patients with NSCLC are at an advanced stage of lung
cancer at diagnosis (23). In
addition, conventional therapeutic techniques show poor efficacy
for NSCLC, and recurrence and metastasis are frequently observed
clinically. Statistical data have also shown that the survival
rates of patients with NSCLC, in terms of the overall 5-year
survival rate, are poor (25).
Therefore, the identification of more efficient anti-NSCLC agents
is required for patients with NSCLC in preclinical and clinical
trials.
Growth, migration and invasion in NSCLC are the most
important drivers for tumor metastasis and poor survival rates in
treatment and recurrence for patients with NSCLC (10,11).
Previous studies have reported that SRPK-1-encoding transcripts are
ubiquitously expressed in several human tissues, and higher
expression levels of SRPK-1 in the precise cellular localization of
NSCLC, pancreatic carcinoma, colon cancer and breast cancer have
been reported previously (13,15).
It has been identified that the majority of SRPK-1 protein is
expressed exclusively within NSCLCDVECs and that SRPK-1 is
positively correlated with the grade of cancer progression.
Furthermore, the downregulation of SRPK-1 increases the apoptosis
of tumor cells (17). These
results suggest that therapeutic agents, through suppressing the
activity of SRPK-1, may be effective as a potential single
anticancer agent or an adjuvant agent in combination with
conventional therapeutic regimens.
In the present study, the design involved
construction of a Chan-SRPK-1 for NSCLC therapy via inhibition of
growth, migration and invasion. The expression of SRPK-1 and
inhibitory effects on NSCLCDVECs were also examined in vitro
and in vivo. Lehman et al (12) reported that two major kinases
responsible for the protein phosphorylation of mitogen-activated
protein kinase (MAPK)3 and MAPK1 were reduced through inhibited
phosphorylation levels of GSK3-β. In the present study, the mRNA
expression levels of the TCF, MMP, CT1 and FBC tumor cell
metastasis-associated proteins were significantly decreased in
NSCLCDVECs treated with ChanSRPK-1, compared with levels in
untreated cells.
The ability of SRPK-1 to enhance proliferation and
survival via the regulation of multiple signaling pathways in tumor
cells has been exploited and suggested as an opportunity to develop
novel anticancer therapeutics targeting SRPK-1 (26). A previous study reported that
SRPK-1 regulates migration and growth in various cancer cells
(27). In the present study, it
was shown that the expression of SRPK-1 was superfluous in
NSCLCDVECs, compared with normal MRC-5 lung cells. It was found
that ChanSRPK-1 interrupted the signaling pathway involved in tumor
cell growth, migration and invasion regulated by SRPK-1. In
addition, ChanSRPK-1 treatment at a concentration of 400 ng/ml for
24 h markedly suppressed NSCLCDVEC migration, compared with that in
the untreated cells in vitro, and inhibited tumor metastasis
in tumor-bearing mice in vivo. Primary data analysis of
tumor diameter and long-term survival showed that injection with
ChanSRPK-1 once a day led to tumor regression and resulted in a
survival rate of 50% in a 120-day period in tumor-bearing mice.
Data also indicated that treatment with ChanSRPK-1 against NSCLC
was sufficient to partially protect the animals via the eradication
of tumors in experimental mice, which translated into long-term
survival and tumor-free living in the mice of the lung cancer
model.
In conclusion, the present study introduced
ChanSRPK-1 as an anticancer candidate via once daily administration
through intravenous injection. In the NSCLC mouse model, the
results demonstrated that, ChanSRPK-1 downregulated the expression
of SRPK-1, which led to the reversal of TCF-induced cell migration.
Taken together, the data obtained in the present study suggested
the beneficial effects of cellular targeted therapy, which can
assist in further elucidating the clinical value of applying such a
regimen.
References
|
1
|
Blair HA and Deeks ED: Albumin-bound
paclitaxel: A review in non-small cell lung cancer. Drugs.
75:2017–2024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeCotiis C, Hu Y, Greenberg AK, Huie M,
Tsay JC, Pass H, Goldberg JD and Rom WN: Inflammatory cytokines and
non-small cell lung cancer in a CT-scan screening cohort:
Background review of the literature. Cancer Biomark. 16:219–233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keating GM: Nivolumab: A review in
advanced squamous non-small cell lung cancer. Drugs. 75:1925–1934.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Wekken AJ, Saber A, Hiltermann TJ,
Kok K, van den Berg A and Groen HJ: Resistance mechanisms after
tyrosine kinase inhibitors afatinib and crizotinib in non-small
cell lung cancer, a review of the literature. Crit Rev Oncol
Hematol. 100:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brody H: Lung cancer. Nature. 513
Suppl:S12014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moro-Sibilot D, Smit E, de Castro Carpeño
J, Lesniewski-Kmak K, Aerts JG, Villatoro R, Kraaij K, Nacerddine
K, Dyachkova Y, Smith KT, et al: Non-small cell lung cancer
patients with brain metastases treated with first-line
platinum-doublet chemotherapy: Analysis from the European FRAME
study. Lung Cancer. 90:427–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barnett SA, Downey RJ, Zheng J, Plourde G,
Shen R, Chaft J, Akhurst T, Park BJ and Rusch VW: Utility of
routine PET imaging to predict response and survival after
induction therapy for non-small cell lung cancer. Ann Thorac Sur.
101:1052–1059. 2016. View Article : Google Scholar
|
|
8
|
Xie FJ, Lu HY, Zheng QQ, Qin J, Gao Y,
Zhang YP, Hu X and Mao WM: The clinical pathological
characteristics and prognosis of FGFR1 gene amplification in
non-small-cell lung cancer: A meta-analysis. OncoTargets Ther.
9:171–181. 2016. View Article : Google Scholar
|
|
9
|
Lim SH, Sun JM, Lee SH, Ahn JS, Park K and
Ahn MJ: Pembrolizumab for the treatment of non-small cell lung
cancer. Expert Opin Biol Ther. 16:397–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Müller B, Bovet M, Yin Y, Stichel D, Malz
M, González-Vallinas M, Middleton A, Ehemann V, Schmitt J, Muley T,
et al: Concomitant expression of far upstream element (FUSE)
binding protein (FBP) interacting repressor (FIR) and its splice
variants induce migration and invasion of non-small cell lung
cancer (NSCLC) cells. J Pathol. 237:390–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Q, Yue J, Zhang C, Gu X, Chen H and
Xu L: Inactivation of M2 AChR/NF-κB signaling axis reverses
epithelial-mesenchymal transition (EMT) and suppresses migration
and invasion in non-small cell lung cancer (NSCLC). Oncotarget.
6:29335–29346. 2015.PubMed/NCBI
|
|
12
|
Lehman M: Improving therapeutic outcomes
in non-small cell lung cancer not suitable for curative intent
therapy- A review of the role of radiation therapy in an era of
increasing systemic therapy options. Clin Oncol (R Coll Radiol).
28:327–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polo V, Zago G, Frega S, Canova F, Bonanno
L, Favaretto A, Bonaldi L, Bertorelle R, Conte P and Pasello G:
Non-small cell lung cancer in a very young woman: A case report and
critical review of the literature. Am J Case Rep. 16:782–789. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XH, Song JW, Liu JL, Wu S, Wang LS,
Gong LY and Lin X: Serine-arginine protein kinase 1 is associated
with breast cancer progression and poor patient survival. Med
Oncol. 31:832014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu H, Hu X, Zhu Y, Jiang G and Chen S:
Up-regulation of SRPK1 in non-small cell lung cancer promotes the
growth and migration of cancer cells. Tumour Biol. 37:7287–7293.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krishnakumar S, Mohan A, Kandalam M,
Ramkumar HL, Venkatesan N and Das RR: SRPK1: A cisplatin sensitive
protein expressed in retinoblastoma. Pediatr Blood Cancer.
50:402–406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mavrou A, Brakspear K, Hamdollah-Zadeh M,
Damodaran G, Babaei-Jadidi R, Oxley J, Gillatt DA, Ladomery MR,
Harper SJ, Bates DO and Oltean S: Serine-arginine protein kinase 1
(SRPK1) inhibition as a potential novel targeted therapeutic
strategy in prostate cancer. Oncogene. 34:4311–4319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayes GM, Carrigan PE and Miller LJ:
Serine-arginine protein kinase 1 overexpression is associated with
tumorigenic imbalance in mitogen-activated protein kinase pathways
in breast, colonic, and pancreatic carcinomas. Cancer Res.
67:2072–2080. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishio M, Horai T, Horiike A, Nokihara H,
Yamamoto N, Takahashi T, Murakami H, Yamamoto N, Koizumi F, Nishio
K, et al: Phase 1 study of lenvatinib combined with carboplatin and
paclitaxel in patients with non-small-cell lung cancer. Br J
Cancer. 109:538–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sultani M, Azad T Mokhtari, Eshragian M,
Shadab A, Naseri M, Eilami O and Yavarian J: Multiplex SYBR green
real-time PCR assay for detection of respiratory viruses.
Jundishapur J Microbiol. 8:e190412015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han S, Bui NT, Ho MT, Kim YM, Cho M and
Shin DB: Dexamethasone inhibits TGF-β1-induced cell migration by
regulating the ERK and AKT pathways in human colon cancer cells via
CYR61. Cancer Res Treat. 48:1141–1153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fenton-Ambrose L and Kazerooni EA:
Preventative care: Lung-cancer screens now worth the cost. Nature.
514:352014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim DS, Park KM, Won YS, Kim JY, Lee JK,
Kim JG, Oh ST, Jung SS and Kang WK: Occurrence and prognosis of
symptomatic venous thromboembolism in colorectal cancer surgery
patients. Vasc Specialist Int. 30:49–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tauhardt E, Reissig A, Winkens T and
Freesmeyer M: Early detection of disease progression after
palliative chemotherapy in NSCLC patients by (18)F-FDG-PET.
Nuklearmedizin. 53:197–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gold M, Dunn LB, Phoenix B, Paul SM,
Hamolsky D, Levine JD and Miaskowski C: Co-occurrence of anxiety
and depressive symptoms following breast cancer surgery and its
impact on quality of life. Eur J Oncol Nurs. 20:97–105. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang W, Chang HY, Fei T, Wu H and Chen
YG: GSK3 beta mediates suppression of cyclin D2 expression by tumor
suppressor PTEN. Oncogene. 26:2471–2482. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoon C, Kim D, Kim S, Park GB, Hur DY,
Yang JW, Park SG and Kim YS: MiR-9 regulates the
post-transcriptional level of VEGF165a by targeting SRPK-1 in
ARPE-19 cells. Graefes Arch Clin Exp Ophthalmol. 252:1369–1376.
2014. View Article : Google Scholar : PubMed/NCBI
|