Introduction
The metabolism of bone tissues, also known as bone
remodeling, is a cyclic process involving constant resorption of
old bone by osteoclasts and formation of new bone by osteoblasts.
Overactive bone resorption or dysfunction of bone formation
perturbs the balance between bone resorption and formation,
damaging the structural integrity and strength of bone, resulting
in the loss of structural integrity and bone mass, manifesting as
osteoporosis (1). An imbalance
between osteoclast and osteoblast activity is also involved in the
pathogenesis of common rheumatic diseases, including rheumatoid
arthritis (RA), ankylosing spondylitis (AS), osteoarthritis and
osteoporosis (1). Thus, one of the
targets for treating these diseases is to restore this balance and
prevent bone destruction by inhibiting osteoclast or inducing
osteoblast functions.
Osteoblasts differentiate from bone marrow stromal
cells. The process of osteoblast differentiation consists of 3
steps: Cell proliferation, extracellular matrix formation and
maturation, and bone matrix mineralization (2). Each differentiation period involves
the expression and regulation of distinct genes. During osteoblast
proliferation, the expression of type I collagen peaks,
facilitating bone matrix maturation and mineralization. During bone
matrix maturation, osteoblast proliferation is reduced and
expression of alkaline phosphatase (ALP) increases. The peak of ALP
activity marks the maturation of the bone matrix. During bone
matrix mineralization, ALP activity decreases while osteocalcin
(OCN) expression increases, and the formation of bone nodules
indicates bone matrix mineralization (3). Bone morphogenetic protein-2 (BMP-2)
is the only cytokine that can independently induce ectopic bone
formation. BMP-2 has been reported to be involved in the induction
of pluripotent stem cell differentiation into bone cells, and is
associated with multiple signal pathways that promote osteogenesis
(4).
During bone formation, cytokines synthesized and
secreted by osteoblasts regulate the differentiation and maturation
of osteoclasts (5). The
osteoprotegerin (OPG)/receptor activator of nuclear factor-κB
(NF-κB; RANK)/receptor activator of NF-κB ligand (RANKL) system has
been reported to regulate osteoclast function and bone remodeling
(6). RANKL secreted by osteoblasts
and bone marrow stromal cells binds to RANK expressed on the
surface of osteoclast precursor cells or osteoclasts, inducing
osteoclast differentiation and increasing bone resorption. OPG
secreted by osteoblasts and bone marrow stromal cells competitively
binds to RANKL, inhibiting RANKL and RANK binding. Thus, the
balance of OPG and RANKL expression regulates osteoclast
differentiation, activation and function (6). RANKL expression and soluble RANKL
(sRANKL) release induce bone resorption and destruction (7). OPG and RANKL expression regulates
osteoclast activation, and this further influences bone remodeling
(8).
The major component of technetium
methylenediphosphonate (99Tc-MDP) injection is the
chelate of stannous chloride-reduced technetium (agent A) with
methylene diphosphonate (agent B). 99Tc-MDP is widely
used in the treatment of many diseases, including RA, AS,
osteonecrosis, osteoarthritis, osteoporosis, Graves' eye disease,
psoriatic arthritis, bone metastases and multiple myeloma (9–12).
However, previous studies have also reported additional
pharmacological effects of 99Tc-MDP, including
inhibition of inflammatory reactions, immune regulation and
regulation of bone metabolism (11,13–15).
As the synovial membrane is semi-permeable, generic drugs cannot
penetrate it, but MDP is able to carry 99Tc into the
joint cavity and facilitate its function close to the lesion. When
99Tc-MDP enters the joint cavity and reaches an area of
synovitis or abnormal bone, it binds immature collagen or is
absorbed by hydroxyapatite crystals, thereby persisting and
exerting a long-lasting therapeutic effect.
However, the mechanism by which 99Tc-MDP
influences bone metabolism has not been elucidated, and to the best
of our knowledge, no study has reported the effect of
99Tc-MDP on the proliferation and function of
osteoblasts cultured in vitro. The aim of the present study
was to investigate the effect of 99Tc-MDP on in
vitro-cultured osteoblasts, and to explore the mechanisms
responsible for its inhibition of bone destruction, providing a
theoretical basis for the use of 99Tc-MDP in the
treatment of bone-destructive diseases.
Materials and methods
Isolation and culture of human
osteoblasts
Iliac cancellous bone was sampled from 8 patients
aged between 30 and 50 years, presenting with no metabolic bone
diseases but receiving autologous bone transplantation to repair
fracture in the West China Hospital of Sichuan University (Chengdu,
China). The present study was been approved by the ethics committee
of the West China Hospital of Sichuan University (Chengdu, China)
and all patients gave their informed consent.
Iliac cancellous bone samples were stripped of
periosteum and soft tissues, washed with sterilized phosphate
buffered saline (PBS) 3 times, cut into 1 mm2 sections,
washed in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and PBS and incubated at 37°C
for 8 min with erythrocyte lysate (840 mg NaHCO3, 37.2
mg EDTA, 8.023 g ammonia chloride, 1000 ml distilled water; pH
7.2). The suspension was subsequently centrifuged at 500 × g for 10
min at room temperature, and the precipitate was rinsed until it
appeared white. The precipitate was pre-digested in 0.25% EDTA
containing trypsin (HyClone; GE Healthcare Life Sciences, Chalfont,
UK) for 20 min at 37°C. Digestion was terminated with fetal bovine
serum (FBS; Yuanheng Jinma Biotechnology Co., Ltd., Beijing,
China), and the supernatant was discarded. Type I collagenase
(0.1%; BD Biosciences, Franklin Lakes, NJ, USA) was added at a
volume ratio of 1:8, and bone cells were digested for 1 h at 37°C.
Bone cells were then centrifuged at 500 × g for 5 min at room
temperature and rinsed in PBS twice, then resuspended in
high-glucose DMEM containing 10% FBS and antibiotics (penicillin
100 IU/ml, streptomycin 100 mg/ml) (Gibco; Thermo Fisher
Scientific, Inc.) and passed through a 200-mesh stainless steel
screen (75 µm pore size) to remove non-osteoblasts and impurities.
The filtered suspension was further digested by type I collagenase
3 times. The cell suspension was counted using a hemocytometer and
trypan blue staining. Cells were ≥95% viable and cultured at
5×104 cells/ml in a sterile Petri dish at 37°C with 5%
CO2 and 95% humidity. The medium was changed every 48 h
and cells were passaged with a 1:2 division using 0.25%
trypsin/0.02% EDTA.
Preparation of 99Tc-MDP and
β fibroblast growth factor (β-FGF) working solutions
99Tc-MDP (Chengdu Yunke Pharmaceutical
Co., Ltd., Chengdu, China) was prepared according to the
manufacturer's protocol. Agent A (5 ml, containing 0.05 µg of
99Tc) and B (containing 5 mg MDP and 0.5 mg stannous
chloride) were mixed at room temperature for 5 min. The stock
solution was then adjusted to 1 ml/mg MDP. The working solutions
were freshly prepared prior to use. β-FGF (PeproTech, Inc., Rocky
Hill, NJ, USA) was diluted in complete medium (high-glucose DMEM
supplemented with 10% FBS and antibiotics) to 10 ng/ml.
CCK-8 measurement of cell
proliferation
Passage 2 osteoblasts were cultured at
2.5×103 cells/well in a 96-well plate for 24 h.
Osteoblasts were subsequently synchronized by serum starvation (24
h in DMEM containing 0.1% FBS), then incubated with medium
supplemented with 10−4-10−12 M
99Tc-MDP, 10 ng/ml β-FGF or medium alone. Each condition
was replicated five times. Proliferation was measured at 24, 48 and
72 h with a CCK-8 kit (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol.
Bromodeoxyuridine (BrdU) measurement
of cell proliferation
Passage 2 osteoblasts were cultured at
1×104 cells/well in a 24-well plate pre-covered with
coverslips for 24 h. Osteoblasts were subsequently synchronized as
previously described, then incubated with 10−8 M
99Tc-MDP, 10 ng/ml β-FGF or medium (high-glucose DMEM
supplemented with 10% FBS and antibiotics) alone for 72 h. Each
condition was replicated three times. The medium was then aspirated
and 10 µg/ml BrdU (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was added for 4 h. The slides were then washed with PBS,
fixed in 4% paraformaldehyde at 4°C for 30 min, then washed with
0.1 M PBS containing 1% Triton X-100 (Amresco, Inc., Framingham,
MA, USA), incubated in 1 M ice-cold HCl for 10 min, then 2 M HCl
for 10 min at room temperature and incubated at 37°C for 20 min.
Slides were then washed with PBS, incubated in 0.1 M sodium borate
(pH=8.6) at room temperature for 12 min, washed in 0.1 M PBS
containing 1% Triton X-100 and blocked in 5% FBS containing 1 M
glycine for 1 h. Slides were incubated with mouse anti-BrdU
immunoglobulin G (IgG; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA; catalog no. sc-70443; 1:100) and RNase (Ambion; Thermo Fisher
Scientific, Inc.) at 4°C overnight, then washed in 0.1 M PBS
containing 1% Triton X-100, and incubated with Cy3 goat-anti-mouse
IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA; catalog no. 115-165-205; 1:200) at 37°C or room temperature
for 1 h, then washed in PBS and mounted with antifade medium
(Thermo Fisher Scientific, Inc. P36934). The sections were
preserved at 4°C in the dark, and the proportion of BrdU-positive
cells was calculated by counting 10 randomly selected fields under
a fluorescence microscope, at ×200 magnification.
Flow cytometric analysis of cell cycle
and apoptosis
Passage 2 osteoblasts were cultured at
4×105 cells/dish in 100 mm dishes for 24 h. Osteoblasts
were then synchronized and incubated with 10−8 M
99Tc-MDP, 10 ng/ml β-FGF or medium (high-glucose DMEM
supplemented with 10% FBS and antibiotics) alone for 48 h. Each
condition was replicated three times. Cells were then digested with
trypsin, washed with PBS and collected by centrifuging at 500 × g
for 5 min at room temperature. Pre-cooled ethanol (70%, 1 ml) was
added to the precipitate and the cell suspension was fixed at 4°C
overnight. Following centrifugation at 500 × g for 5 min at room
temperature, the precipitate was resuspended in 3 ml PBS, passed
through a 400-mesh screen and stained with 1 ml propidium iodide
(PI) at 4°C for 30 min in the dark. The cell cycle was assessed by
flow cytometry, and 3×104 cells were collected for each
sample. Modfit software Version 4.0 (Verity Software House, Inc.,
Topsham, ME, USA) was used for data acquisition, processing,
calculation of apoptotic cell proportion, and the determination of
cell cycle distribution. The S-phase fraction (SPF) and
proliferation index [proliferation index=(S +
G2/M)/(G0/G1 + S +
G2/M) ×100%] were used to evaluate the speed of
proliferation.
Determination of alkaline phosphatase
activity
Passage 2 osteoblasts were cultured at
5×104 cells/well in 12-well plates for 24 h. Osteoblasts
were then synchronized and incubated with
10−4-10−12 M 99Tc-MDP, 10 ng/ml
β-FGF or medium (high-glucose DMEM supplemented with 10% FBS and
antibiotics) alone. Each condition was replicated three times.
Medium was changed every 48 h, and cells were collected 3, 6 and 9
days later, washed in PBS and treated with 80 µl of lysate (50 mM
Tris-HCL; pH 7.4; 150 mM NaCl, 1% TritonX-100, 0.5% sodium
deoxycholate, 0.1% SDS; stored in the dark at 4°C) at 4°C for 30
min, then centrifuged at 5,000 × g at 4°C for 30 min. The
supernatant was collected and 30 µl was added to each well of a
96-well plate. ALP activity was determined using the p-nitrophenyl
phosphate (pNPP) method, with Alkaline Phosphatase Assay Kit
(Beyotime Institute of Biotechnology, Haimen, China; P0321),
according to the manufacturer's protocol; optical density at 520 nm
was measured using a BioTek Synergy micro-plate reader (Thermo
Fisher Scientific, Inc.). Protein content was quantified using the
Thermo Scientific™ Pierce™ Coomassie Plus™ (Bradford) Protein Assay
(Thermo Fisher Scientific, Inc.) (16).
Mineralized nodule counts
Passage 2 osteoblasts were cultured at
1×104 cells/well in 24-well plates for 24 h. Osteoblasts
were then synchronized and incubated with 10−8 M
99Tc-MDP, 10 ng/ml β-FGF or medium (high-glucose DMEM
supplemented with 10% FBS and antibiotics) alone. Each condition
was replicated three times. Medium was changed every 48 h, and at
14 days complete medium containing 10 mM β-glycerophosphate and 50
mg/l ascorbic acid was added. Cells were subsequently washed in
PBS, fixed with 2.5% glutaraldehyde at room temperature for 15 min,
rinsed in PBS (pH 4.2), incubated with 2% Alizarin Red at 37°C for
10 min, and washed in PBS (pH 4.2). Using a polyester film with 0.2
mm2 grid marks to assist counting, red mineralized
nodules >200 µm with a clear boundary were counted in 10
randomly selected high power fields under a light microscope (x40
magnification) by two independent investigators blinded to the
grouping.
Reverse transcription-quantitative
polymerase chain reaction (RT-PCR)
Passage 2 osteoblasts were cultured at
1×105 cells/well in a 6-well plate for 24 h. Osteoblasts
were subsequently synchronized and incubated with 10−8 M
99Tc-MDP, 10 ng/ml β-FGF or medium (high-glucose DMEM
supplemented with 10% FBS and antibiotics) alone for 72 h. Each
condition was replicated three times. Total RNA extraction was
performed using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA (1 µg) was then reverse transcribed
into cDNA (20 µl reaction volume) using First Strand cDNA Synthesis
Kit (Fermentas; Thermo Fisher Scientific, Inc.) as previously
described (17). Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as an internal
reference. Forward and reverse primers of OCN, BMP-2, OPG, RANKL
and GAPDH are listed in Table I.
Primers were designed by Primer Premier 5.0 (PREMIER Biosoft, Palo
Alto, CA, USA) and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). SYBR Green I (Takara Bio, Inc., Otsu, Japan) was
used for fluorescent detection according to the manufacturer's
protocol. The thermocycler conditions used were as follows: 94°C
pre-denaturation for 120 sec, 94°C denaturation for 20 sec,
annealing at 58, 54, 54, 54 and 50°C for OCN, BMP-2, OPG, RANKL and
GAPDH, respectively, for 20 sec, 72°C elongation for 40 sec, and 45
cycles. The melting curve was plotted to determine the specificity
of amplified products. Relative OCN, BMP-2, OPG, RANKL and GAPDH
mRNA expression levels were determined using the 2−ΔΔCq
method (18).
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Genes | Sequence | Target sequence
length (bp) |
|---|
| OCN | F:
5′-GTGCAGCCTTTGTGTCCAAG-3′ | 158 |
|
| R:
5′-GTCAGCCAACTCGTCACAGT-3′ |
|
| BMP-2 | F:
5′-CGGTCTCCTAAAGGTCGACCAT-3′ | 120 |
|
| R:
5′-CGAACTTCCTGCGGCCCAGCT-3′ |
|
| OPG | F:
5′-TCAGAAAGGAAATGCAACACA-3′ | 205 |
|
| R:
5′-CCGTTTTATCCTCTCTACACT-3′ |
|
| RANKL | F:
5′-GCCTCCCGCTCCATGTTC-3′ | 101 |
|
| R:
5′-TTAGGATCCATCTGCGCTC-3′ |
|
| GAPDH | F:
5′-TGACATCAAGAAGGTGGTGA-3′ | 177 |
|
| R:
5′-TCATACCAGGAAATGAGCTT-3′ |
|
ELISA
Passage 2 osteoblasts were cultured at
1×105 cells/well in a 6-well plate for 24 h. Osteoblasts
were then synchronized and incubated with 10−8 M
99Tc-MDP, 10 ng/ml β-FGF or medium (high-glucose DMEM
supplemented with 10% FBS and antibiotics) alone for 72 h. Each
condition was replicated three times. The OPG and RANKL contents in
supernatants were assessed by specific ELISA kits (Cusabio Biotech
Co., Ltd., College Park, MD, USA; catalog nos. CSB-E04692 h and
CSB-E05125 h, respectively) was performed according to the
manufacturer's protocol.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). Multiple sets of data were compared by
one-way analysis of variance (ANOVA). If H0 was refused,
the Least Significant Difference-t test was used for multiple
comparisons. Different conditions were compared by ANOVA with
Dunnett's t-test and comparisons of level data were made by the
rank sum test. Test level α (bilateral) was defined at 0.05, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
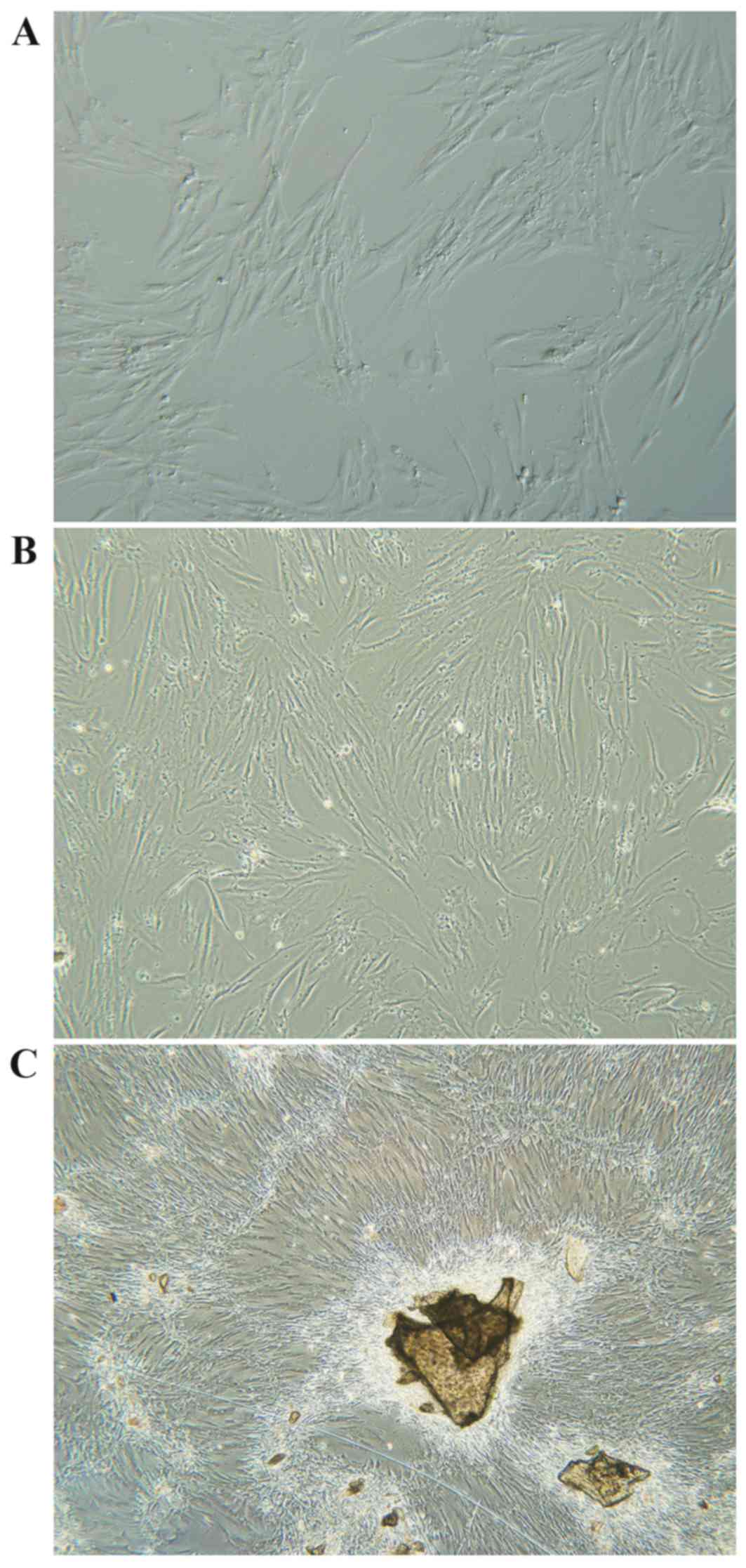
Osteoblast culturing
Primary human osteoblasts were isolated from iliac
cancellous bone and cultured ex vivo. Following isolation, cells
were spherical, evenly distributed, and surrounded by translucent
membranes. Osteoblasts began to attach to the dish 12–24 h post
inoculation, and cells swelled and formed a triangle shape with a
visibly enlarged nucleus. Between 24–120 h most cells were attached
and cell shape varied from polygonal to spindle, fusiform and
triangle (Fig. 1A and B).
Projections increased and enlarged with extended culture. The
nuclei were large, clear, round or oval-shaped and contained
abundant cytoplasm and 1–3 nucleoli. Cell secretions also gradually
increased with time, and by day 7–12 osteoblasts were spindle or
cord-shaped and grew to ~100% confluence, forming a single cell
layer of clustered and fused cells, with unclear cell boundaries
(Fig. 1C).
Impact of 99Tc-MDP on
osteoblast cell proliferation
When incubated with 10−4 M
99Tc-MDP, osteoblast proliferation was significantly
inhibited at 24, 48 and 72 h compared with controls (P=0.007,
P=0.007 and P=0.014, respectively; Table II). However, in the presence of
lower concentrations of 99Tc-MDP
(10−5-10−9 M), osteoblast proliferation was
significantly increased at 24, 48 and 72 h compared with controls
(P<0.05; Table II), and in the
presence of 10−5-10−12 M 99Tc-MDP
osteoblast proliferation was significantly enhanced at 72 h
compared with controls (P<0.05; Table II). However, the result was not
significantly associated with time of intervention (P>0.05;
Table II).
 | Table II.Detection of osteoblast proliferation
using the CCK-8 assay. |
Table II.
Detection of osteoblast proliferation
using the CCK-8 assay.
|
| Percent of controls
(%) |
|---|
|
|
|
|---|
|
| Concentration | 24 h | 48 h | 72 h |
|---|
| Control |
| 100.00±3.69 | 100.00±3.91 | 100.00±2.68 |
| β-FGF (ng/ml) | 10 |
111.77±3.25a |
125.58±5.24a |
123.05±7.99a |
| 99Tc-MDP
(M) |
10−4 |
92.72±2.40a |
73.80±8.27a |
69.79±5.26a |
|
|
10−5 |
114.91±2.86a |
118.83±8.74a |
104.81±2.60a |
|
|
10−6 |
109.62±2.05a |
120.03±8.24a |
105.61±5.03a |
|
|
10−7 |
115.05±4.78a |
129.86±7.27a |
103.91±2.05a |
|
|
10−8 |
124.70±3.12a |
127.95±8.13a |
130.85±8.10a |
|
|
10−9 |
113.85±4.17a |
115.80±3.65a |
124.18±6.80a |
|
|
10−10 |
119.41±5.51a | 111.51±2.52 |
121.36±7.89a |
|
|
10−11 | 105.26±3.21 | 117.92±7.51 |
113.52±8.00a |
|
|
10−12 | 103.61±2.82 |
116.68±7.94a |
117.20±8.41a |
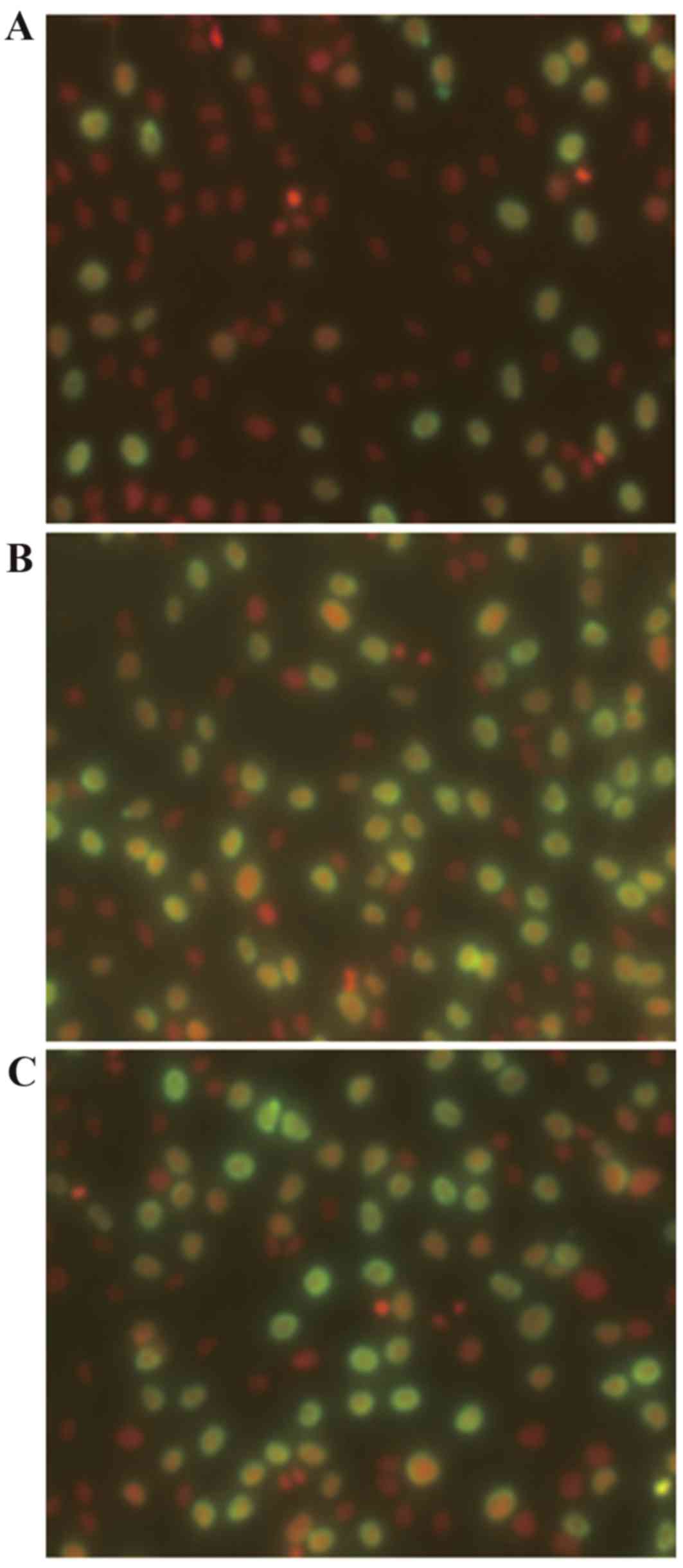
The proportion of cells in the proliferative phase
was assessed by BrdU assay. The fraction of cells in the
proliferative phase was 16.78±3.00% higher in cultures incubated
with 10−8 M 99Tc-MDP for 24 h compared with
cultures incubated in medium alone (P=0.026; Fig. 2 and Table III).
 | Table III.Determination of osteoblast
proliferation using bromodeoxyuridine staining. |
Table III.
Determination of osteoblast
proliferation using bromodeoxyuridine staining.
|
| Concentration | Percent of control
(%) |
|---|
| Control |
| 100.00±2.56 |
| β-FGF (ng/ml) | 10 |
115.87±1.83a |
| 99Tc-MDP
(M) |
10−8 |
116.78±3.00a |
Influence of 99Tc-MDP on
the osteoblast cell cycle
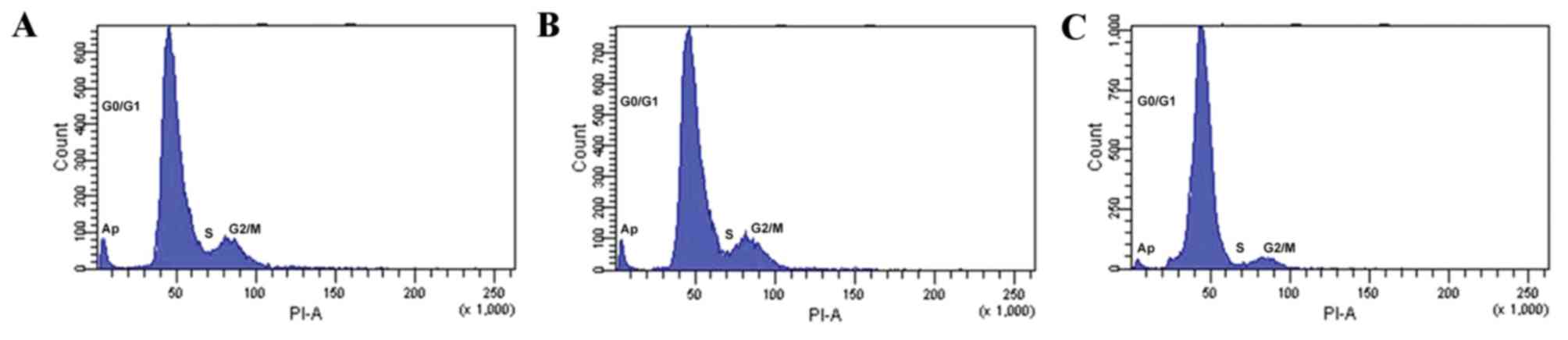
Flow cytometry and PI staining was used to assess
the osteoblast cell cycle stage. Following 48 h incubation with
10−8 M 99Tc-MDP, the fraction of cells in the
S-phase (6.62±1.18%) significantly increased compared with the
control (27.47±2.38%; P=0.004; Fig.
3 and Table IV). The fraction
of cells in G2/M phase was not significantly altered
(P>0.05; Fig. 3 and Table IV), but the fraction of cells in
the G0/G1 phase was significantly reduced
from 90.30±0.69 to 69.17±2.32% compared with the control (P=0.001;
Fig. 3 and Table IV). The proliferation index of
cells incubated with 10−8 M 99Tc-MDP was also
significantly increased from 9.7±0.69 to 30.83±2.32 compared with
controls (P=0.006; Table IV). The
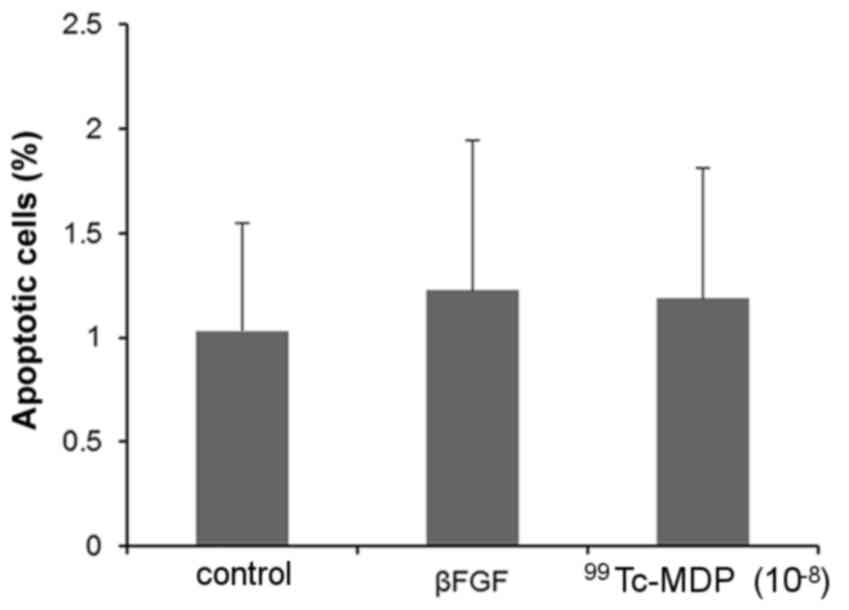
rate of apoptosis in osteoblasts incubated with 10−8 M
99Tc-MDP also increased, but the difference was not
statistically significant (P>0.05; Fig. 4).
 | Table IV.Determination of osteoblast
proliferation using flow cytometry. |
Table IV.
Determination of osteoblast
proliferation using flow cytometry.
|
| Concentration |
G0/G1 (%) | S (%) | G2/M
(%) | Proliferation
index |
|---|
| Control |
| 90.30±0.69 | 6.62±1.18 | 3.07±1.52 | 9.7±0.69 |
| β-FGF (ng/ml) | 10 |
68.74±2.87a |
27.97±3.00a | 3.29±1.21 |
31.26±2.87a |
| 99Tc-MDP
(M) |
10−8 |
69.17±2.32a |
27.47±2.38a | 3.36±1.14 |
30.83±2.32a |
Influence of 99Tc-MDP on
alkaline phosphatase activity
As an early marker of osteoblast differentiation,
ALP activity reflects the differentiation and functional status of
osteoblasts. Higher ALP activity indicates more significant
differentiation of pre-osteoblasts into mature osteoblasts
(19). The level of ALP activity
in osteoblasts was assessed by the pNPP method following 3, 6 and 9
days of incubation with 10−5 to 10−12 M
99Tc-MDP. Significantly enhanced phosphatase activity
was observed following 3, 6 and 9 days of incubation with
10−6 to 10−9 M 99Tc-MDP compared
with controls (P<0.05; Table
V), peaking at 44.80±4.66% over control following incubation
with 10−9 99Tc-MDP for 3 days (Table V). The effect of
99Tc-MDP was not significantly associated with the time
of intervention (P>0.05; Table
V).
 | Table V.Determination of alkaline phosphatase
activity using the p-nitrophenyl phosphate method. |
Table V.
Determination of alkaline phosphatase
activity using the p-nitrophenyl phosphate method.
|
| Percent of controls
(%) |
|---|
|
|
|
|---|
|
| Concentration | 3 d | 6 d | 9 d |
|---|
| Control |
| 100.00±3.25 | 100.00±4.01 | 100.00±2.79 |
| β-FGF (ng/ml) | 10 |
74.28±5.71a |
60.93±4.52a |
50.07±3.71a |
|
99Tc-MDP(M) |
10−4 | 109.98±2.96 | 92.78±4.03 | 91.45±5.22 |
|
|
10−5 | 113.75±2.83 | 108.92±3.58 | 103.24±2.54 |
|
|
10−6 |
117.84±3.65a |
125.85±4.93a |
118.01±3.23a |
|
|
10−7 |
125.04±3.53a |
129.09±5.73a |
123.65±5.26a |
|
|
10−8 |
151.68±4.06a |
131.48±4.04a |
128.01±5.47a |
|
|
10−9 |
144.80±4.66a |
116.34±3.47a |
118.74±4.77a |
|
|
10−10 |
114.21±2.63a | 107.07±3.13 |
115.33±5.13a |
|
|
10−11 |
125.68±4.55a | 104.13±2.41 | 111.05±5.83 |
|
|
10−12 | 109.95±4.14 | 103.17±3.06 | 95.63±3.07 |
Influence of 99Tc-MDP on
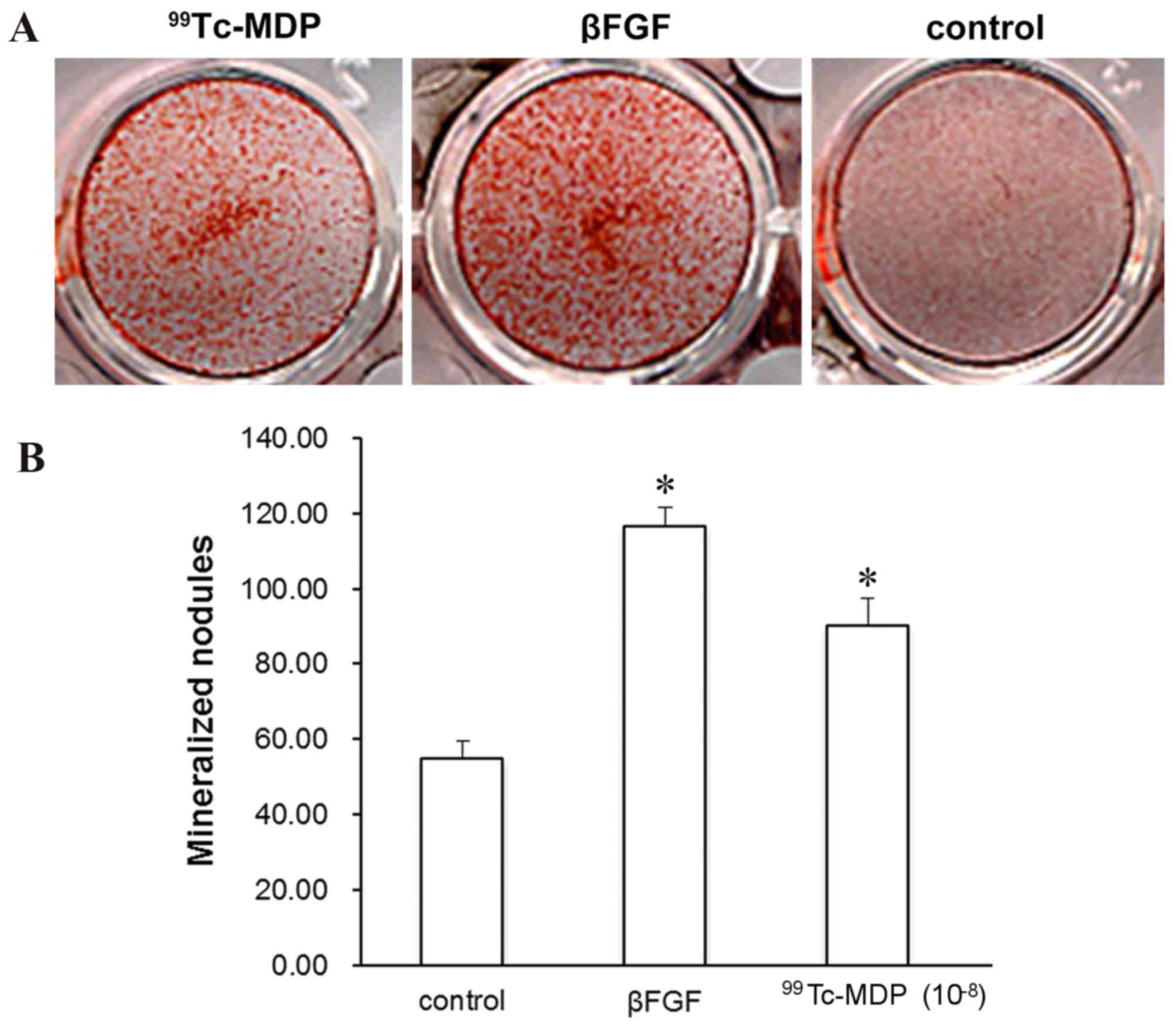
mineralized nodules
The formation of mineralized nodules marks the
mature differentiation of osteoblasts, and represents a
morphological manifestation of osteoblast function. The influence
of 99Tc-MDP on the development of mineralized nodules in
osteoblasts was assessed by light microscopy. Following 14 days of
incubation with 10−8 99Tc-MDP, Alizarin Red staining
revealed the level of mineralized nodules to be significantly
increased compared with controls (P<0.001; Fig. 5).
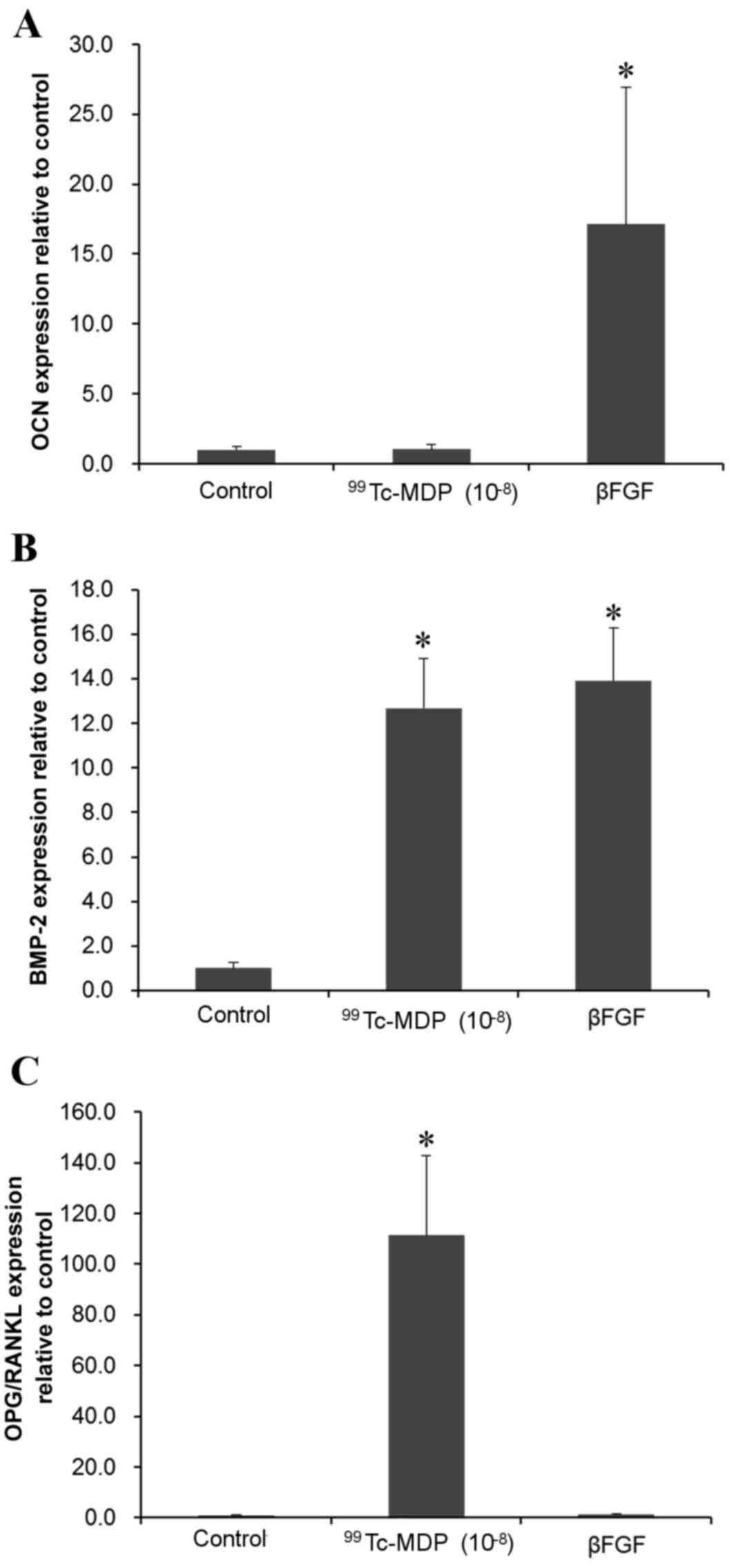
Influence of 99Tc-MDP on
OCN, BMP-2, OPG/RANKL mRNA expression levels
RT-qPCR revealed that incubation with
10−8 M 99Tc-MDP for 72 h did not
significantly affect OCN mRNA expression levels (Fig. 6A) but BMP-2 and OPG/RANKL mRNA
expression levels were significantly increased compared with
controls (P<0.001 and P<0.001, respectively; Fig. 6B and C).
Influence of 99Tc-MDP on
OPG/RANKL secretion
ELISA revealed that secretion of OPG was
significantly increased, by 10.15±5.32%, in osteoblasts incubated
with 10−8 M 99Tc-MDP for 72 h (P=0.002;
Table VI). Although
10−8 M 99Tc-MDP did not significantly affect
the secretion of RANKL, the ratio of OPG to RANKL secretion was
increased by 13.41±3.24% (Table
VI).
 | Table VI.Determination of OPG/RANKL protein
expression using ELISA. |
Table VI.
Determination of OPG/RANKL protein
expression using ELISA.
|
| Percent of controls
(%) |
|---|
|
|
|
|---|
|
|
| OPG | RANKL | OPG/RANKL |
|---|
| Control |
| 100.00±3.11 | 100.00±4.33 | 100.00±3.23 |
| β-FGF (ng/ml) | 10 |
110.66±1.21a |
116.71±5.84a | 94.91±4.68 |
| 99Tc-MDP
(M) |
10−8 |
110.15±5.32a | 97.12±3.08 |
113.41±3.24a |
Discussion
99Tc-MDP has been effectively used in the
treatment of many autoimmune and bone-destructive diseases
(9–15). The primary aims of the present
study were to investigate the influence of 99Tc-MDP on
osteoblasts cultured in vitro, and to explore the mechanisms
responsible for its depression of bone destruction. Primary human
osteoblasts were isolated from iliac cancellous bone and cultured
ex vivo.
A CCK-8 kit was used to measure proliferation of
passage 2 osteoblasts. Whilst high concentrations of
99Tc-MDP (10−4 M and above) inhibited
osteoblast proliferation, at lower concentrations
(10−5-10−12 M) proliferation was enhanced.
BrdU staining and flow cytometry were used to the examine cell
cycle, thereby reflecting cell proliferation. BrdU staining has
been established as a highly sensitive method of measuring
osteoblast differentiation (20,21).
Treatment with 10−8 M 99Tc-MDP significantly
increased SPF and proliferation index, further indicating that
99Tc-MDP promoted osteoblast proliferation. However,
99Tc-MDP did not significantly affect the rate of
apoptosis, indicating that 99Tc-MDP-induced cell
proliferation was not achieved by inhibiting osteoblast
apoptosis.
BMP-2, the most active member of the transforming
growth factor-β family, induces ectopic osteogenesis and is the
only cytokine able to induce bone formation independently (22). BMP-2 also induces osteoblast
differentiation, and has been reported to induce the
differentiation of pluripotent cells isolated from mouse bone
marrow into various mesoderm-derived cells, including muscle, fat
and cartilage cells, while inhibiting the formation of fat and
muscle cells (23). BMP-2 was also
reported to induce differentiation of the MC3T3-E1 cell line into
osteoblasts, stimulate OCN expression and enhance ALP activity
(24). The present study
demonstrated that 10−8 M 99Tc-MDP
significantly induced BMP-2 mRNA expression, enhanced ALP activity
and induced formation of mineralized nodules, indicating that
99Tc-MDP induced osteoblast differentiation.
OCN is another marker of osteoblast maturation, and
reflects osteogenic function (19). OCN mRNA expression levels were
measured by RT-qPCR, and treatment with 10−8 M
99Tc-MDP did not significantly affect OCN mRNA
expression levels within 72 h. Potentially, OCN expression would
reach a detectable level only later in the culture period, for
example the period of bone matrix mineralization.
Decreased OPG/RANKL expression has been reported to
be associated with bone destruction and loss of bone mass in
several rheumatic diseases, including RA, AS and osteoporosis
(25). In many patients with RA,
inflammatory factors regulate the expression of OPG and RANKL and
further influence bone metabolism, and the expression of OPG/RANKL
can be used to predict the level of bone destruction in RA patients
(25). Kim et al (26) demonstrated that in 75% of patients
with AS, bone density declined with disease activity (26). sRANKL serum levels and the ratio of
sRANKL to OPG were also increased in patients with RA and these
changes were associated with bone density and radiological changes,
indicating that the imbalance between RANKL and OPG might be
associated with the development of AS and osteoporosis. The
involvement of the OPG/RANKL signaling pathway in osteoporosis has
been studied intensively, and OPG/RANKL are considered a target for
osteoporosis treatment (27).
99Tc has also previously been reported to conjugate with
methylene diphosphonate, and inhibit receptor activator of NF-κB
ligand-induced osteoclastogenesis (28). In the present study, OPG mRNA
expression levels were significantly increased in osteoblasts
treated with 99Tc-MDP, while the protein expression
levels were not significantly elevated. The level of RANKL mRNA was
not significantly decreased, but the ratio of OPG mRNA to/RANKL
mRNA was significantly elevated. These findings indicate that
99Tc-MDP induced osteoblast expression of OPG and
increased the ratio of OPG to RANKL, thereby inhibiting
differentiation and activation of osteoclasts and
osteoclast-mediated bone destruction.
In conclusion, 99Tc-MDP induced
osteoblast proliferation and differentiation, enhanced osteoblast
growth and matrix mineralization, specifically stimulated
expression of BMP-2 and ALP and thus bone formation, and enhanced
the osteogenic function of osteoblasts. Meanwhile,
99Tc-MDP induced osteoblasts to express OPG and
increased the ratio between OPG/RANKL, thereby inhibiting the
differentiation and activation of osteoclasts and
osteoclast-mediated bone destruction. Overall, the findings of the
present study suggested that 99Tc-MDP may be further
assessed as a therapeutic agent for the treatment of
bone-destructive diseases.
Acknowledgements
The present study was supported by the Scientific
Research Projects of Health and Family Planning Commission of
Sichuan Province, China (grant no. 150078).
References
|
1
|
Thommesen L, Stunes AK, Monjo M, Grøsvik
K, Tamburstuen MV, Kjøbli E, Lyngstadaas SP, Reseland JE and
Syversen U: Expression and regulation of resistin in osteoblasts
and osteoclasts indicate a role in bone metabolism. J Cell Biochem.
99:824–834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein GS and Lian JB: Molecular mechanisms
mediating proliferation/differentiation interrelationships during
progressive development of the osteoblast phenotype. Endocr Rev.
14:424–442. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pockwinse SM, Stein JL, Lian JB and Stein
GS: Developmental stage-specific cellular responses to vitamin D
and glucocorticoids during differentiation of the osteoblast
phenotype: Interrelationship of morphology and gene expression by
in situ hybridization. Exp Cell Res. 216:244–260. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quarto N, Li S, Renda A and Longaker MT:
Exogenous activation of BMP-2 signaling overcomes TGFβ-mediated
inhibition of osteogenesis in Marfan embryonic stem cells and
Marfan patient-specific induced pluripotent stem cells. Stem Cells.
30:2709–2719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi N, Udagawa N and Suda T: A new
member of tumor necrosis factor ligand family,
ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and
function. Biochem Biophys Res Commun. 256:449–455. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofbauer LC and Heufelder AE: Role of
receptor activator of nuclear factor-kappaB ligand and
osteoprotegerin in bone cell biology. J Mol Med (Berl). 79:243–253.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khosla S: Minireview: The OPG/RANKL/RANK
system. Endocrinology. 142:5050–5055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai K, Jin C, Tu S, Xiong Y, Huang R and
Ge J: Intravitreal injection of (99)Tc-MDP inhibits the development
of laser-induced choroidal neovascularization in rhesus monkeys.
Graefes Arch Clin Exp Ophthalmol. 252:1049–1057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai K, Xu L, Jin C, Wu K, Tian Z, Huang C,
Zhong X and Ye H: Technetium-99 conjugated with methylene
diphosphonate (99Tc-MDP) inhibits experimental choroidal
neovascularization in vivo and VEGF-induced cell migration and tube
formation in vitro. Invest Ophthalmol Vis Sci. 52:5702–5712. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Wang L, Liu Y, Akiyama K, Chen C,
Atsuta I, Zhou T, Duan X, Jin Y and Shi S: Technetium-99 conjugated
with methylene diphosphonate ameliorates ovariectomy-induced
osteoporotic phenotype without causing osteonecrosis in the jaw.
Calcif Tissue Int. 91:400–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, He Y and He CS: The development of
Technetium [99Tc] Methylenediphosphonate. China Pharmacy.
6:553–555. 2010.
|
|
13
|
Wang L, Gu Q, Xu Y, Li S, Gui J, Yang J,
Yao Q and Ji Y: Effects of Yunke (technetium-99 conjugated with
methylene diphosphonate; (99)Tc-MDP) and/or colloidal chromic
phosphate phosphonium-32, alone and in combination, in rats with
adjuvant arthritis. Clin Exp Pharmacol Physiol. 35:23–28. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su D, Shen M, Gu B, Wang X, Wang D, Li X
and Sun L: (99)Tc-methylene diphosphonate improves rheumatoid
arthritis disease activity by increasing the frequency of
peripheral γδ T cells and CD4(+) CD25 Foxp3(+) Tregs. Int J Rheum
Dis. 19:586–593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mu R, Chen S and Li ZG: Therapeutic effect
of 99mTc-MDP and its role in proinflammatory cytokines in
rheumatoid arthritis. Chinese Journal of Rheumatology. 1:39–41.
2004.
|
|
16
|
Kanno S, Hirano S and Kayama F: Effects of
phytoestrogens and environmental estrogens on osteoblastic
differentiation in MC3T3-E1 cells. Toxicology. 196:137–145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Im GI, Qureshi SA, Kenney J, Rubash HE and
Shanbhag AS: Osteoblast proliferation and maturation by
bisphosphonates. Biomaterials. 25:4105–4115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diduch DR, Coe MR, Joyner C, Owen ME and
Balian G: Two cell lines from bone marrow that differ in terms of
collagen synthesis, osteogenic characteristics, and matrix
mineralization. J Bone Joint Surg Am. 75:92–105. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bianco P, Riminucci M, Bonucci E, Termine
JD and Robey PG: Bone sialoprotein (BSP) secretion and osteoblast
differentiation: Relationship to bromodeoxyuridine incorporation,
alkaline phosphatase, and matrix deposition. J Histochem Cytochem.
41:183–191. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gratzner HG: Monoclonal antibody to
5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA
replication. Science. 218:474–475. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito Y, Yoshizawa T, Takizawa F, Ikegame
M, Ishibashi O, Okuda K, Hara K, Ishibashi K, Obinata M and
Kawashima H: A cell line with characteristics of the periodontal
ligament fibroblasts is negatively regulated for mineralization and
Runx2/Cbfa1/Osf2 activity, part of which can be overcome by bone
morphogenetic protein-2. J Cell Sci. 115:4191–4200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gori F, Thomas T, Hicok KC, Spelsberg TC
and Riggs BL: Differentiation of human marrow stromal precursor
cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances
osteoblast commitment, and inhibits late adipocyte maturation. J
Bone Miner Res. 14:1522–1535. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rudkin GH, Yamaguchi DT, Ishida K,
Peterson WJ, Bahadosingh F, Thye D and Miller TA: Transforming
growth factor-beta, osteogenin, and bone morphogenetic protein-2
inhibit intercellular communication and alter cell proliferation in
MC3T3-E1 cells. J Cell Physiol. 168:433–441. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geusens PP, Landewé RB, Garnero P, Chen D,
Dunstan CR, Lems WF, Stinissen P, van der Heijde DM, van der Linden
S and Boers M: The ratio of circulating osteoprotegerin to RANKL in
early rheumatoid arthritis predicts later joint destruction.
Arthritis Rheum. 54:1772–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HR, Lee SH and Kim HY: Elevated serum
levels of soluble receptor activator of nuclear factors-kappaB
ligand (sRANKL) and reduced bone mineral density in patients with
ankylosing spondylitis (AS). Rheumatology (Oxford). 45:1197–1200.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamdy NA: Targeting the RANK/RANKL/OPG
signaling pathway: A novel approach in the management of
osteoporosis. Curr Opin Investig Drugs. 8:299–303. 2007.PubMed/NCBI
|
|
28
|
Gong W, Dou H, Liu X, Sun L and Hou Y:
Technetium-99 conjugated with methylene diphosphonate inhibits
receptor activator of nuclear factor-κB ligand-induced
osteoclastogenesis. Clin Exp Pharmacol Physiol. 39:886–893. 2012.
View Article : Google Scholar : PubMed/NCBI
|