Introduction
Chronic stress exposure induces pathological changes
in the central nervous system that affect both emotion and
cognition (1). The
hypothalamic-pituitary-adrenal (HPA) axis is activated and
glucocorticoids (corticosterone in rodents and cortisol in humans)
are released at higher levels in response to stress. Glucocorticoid
receptors (GRs) are located throughout the brain, including the
cortex and hippocampus, which represent regions closely associated
with learning and memory (2).
Glucocorticoids bind to GRs and inhibit cognitive function during
stress (3). Numerous studies have
demonstrated that chronic stress and/or release of stress hormones
lead to the deterioration of cognition through various mechanisms,
including epigenetic regulation (4–6).
Histone deacetylation is a typical epigenetic regulatory mechanism
involved in stress. Many gene transcription events related to
cognition are blocked by histone deacetylation during stress
(7,8). Histone deacetylases (HDACs) are vital
for the catalysis of histone deacetylation (9,10).
The relationship between specific HDAC proteins and chronic stress
with regards to cognitive impairment remains unclear.
Histone deacetylase-2 (HDAC2) is a member of the
class I HDAC family. Although HDAC2 is diffusely distributed in the
cerebrum, the most densely stained neurons are located in the
CA1-CA3 regions of the hippocampus (11). A recent study has demonstrated that
HDAC2 upregulation exerts an important epigenetic blockade of
cognition (12). HDAC2
overexpression exerts negative effects regarding memory because it
suppresses spine formation and decreases the spine density of
hippocampal neurons (12). HDAC2
activity leads to reduced histone H3 and H4 acetylation levels,
which results in memory-related gene silencing, such as
brain-derived neurotrophic factor (BDNF), c-Fos, and glutamate
receptor 1, in mice (12). Both
HDAC inhibitors and HDAC2 knockdown are neuroprotective (13–16).
Moreover, HDAC2 is increased in both animal models and patients
with neurodegenerative diseases, such as Alzheimer's disease (AD),
and may represent a novel drug target (13). Despite the recent attention focused
on HDAC2, the mechanism of HDAC2 upregulation in diseases is
largely unknown (17,18).
GRs and HDAC2 are both expressed in the hippocampus
(19,20). Therefore, there is a possibility
that they co-localize and interact. In the present study, it is
demonstrated that the administration of the stress hormone
corticosteroid or chronic stress increased HDAC2 levels. The
pathological changes and potential mechanisms were subsequently
investigated, and it was determined that a stress-induced increase
in HDAC2 was accompanied by cellular dysfunction and cognitive
decline in mice. HDAC2 knockdown partially prevented stress-induced
cellular injuries and partially restored the levels of histone H3K9
acetylation and phosphoinositide 3-kinase/protein kinase B pathway
phosphorylation. The current findings indicated that HDAC2 is
involved in stress-induced cognitive impairment. To the best of the
authors' knowledge, this is the first study to demonstrate that
HDAC2 is directly involved in stress-related cognitive decline.
Materials and methods
Cell culture and treatments
Mouse neuroblastoma N2a cells (China National
Infrastructure of Cell Line Resource, Beijing, China) were used and
cultured in minimum essential medium (MEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
streptomycin and 100 U/ml penicillin (Gibco; Thermo Fisher
Scientific, Inc.) in a 37°C humidified incubator with 95% air and
5% CO2. The cells were resuspended and plated into fresh
dishes prior to treatment. For stress hormone administration,
following cell attachment, the medium was replaced by fresh MEM
with corticosteroids (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at different final concentrations and incubated for an
additional 24–48 h prior to collection. For HDAC2 silencing, N2a
cells were transfected with a lentivirus-HDAC2-short hairpin
(sh)RNA plasmid (LV-HDAC2-shRNA; Biowit Technologies, Ltd.,
Shenzhen, China), which encoded shRNA that targeted the mouse HDAC2
transcript. The HDAC2 expression levels were confirmed by western
blot analysis.
Cell MTT assay
MTT (Sigma-Aldrich; Merck KGaA) was used to assess
cell viability as described by Wang et al (21). N2a cells were seeded into a 96-well
plate at 1×104 cells/ml and 200 µl/well. The medium was refreshed
following cell attachment, and the cells were exposed to
corticosteroids at various concentrations (1–10 µM). Following a 24
h incubation, 20 µl MTT solution (5 mg/ml) was added to each well
for an additional 4 h culture at 37°C. Cells without corticosteroid
treatment were treated with culture medium under the same culture
conditions and were used as the control group. The media were
emptied, and 200 µl dimethylsulfoxide were added to each well to
dissolve the crystals. The absorbance was assessed at 550 nm to
determine the cell viability; four vice holes were used per
dose.
Cell morphological analysis
The N2a cells were treated with a 2 µM solution of
corticosteroids (cat. no. C2505; Sigma-Aldrich; Merck KGaA) for 48
h, and the morphology was analyzed and compared with the control
cells. An inverted phase-contrast microscope (Olympus Corporation,
Tokyo, Japan) and Image-Pro Plus software (version, 6.0; Media
Cybernetics, Inc., Rockville, MD, USA) were used to evaluate
morphological changes. The cell density and length of the
neurite-like outgrowths were measured, and the cell size, including
the number of primary dendrites, were determined as previously
described (22). Then, >100
cells were counted per group at random at the same magnification
(x40), and at least 3 cultures were used per group.
Immunofluorescence staining
Corticosteroid-treated and control cells were fixed
with 4% paraformaldehyde for 10 min at room temperature; the frozen
brain sections (8 µm) were fixed for 20 min. The slides were
subsequently incubated with 0.5% Triton followed by 3% hydrogen
peroxide (H2O2) for 10 min each. The slides
were then incubated with mouse anti-HDAC2 (1:200; cat. no. ab51832)
and rabbit anti-PSD95 primary antibodies (1:200; cat. no. ab18258),
which were both obtained from Abcam (Cambridge, MA, USA), overnight
at 4°C. The following day, the slides were incubated with goat
anti-mouse and goat anti-rabbit fluorescein-conjugated IgG
secondary antibodies (1:100; cat. nos. ZB-0312 and ZB-0311,
respectively; Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) for 40 min at room temperature. The slides were
examined via a laser scanning confocal microscope (Olympus
Corporation) and analyzed using ImageJ 3.1 software (National
Institutes of Health, Bethesda, MD, USA).
Protein extraction and western blot
analysis
To obtain the total protein, N2a cells maintained in
plates or hippocampal tissue were lysed with a mixture of lysis
buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA and 0.5% Triton
X-100) and protease inhibitors on ice for 30 min. The lysates were
clarified via centrifugation at 13,000 × g for 30 min at 4°C. The
supernatant was discarded prior to protein analysis and western
blotting. A total of 40 µg total proteins from each sample were
separated by 10% SDS-PAGE, and transferred to a polyvinylidene
difluoride membrane (0.45 µm). The membrane was incubated with 5%
fat-free milk to block the blots for 1 h at room temperature prior
to incubation with primary antibodies [HDAC2, 1:1,000, cat. no.
ab51832; PSD-95, 1:1,000, cat. no. ab18258; acetyl-H3K9, 1:500,
cat. no. ab10812; H3, 1:500, cat. no. ab1791; acetyl-H4K8, 1:500,
cat. no. ab15823; H4, 1:500, cat. no. ab10158; all obtained from
Abcam; Akt, 1:1,000, cat. no. 4691; p-Akt (Ser 473), 1:1,000, cat.
no. 4058; PI3K, 1:500, cat. no. 4292; and p-PI3K-p85, 1:500, cat.
no. 4228; all obtained from Cell Signaling Technology, Inc.,
Danvers, MA, USA] overnight at 4°C. The blots were probed with
horseradish peroxidase-conjugated GAPDH (1:15,000; KC-5G5;
Kangcheng Technology Co., Ltd.), and goat anti-mouse and goat
anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibodies (1:15,000; cat. nos. ZB-2305 and ZB-2301, respectively;
Zhongshan Golden Bridge Biotechnology Co., Ltd.) the following day
for 1 h at room temperature and the protein expression levels were
visualized via X-ray films and quantified using ImageJ 3.1
software. GAPDH, total H3, total H4, total AKT, and total PI3K were
used as loading controls for HDAC2 and PSD-95, acetyl-H3K9,
acetyl-H4K8, p-Akt and p-PI3K, respectively.
Animals and the chronic restraint
stress procedure
In total, 20 female C57BL/6J mice (12-months-old;
weight, 20–30 g) were used because older mice are more sensitive to
stress (23) and mice were divided
into 2 groups randomly (n=10 per group). The mice were maintained
in a temperature- and humidity-controlled room on a 12 h light-dark
cycle and had free access to food and water. The use of animals was
approved by the Animal Care and Use Committee of The Institute of
Laboratory Animal Science of Peking Union Medical College (Beijing,
China; ILAS-GC-2012-004). Chronic restraint stress was performed
and modified according to Yang et al (24). Briefly, the stressed mice were
restrained in cylinder tubes to limit their autonomous actions for
7 h/day (from 11:00 p.m. to 6:00 a.m.) for 28 consecutive days in a
behavior science laboratory. The control mice remained in their
home cages without stress exposure.
Determination of blood
corticosteroids
Fresh serum was obtained immediately following
euthanasia at 8:30-9:30 a.m., and corticosteroids were measured
with a corticosteroid ELISA kit (ADI-900-097; Enzo Life Sciences,
Inc., Farmingdale, NY, USA) according to the instruction
manual.
Morris water maze (MWM) test
The MWM procedure was similar to previous
descriptions (25) and was
conducted for 7 days following the completion of the stress
protocol. Briefly, the mice were trained twice per day on days 1–6
during the learning period and examined via a probe test on day 7.
The platform was hidden starting on day 2 and was absent on day 7.
Each swimming cycle was 60 sec. The mice were considered to have
located the platform when they remained on it for at least 5 sec.
The automatic tracking system Noldus Ethovision XT (Noldus
Information Technology BV, Wageningen, Netherlands) was used to
monitor and analyze the data.
Statistical analysis
The data were analyzed using SPSS software (version,
13.0; SPSS, Inc., Chicago, IL, USA). The data are expressed as the
means ± standard error of the mean, and P<0.05 was considered to
indicate a statistically significant difference. T-tests were used
to evaluate the differences between two groups, and one-way
analyses of variance (ANOVA) followed by Fisher's least significant
difference test were performed to evaluate the differences among
multiple groups. Repeated measures and multivariate ANOVAs were
used for the MWM.
Results
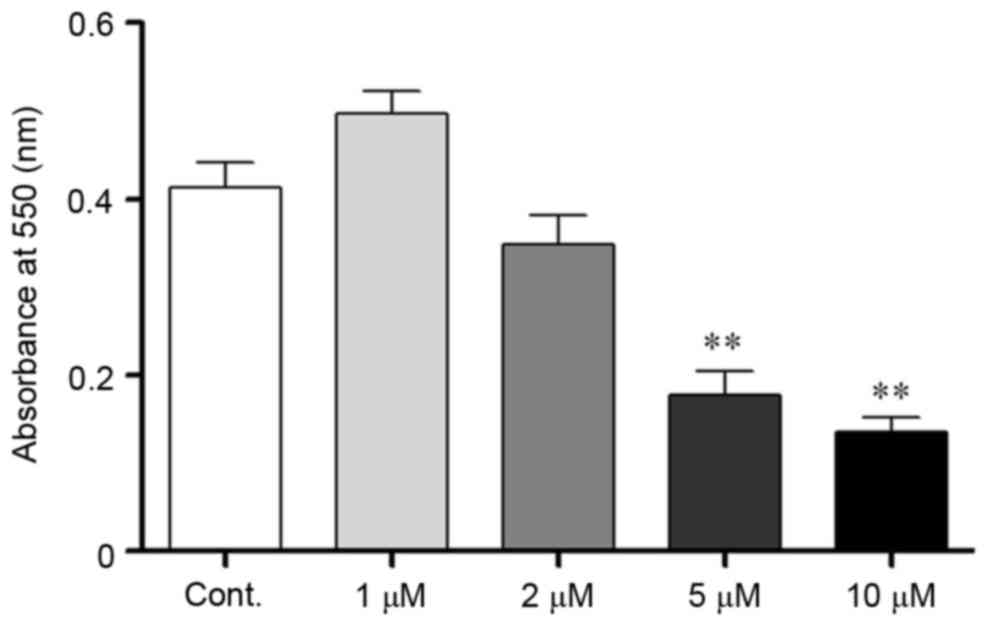
Corticosteroid administration
decreased N2a cell viability
Corticosteroids were used as a stressor agent to
mimic stress conditions in vitro. To determine a suitable
corticosteroid concentration, the MTT assay was used to evaluate
N2a cell viability. Cells were incubated with corticosteroids at a
series of concentrations (1, 2, 5 and 10 µM) for 24 h. The
absorbance at 550 nm was measured following incubation, and the
optical density (OD) value in the defined conditions reflected the
number of viable cells present. The OD value exhibited a decreasing
trend when the corticosteroid concentration was >1 µM, which
indicated that the cells were injured. When the concentration
increased to 5 µM, the cell viability significantly decreased,
which indicated substantial cell death. Taken together, the stress
hormone corticosteroid exhibited debilitative effects on N2a cells,
and at a higher dose, it was associated with cell lethality
(P<0.05; Fig. 1). Therefore, 2
µM was chosen as a suitable dose to induce injuries without
excessive cell apoptosis in the present study.
Corticosteroid treatment disturbed N2a
neurite outgrowth and connections
In the current study, the authors chose a continuous
48 h treatment of 2 µM corticosteroids for the experimental
conditions to mimic chronic stress. These conditions induced
significant changes in cell morphology and density (P<0.05;
Fig. 2A-C). The cell number
significantly decreased in these experimental conditions and
resulted in a lower cell density, which reflected an effect of
corticosteroid growth inhibition on the N2a cells (P<0.05;
Fig. 2B). In addition to the
reduced cell density, the N2a cells treated with corticosteroid had
shorter neurites, and the longest neurite length of the cells
significantly decreased after corticosteroid treatment compared
with the control cells (P<0.05; Fig. 2C). There were no significant
changes in the average neurite number per cell (P>0.05; Fig. 2D). The decreased cell number and
neurite outgrowth resulted in a loosened cellular attachment, which
reflected a weakened connection and communication among cells. In
addition to cell growth inhibition, cell function was also injured
after corticosteroid incubation. Postsynaptic density protein 95
(PSD95), a scaffold protein, is a well-established
synapse-associated marker that is crucial for synaptic plasticity
(26). PSD95 is located in the
postsynaptic density domain, and its expression is important for
synaptic function. The PSD95 protein was located at the neurite
outgrowth of the N2a cells (Fig.
2G). During the experimental conditions, the PSD95 expression
level decreased as detected by western blot analysis (P<0.05;
Fig. 2E and F). Furthermore,
immunofluorescence staining indicated decreased PSD95 expression
following corticosteroid incubation (P<0.05; Fig. 2G and H). The downregulated PSD95
verified the functional impairment of the N2a cells.
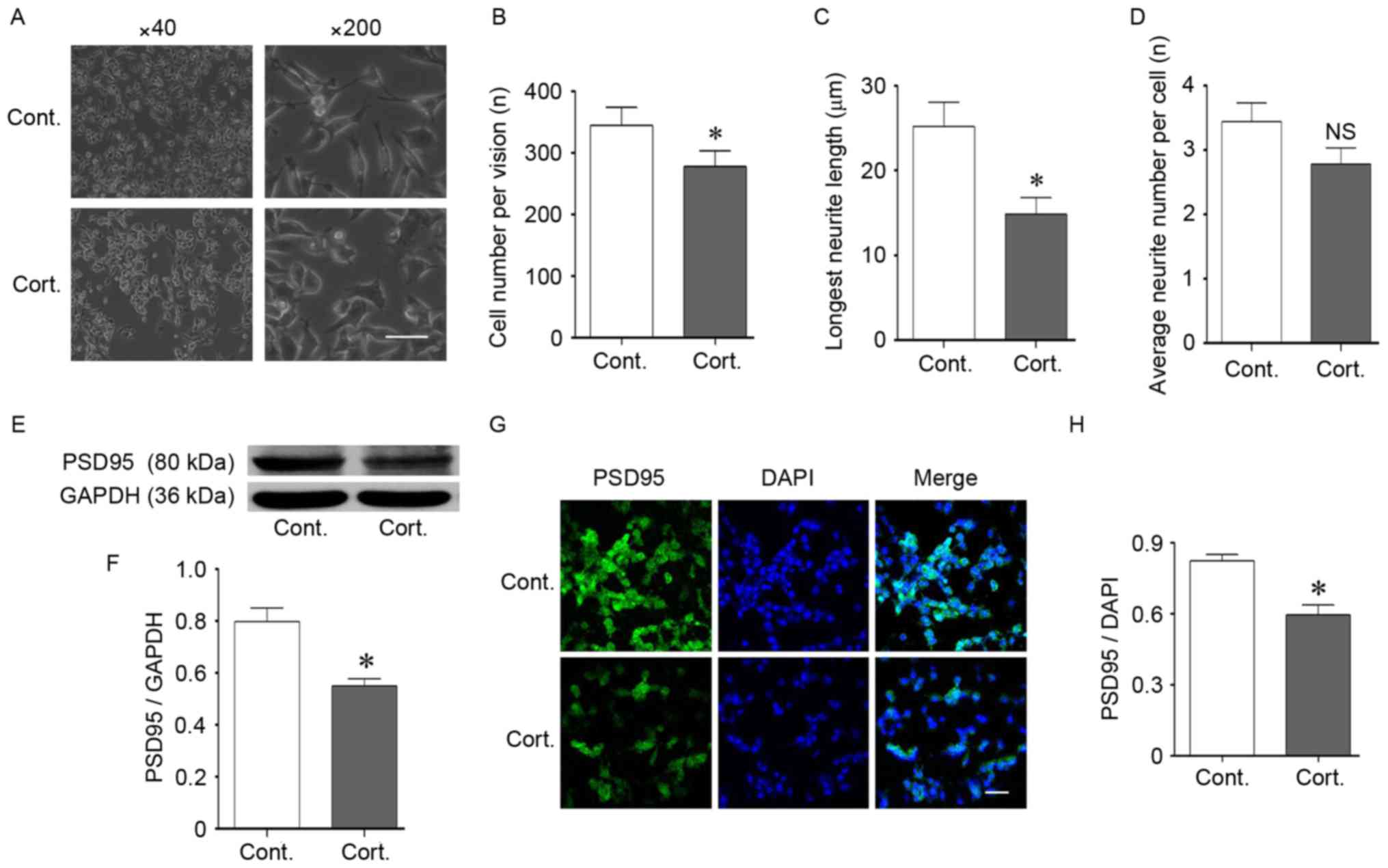 | Figure 2.Corticosteroid administration induced
injury in N2a cells. (A-D) Cell morphological changes following
corticosteroid administration at a dose of 2 µM for 48 h. (A and B)
Corticosteroid treatment reduced the cell density. The cell number
was reduced following treatment compared with the control cells. (A
and C) Corticosteroid treatment shortened the neurite outgrowth
length of the N2a cells, and the longest neurite length was
significantly decreased compared with the control cells. (A and D)
There was no significant difference in the average neurite number
per cell. (E and F) The expression of synapse-associated protein
PSD95 decreased. Western blotting indicated a decrease in PSD95
after 48 h of corticosteroid treatment at a dose of 2 µM. GAPDH was
used as the loading control. (G and H) The location and decreased
expression of PSD95 were demonstrated by immunofluorescence
staining. DAPI was used to stain the nucleus. (G) PSD95 was located
at the neurite outgrowth of N2a cells. (G and H) Corticosteroid
treatment decreased the fluorescent intensity of PSD95 in N2a
cells. For all panels, at least 100 cells in each culture were
evaluated, and at least 3 cultures were used per group. The
magnification and scale are as follows: (A) magnification, ×40; (G)
magnification, ×200; bar, 20 µm. Data are presented as the mean ±
standard error of the mean. *P<0.05 vs. control cells; NS,
P>0.05 vs. control cells. N2a, neuro-2a; PSD95, postsynaptic
density 95; cont, control cells; cort, corticosteroid treated
cells. |
Corticosteroids induced the
deacetylation of histone H3K9 and HDAC2
The acetylation of histone residues, including H3K9,
H3K14, H4K8 and H4K12, enhances gene expression related to learning
and memory (27). In previous
studies of the authors, decreased acetylation levels of histone
H3K9 and H4K8 were identified in a mouse model of AD, which is
characterized by cognitive impairment (unpublished data).
Glucocorticoids can also cause cognitive impairments in mice via a
reduction in histone acetylation (28). In the present study, the
acetylation levels of H3K9 were significantly decreased following
corticosteroid treatment (P<0.05; Fig. 3E and F), consistent with abnormal
epigenetic regulation involved in stress, even though H4K8
acetylation remained stable (P>0.05; Fig. 3G-I). Histone deacetylation leads to
more compact chromosomes and reduces gene transcription, which thus
reduces proteins crucial for learning and memory. Histone
deacetylation is catalyzed by histone deacetylases (HDACs), and the
protein levels indicated both dose-dependent and time-dependent
augmentation of HDAC2 (P<0.05; Fig.
3A-D). HDAC2 was prominently increased following 48 h of
corticosteroid treatment at the same dose of 2 µM and exhibited a
persistent increase with an increase in dose, when compared with
the control group. The levels of HDAC2 were determined using
immunofluorescence staining. HDAC2 was expressed in the nucleus
(Fig. 3G). At 48 h of
corticosteroid incubation at a dose of 2 µM, there was an
augmentation of HDAC2 compared with the control cells (Fig. 4G and H). This corresponding
upregulation of HDAC2 and cell injuries suggested that HDAC2 may be
a negative mediator in corticosteroid-induced neural damage.
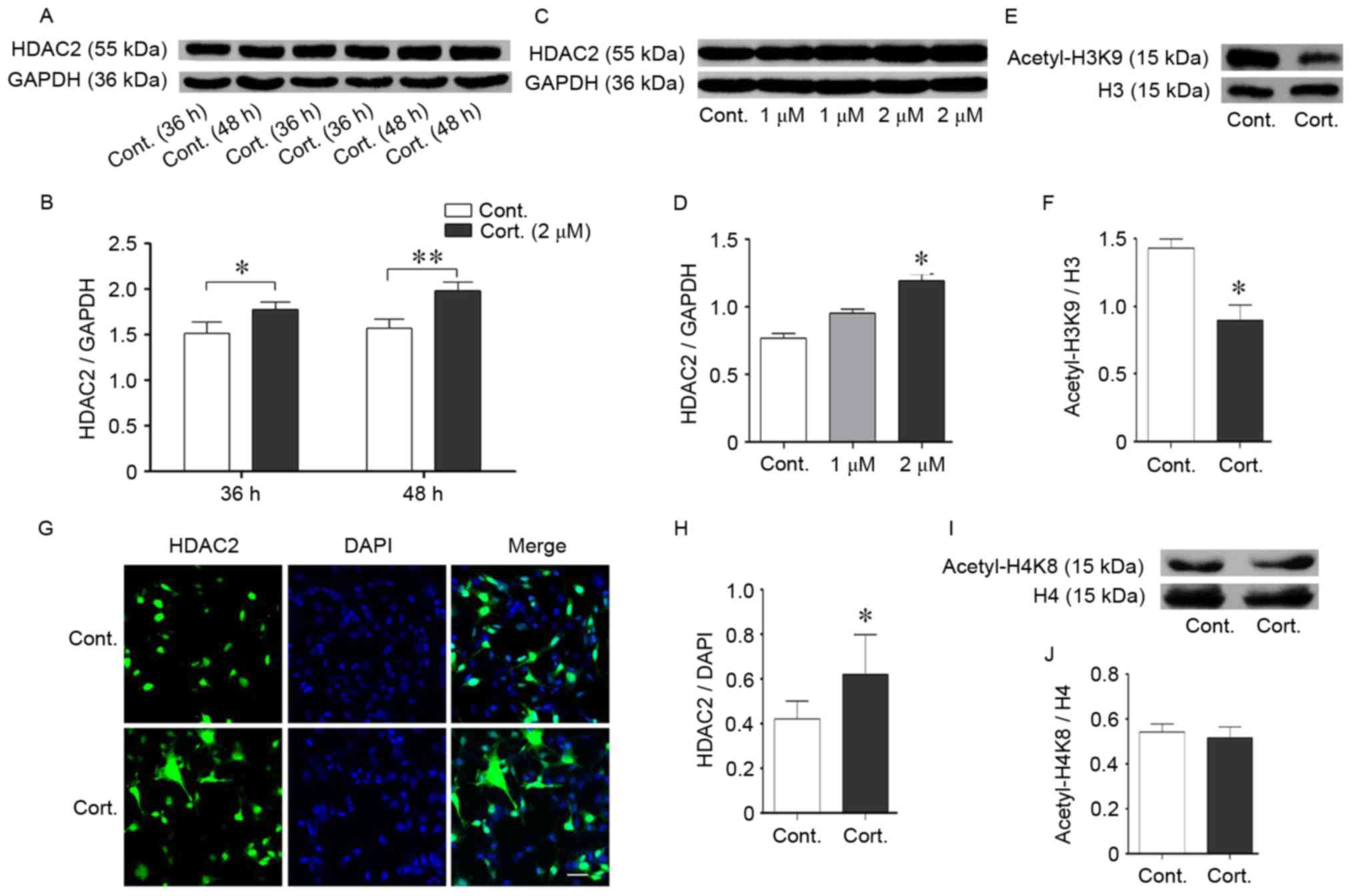 | Figure 3.Corticosteroid treatment increased
HDAC2 expression. (A and B) Time-dependent effects on HDAC2 protein
expression in N2a cells. The cells were incubated with
corticosteroid at 2 µM for the indicated times from 24–48 h prior
to collection. The HDAC2 protein levels were measured by western
blot analysis. The HDAC2 protein increased over time; GAPDH was
used as the loading control. (C and D) Dose-dependent expression
effects on the HDAC2 protein in N2a cells. The cells treated with
an increased dose of corticosteroid from 1–2 µM for 48 h exhibited
enhanced HDAC2 protein levels as evaluated by western blot
analysis. HDAC2 increased with an increase in the corticosteroid
dose. GAPDH was used as the loading control. (G and H)
Immunofluorescence staining indicated the subcellular location of
HDAC2 protein. HDAC2 was located in the nucleus of N2a cells.
Differences in fluorescent staining were identified between groups,
and an increasing trend was apparent. DAPI was used to stain the
nucleus. (E and F, I and J) Acetylated levels of histone residues
related to cognition. Corticosteroid incubation (2 µM, 48 h)
significantly decreased the acetylated level of H3K9 but not H4K8.
(E and F) The histone H3K9 acetylation levels were reduced
following corticosteroid treatment compared with the control cells.
Total histone H3/H4 was used as the loading control. Data are
presented as the mean ± standard error of the mean. *P<0.05,
**P<0.01 vs. control cells. At least 3 cultures were used per
group. HDAC2, histone deacetylase 2; N2a, neuro-2a; H4K8, histone 4
lysine 8; H3K9, histone 3 lysine 9; cont, control cell; cort,
corticosteroid treated cells. |
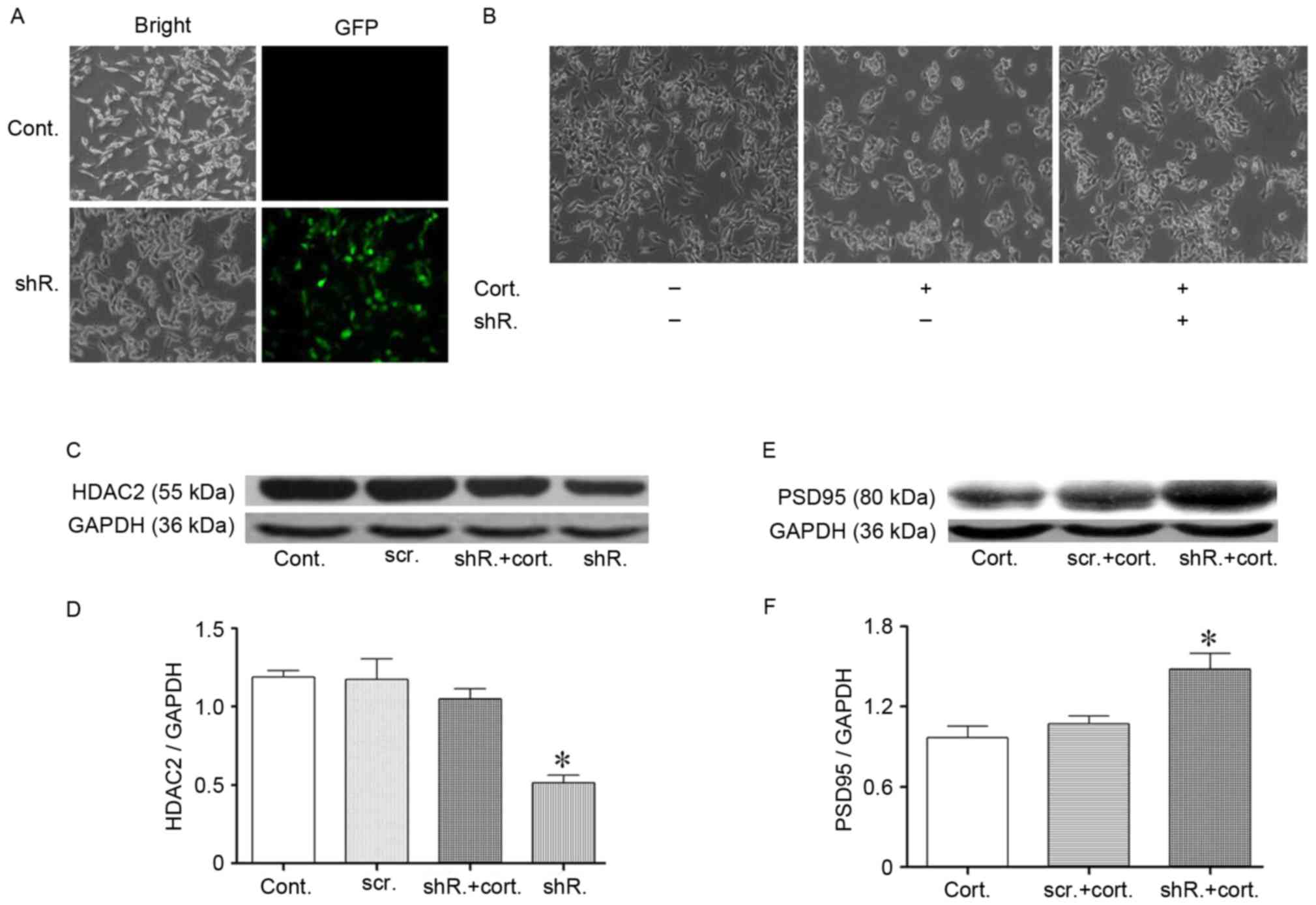 | Figure 4.Specific HDAC2 knockdown partially
resisted corticosteroid-induced N2a cell injury. (A)
Lentivirus-shRNA transfection targeted the HDAC2 protein of N2a
cells. The N2a cell transfection efficiency was confirmed by the
presence of green fluorescence in the cytoplasm. (C and D) The
HDAC2 protein expression level following transfection was detected
by western blotting. The HDAC2 protein significantly decreased 48 h
after the transfection efficiency evaluation. The lentivirus-shRNA
transfection that targeted HDAC2 protein was resistant to the
corticosteroid-induced HDAC2 upregulation in N2a cells. HDAC2 did
not increase during corticosteroid administration following
transfection. GAPDH was used as the loading control. (B)
Morphological recovery of HDAC2 knockdown N2a cells following
corticosteroid administration (2 µM, 48 h). The cells exhibited a
partially restored density and the longest neurite length following
transfection. (E and F) The PSD95 levels exhibited a trend toward
recovery following HDAC2 knockdown in N2a cells. Data are presented
as the mean ± standard error of the mean. *P<0.05 vs. control
cells or between two groups. At least 3 cultures were used per
group. HDAC2, histone deacetylase 2; N2a, neuro-2a; shRNA, short
hairpin RNA; PSD95, postsynaptic density 95; cont, control cells;
cort, corticosteroid treated cells (2 µM, 48 h); shR, transfected
cells; scr, scramble shRNA transfected cells; GFP, green
fluorescence protein. |
HDAC2 acts as a negative regulator
involved in corticosteroid-induced injuries via the PI3K/AKT
signaling cascade
Based on previous results, the authors hypothesized
that HDAC2 mediates cell injuries following incubation with
corticosteroids. To test this hypothesis, further studies were
performed. To knockdown HDAC2, cells were transfected with
LV-shRNA-mHdac2. Lentivirus vectors that expressed Hdac2 shRNA were
added to the media, with the multiplicity of infection =
4.6IU/cell. Polybrene was used as the transfection enhancer. The
vectors also carried the gene encoding green fluorescent protein,
and successfully transfected cells were recognized via green
fluorescence. Scrambled shRNA was used as a control. Green
fluorescence was present after transfection, and the efficiency was
>50% (Fig. 4A). Western blot
analysis indicated a significant reduction in HDAC2 expression
following transfection (P<0.05; Fig. 4C and D). HDAC2 silencing was
maintained during corticosteroid treatment, as presented in
Fig. 4C and D. The transfected
cells did not exhibit an increase in HDAC2 following corticosteroid
treatment compared with the control cells.
Cell morphology was assessed again following
transfection. Consistent with the authors' hypothesis, knockdown of
HDAC2 restored cell density in the stressed cells (Fig. 4B). In addition, the cell neurite
outgrowth length was partially restored (Fig. 4B). These morphological improvements
in the cells provided an indicator of improved cell viability. In
addition, PSD95 expression was partially restored, which was
evaluated by western blot analysis (Fig. 4E and F). HDAC2 knockdown was
followed by an improvement of cell function, and the cell recovery
mechanism was subsequently explored. As previously discussed, the
acetylation of histones, such as histone H3K9 acetylation, was
reduced. Following corticosteroid treatment, transfected cells
exhibited an augmentation of acetylated H3K9 compared with the
control cells, as demonstrated by western blot analysis (P<0.05;
Fig. 5A and B), which may explain,
in part, the restoration of protein PSD95 expression.
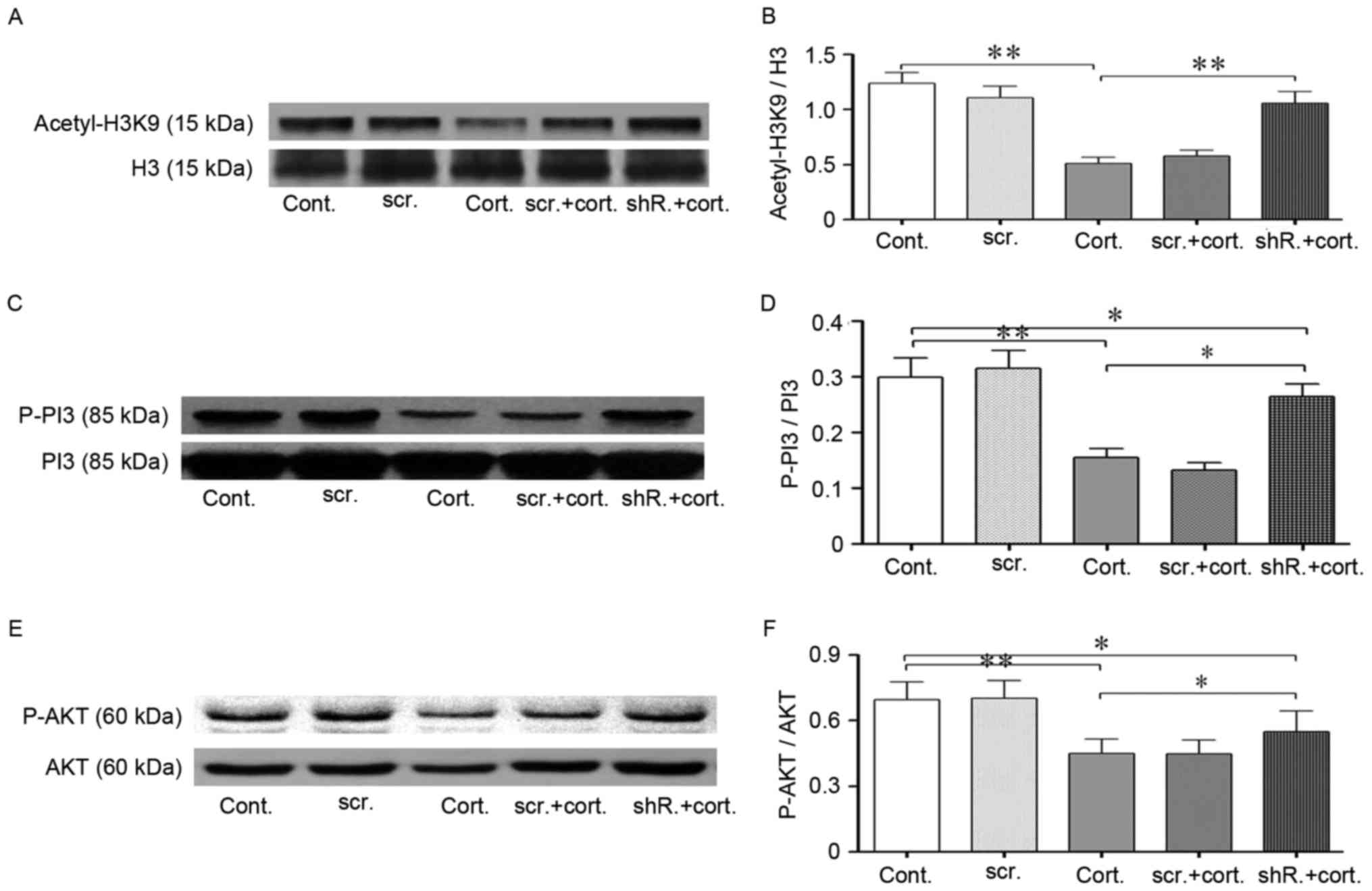 | Figure 5.LV-shRNA-HDAC2 knockdown partially
restored the reduced acetylation level of histone H3K9 and
increased PI3K-AKT signaling pathway phosphorylation. (A and B)
Western blot analysis indicated restored acetylation of H3K9
following lentivirus-shRNA-HDAC2 transfection. Corticosteroid
treatment (2 µM, 48 h) significantly decreased the histone
acetylation levels of H3K9 in neuro-2a (N2a) cells. HDAC2-shRNA
transfected cells exhibited increased histone H3K9 acetylation
levels during corticosteroid treatment compared with the
untransfected cells in the same condition. Total H3 was used as the
loading control. (C-F) HDAC2 knockdown increased PI3K/AKT
phosphorylation. (C and D) In the same treatment conditions, the
phosphorylation of PI3K p85 in HDAC2-shRNA transfected cells was
substantially increased compared with the untransfected cells.
Total PI3K was used as the loading control. (E and F) Following
corticosteroid incubation, AKT (ser473) phosphorylation in
HDAC2-shRNA transfected cells was partially restored compared with
the untransfected cells. Total AKT was used as the loading control.
Data are presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01 between two groups. At least 3 cultures
were used per group. shRNA, short hairpin RNA; HDAC1, histone
deacetylase 2; H3K9 histone 3, lysine 9; PI3K, phosphoinositide
3-kinase; AKT, protein kinase B; n2a, neuro-2a; cont, control
cells; cort, corticosteroid treated cells; scr, scramble shRNA
transfected cells; shR, transfected cells. |
Next, the phosphorylation status of the PI3K/AKT
signaling cascade was assessed. HDAC2 knockdown improved the
protective phosphorylation of this signaling pathway (P<0.05;
Fig. 5C-F). The phosphorylation of
the PI3K subunit p85 is an activated form that phosphorylates
downstream AKT, and the activated PI3K/AKT cascade was beneficial
for neurons (29). The
phosphorylation levels of p85 and serine 473 of AKT decreased
dramatically during corticosteroid incubation; however, this trend
could be partially reversed by HDAC2 knockdown.
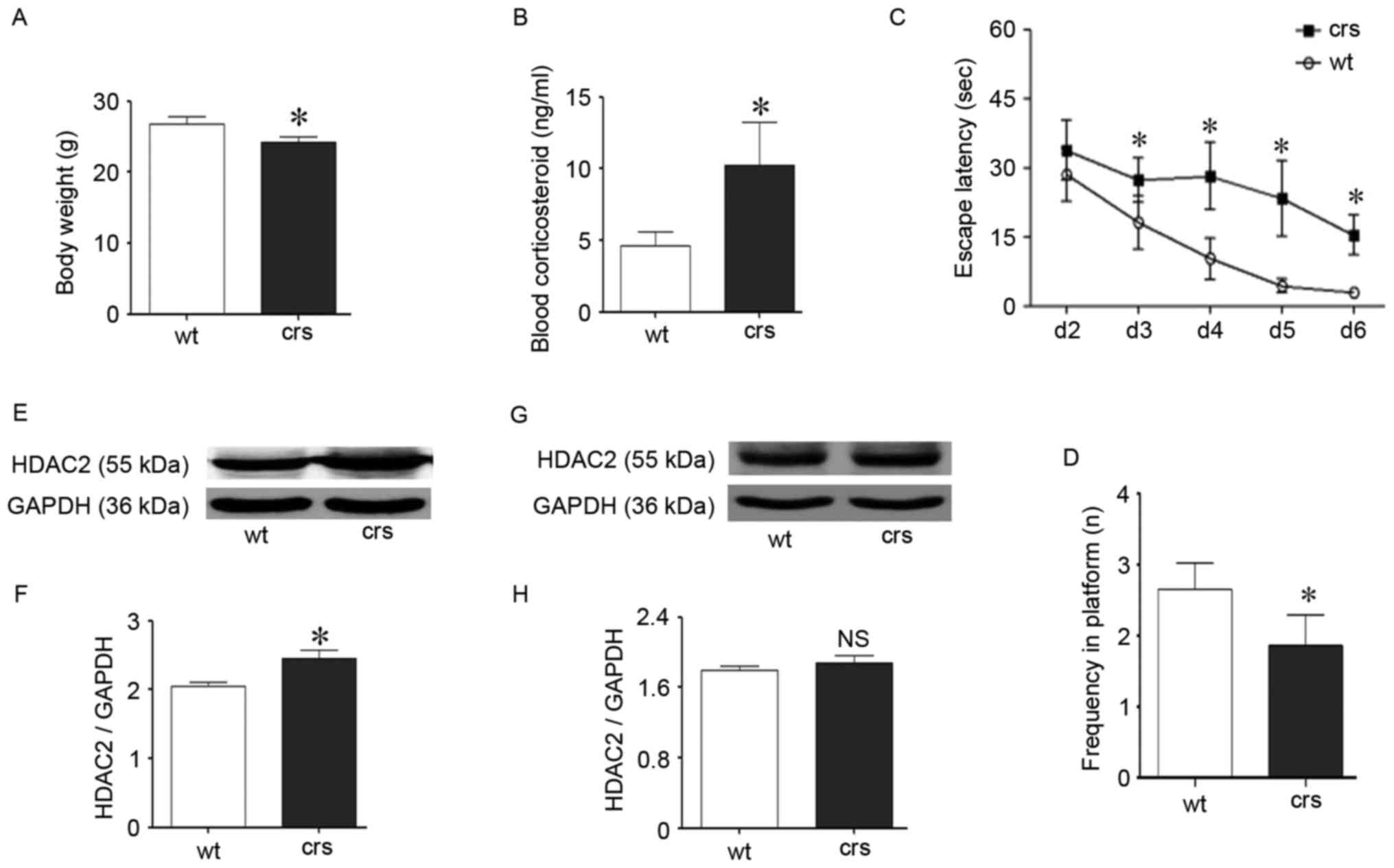
Chronic restraint stress impaired
cognition in mice and up-regulated HDAC2
The chronic restraint procedure is a reliable model
of chronic stress and has been widely used (30,31).
Following stress, the HPA axis is activated, and the main stress
hormone glucocorticoid is overexpressed, which exerts negative
effects on cognition. On average, an increased blood corticosteroid
level was identified in the stressed mice compared with the control
mice (P<0.05; Fig. 6B), which
indicates the involvement of stress hormones during stress and is
consistent with previous studies (32). The stressed mice simultaneously
exhibited lower body weights (P<0.05; Fig. 6A), which indicates disturbed living
states. The MWM test is a classical behavior detection method that
was used to evaluate cognitive performance and identify cognitive
impairment in the chronic stressed mice (Fig. 6C and D). In the learning period,
there was a significant difference in the escape latency between
groups starting on day 3 (P<0.05; Fig. 6C). Specifically, the stressed mice
took a longer time to find the platform compared with the wild-type
control mice starting on day 3 in the learning period. The weakened
learning abilities of the stressed mice lasted for four days to the
end of this test period. During the exploratory period, the
frequency of crossing the platform zone in the stressed group was
also significantly less than the control group (P<0.05; Fig. 6D), and the stressed mice exhibited
a deteriorated ability to remember the exact platform position.
These results confirmed that chronic stress could cause cognitive
decline via glucocorticoid upregulation, which is consistent with
previous reports (33,34).
Following the MWM trials, both the stressed and
control mice were euthanized. Proteins from the hippocampus and
cortex related to cognition were independently extracted. Following
the identification of cognitive decline during stress, HDAC2 was
assessed via western blotting for its specific role in blocking
cognition. Interestingly, there was a corresponding change in HDAC2
during chronic stress. The hippocampal HDAC2 protein levels were
significantly increased compared with the wild-type control mice
(P<0.05; Fig. 6E and F);
however, cortical HDAC2 was unchanged (P>0.05, Fig. 6G and H). The hippocampus is the
predominate region related to cognition, and the upregulation of
hippocampal HDAC2 following stress confirmed that HDAC2 is involved
in chronic stress-induced cognitive impairment in vivo.
Discussion
The current findings indicated that both stress
hormones and chronic restraint stress induce neural injuries and
increase HDAC2. HDAC2 knockdown may partially resist these
pathological changes via an upregulation of histone H3K9
acetylation and PI3K/AKT pathway phosphorylation in N2a cells.
Chronic stress is harmful for cognition and is a
risk factor for the onset of neurodegenerative diseases. The injury
mechanisms during stress have been well established (3). The HPA axis is activated during
stress, which is followed by the over-release of glucocorticoids
(corticosterone in rodents and cortisol in humans). In the present
study, the blood glucocorticoid levels of the stressed mice were
significantly upregulated as expected. Epigenetic mechanisms,
especially histone acetylation/deacetylation, have been
demonstrated to be involved in cognitive regulation and indicate
novel regulation patterns associated with stress. Chronic stress
can reduce global histone acetylation levels (35). The glucocorticoid receptor, which
comprises the key stress hormone receptor, can directly regulate
histone acetylation levels, including histone H3K14 (36) and H4K12 (37). Many histone residues, such as
H2BK5, H3K9, H3K14, H4K5 and H4K12, are important for learning,
memory and synaptic plasticity (38). Histone acetylation makes the
transcription of cognition-related genes substantially easier;
these genes include PSD95 and BDNF, which facilitate learning and
memory (39,40).
N2a cells are broadly utilized in neuroscience
research, as well as in stress related fields. Stress hormones,
such as dexamethasone and corticosteroid, have also been used to
mimic stress conditions (41). In
general, a 48 h or 72 h administration is sufficient to induce long
lasting observations according to the growth curve of N2a cells. In
the present study, a 48 h corticosteroid treatment was sufficient
to induce pathological and molecular changes. Corticosteroid caused
neural impairments and reduced the histone H3K9 acetylation level.
At the same time, PSD95 was downregulated, which supported
epigenetic dysregulation during stress. Histone deacetylases are
necessary to catalyze histone deacetylation. Glucocorticoid
receptors can interact with transcription factors, such as histone
acetylases and histone deacetylases (42,43),
which indicate that chronic stress may exert epigenetic regulation
via HDACs. HDAC2, a member of the class I HDAC family, is a key
negative regulator involved in cognition (12,16,44,45).
HDAC2 and glucocorticoid receptors are both located in the
hippocampus (11,19), and a glucocorticoid receptor
recognition element has been identified in the HDAC2 proximal
promoter region (13). These
findings suggested that HDAC2 may be involved in stress-related
cognitive decline, a hypothesis that was preliminary demonstrated
in the present study. Furthermore, increased HDAC2 protein levels
were induced during chronic stress conditions both in vitro
and in vivo, in parallel with injuries. Following HDAC2
knockdown, both the histone H3K9 acetylation and PSD95 protein
levels in N2a cells could be partially restored. Taken together,
HDAC2 is a negative regulator of cognition during stress via
histone deacetylation.
The PI3K/AKT signaling pathway has been explored in
depth in the field of learning and memory. AKT (ser-473)
phosphorylation induces its active form. The active form of the
PI3K/AKT signaling pathway can inhibit neural apoptosis and improve
neural morphology, which enhances neuronal communication and
synaptic plasticity (46). In
addition, PI3K/AKT signaling pathway activation mediates the
protective functions of several nerve growth factors, such as
insulin growth factor-1 and BDNF. The expression of several
dendritic spine density and presynaptic markers in hippocampal
neurons is dependent on PI3K/AKT signaling pathway activation
(47). Glucocorticoids may damage
this signal transduction (48).
Furthermore, a decrease in PI3K/AKT signaling pathway
phosphorylation may account, in part, for the reduced PSD95.
HDAC2 can regulate AKT activity. HDAC2 has been
demonstrated to provide critical support regarding malignant
progression via AKT activation in hepatocellular carcinoma
(49). In a previous study of the
authors, it was demonstrated that a HDAC2 conditional knockout
significantly improved cognition via the upregulation of AKT
phosphorylation in a mouse model of AD (unpublished data). Insulin
resistance has been identified as a risk factor for cognition
decline and is involved in the progression of AD. The PI3/AKT
pathway is one of the most important pathways in insulin signaling
and may exert an important role in cognition. HDAC2 inhibition has
been demonstrated to improve insulin signaling transduction
(50). As previously discussed,
the present study assessed the phosphorylation modifications in the
PI3/AKT signaling pathway before and after HDAC2 knockdown. A
significant upregulation of protectable p-PI3K and p-AKT following
HDAC2 silencing was identified during stress, which indicates that
HDAC2 could exhibit a role in impairment via PI3/AKT signaling
pathway modification.
Despite these interesting findings, several
limitations of the current study should be considered in the
interpretation of these findings. The present study used female
mice to conduct these experiments because chronic stress is a risk
factor of AD with HDAC2 upregulation, and females are more
susceptible to this disease. However, one potential issue is that
estrogen can affect cognition. As age increases, both estrogen
levels and cognition decline. Furthermore, the female mice in the
current study were housed in the absence of male mice; thus, the
effect of the estrous cycle was likely minimal. Additional studies
in male mice should be conducted to examine sex-dependent
differences in these findings.
In conclusion, HDAC2 is stress-related and acts as a
negative mediator via histone deacetylation and the modification of
PI3K/AKT pathway phosphorylation. These findings contribute novel
insights into the understanding of the epigenetic mechanisms
affected by chronic stress. However, in some neurodegenerative
diseases, such as AD, HDAC2 is significantly increased with limited
knowledge regarding the mechanisms. Thus, chronic stress-induced
increases in HDAC2 protein levels may represent a new research
direction, as well as a novel treatment target.
Glossary
Abbreviations
Abbreviations:
|
HDAC2
|
histone deacetylase-2
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
AKT
|
protein kinase B
|
|
HPA
|
hypothalamic-pituitary-adrenal
|
|
GRs
|
glucocorticoid receptors
|
References
|
1
|
Nicolaides NC, Kyratzi E,
Lamprokostopoulou A, Chrousos GP and Charmandari E: Stress, the
stress system and the role of glucocorticoids.
Neuroimmunomodulation. 22:6–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Q, van Heerikhuize J, Aronica E,
Kawata M, Seress L, Joels M, Swaab DF and Lucassen PJ:
Glucocorticoid receptor protein expression in human hippocampus;
stability with age. Neurobiol Aging. 34:1662–1673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lucassen PJ, Pruessner J, Sousa N, Almeida
OF, van Dam AM, Rajkowska G, Swaab DF and Czéh B: Neuropathology of
stress. Acta Neuropathol. 127:109–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferland CL and Schrader LA: Regulation of
histone acetylation in the hippocampus of chronically stressed
rats: A potential role of sirtuins. Neuroscience. 174:104–114.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kenworthy CA, Sengupta A, Luz SM, Ver
Hoeve ES, Meda K, Bhatnagar S and Abel T: Social defeat induces
changes in histone acetylation and expression of histone modifying
enzymes in the ventral hippocampus, prefrontal cortex, and dorsal
raphe nucleus. Neuroscience. 264:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferland CL, Harris EP, Lam M and Schrader
LA: Facilitation of the HPA axis to a novel acute stress following
chronic stress exposure modulates histone acetylation and the
ERK/MAPK pathway in the dentate gyrus of male rats. Endocrinology.
155:2942–2952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Radley JJ, Kabbaj M, Jacobson L,
Heydendael W, Yehuda R and Herman JP: Stress risk factors and
stress-related pathology: Neuroplasticity, epigenetics and
endophenotypes. Stress. 14:481–497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnsson A, Durand-Dubief M, Xue-Franzen
Y, Rönnerblad M, Ekwall K and Wright A: HAT-HDAC interplay
modulates global histone H3K14 acetylation in gene-coding regions
during stress. EMBO Rep. 10:1009–1014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hildmann C, Riester D and Schwienhorst A:
Histone deacetylases-an important class of cellular regulators with
a variety of functions. Appl Microbiol Biotechnol. 75:487–497.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clayton AL, Hazzalin CA and Mahadevan LC:
Enhanced histone acetylation and transcription: A dynamic
perspective. Mol Cell. 23:289–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao ZG, Liu Y, Zhang L, Huang L, Ma CM, Xu
YF, Zhu H and Qin C: Co-location of HDAC2 and insulin signaling
components in the adult mouse hippocampus. Cell Mol Neurobiol.
32:1337–1342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan JS, Haggarty SJ, Giacometti E,
Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X,
Mazitschek R, et al: HDAC2 negatively regulates memory formation
and synaptic plasticity. Nature. 459:55–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gräff J, Rei D, Guan JS, Wang WY, Seo J,
Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, et al: An
epigenetic blockade of cognitive functions in the neurodegenerating
brain. Nature. 483:222–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xuan A, Long D, Li J, Ji W, Hong L, Zhang
M and Zhang W: Neuroprotective effects of valproic acid following
transient global ischemia in rats. Life Sci. 90:463–468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hommet C, Mondon K, de Toffol B and
Constans T: Reversible cognitive and neurological symptoms during
valproic acid therapy. J Am Geriatr Soc. 55:6282007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morris MJ, Mahgoub M, Na ES, Pranav H and
Monteggia LM: Loss of histone deacetylase 2 improves working memory
and accelerates extinction learning. J Neurosci. 33:6401–6411.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HS, Chang YG, Bae HJ, Eun JW, Shen Q,
Park SJ, Shin WC, Lee EK, Park S, Ahn YM, et al: Oncogenic
potential of CK2α and its regulatory role in EGF-induced HDAC2
expression in human liver cancer. FEBS J. 281:851–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nott A, Watson PM, Robinson JD, Crepaldi L
and Riccio A: S-Nitrosylation of histone deacetylase 2 induces
chromatin remodelling in neurons. Nature. 455:411–415. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saaltink DJ and Vreugdenhil E: Stress,
glucocorticoid receptors, and adult neurogenesis: A balance between
excitation and inhibition? Cell Mol Life Sci. 71:2499–2515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolber BJ and Muglia LJ: Defining brain
region-specific glucocorticoid action during stress by conditional
gene disruption in mice. Brain Res. 1293:85–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang P, Jiang S, Cui Y, Yue Z, Su C, Sun
J, Sheng S and Tian J: The n-terminal 5-MER peptide analogue P165
of amyloid precursor protein exerts protective effects on SH-SY5Y
cells and rat hippocampus neuronal synapses. Neuroscience.
173:169–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scott HJ, Stebbing MJ, Walters CE,
McLenachan S, Ransome MI, Nichols NR and Turnley AM: Differential
effects of SOCS2 on neuronal differentiation and morphology. Brain
Res. 1067:138–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baumann VR: Stress sensitivity and
adaptation. Z Gesamte Inn Med. 30:15–16, passim. 1975.(In German).
PubMed/NCBI
|
|
24
|
Yang X, Han ZP, Zhang SS, Zhu PX, Hao C,
Fan TT, Yang Y, Li L, Shi YF and Wei LX: Chronic restraint stress
decreases the repair potential from mesenchymal stem cells on liver
injury by inhibiting TGF-β1 generation. Cell Death Dis.
5:e13082014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, He Z, Guo L, Huang L, Wang J and He
W: Behavioral alterations associated with a down regulation of HCN1
mRNA in hippocampal cornus ammon 1 region and neocortex after
chronic incomplete global cerebral ischemia in rats. Neuroscience.
165:654–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ricobaraza A, Cuadrado-Tejedor M,
Pérez-Mediavilla A, Frechilla D, Del Rio J and Garcia-Osta A:
Phenylbutyrate ameliorates cognitive deficit and reduces tau
pathology in an Alzheimer's disease mouse model.
Neuropsychopharmacology. 34:1721–1732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gräff J, Kim D, Dobbin MM and Tsai LH:
Epigenetic regulation of gene expression in physiological and
pathological brain processes. Physiol Rev. 91:603–649. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stankiewicz AM, Swiergiel AH and Lisowski
P: Epigenetics of stress adaptations in the brain. Brain Res Bull.
98:76–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buttrick GJ and Wakefield JG: PI3-K and
GSK-3: Akting together with microtubules. Cell Cycle. 7:2621–2625.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu N, Wang LH, Guo LL, Wang GQ, Zhou XP,
Jiang Y, Shang J, Murao K, Chen JW, Fu WQ and Zhang GX: Chronic
restraint stress inhibits hair growth via substance P mediated by
reactive oxygen species in mice. PLoS One. 8:e615742013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong JY, Lee DH and Kang SS: Effects of
chronic restraint stress on body weight, food intake, and
hypothalamic gene expressions in mice. Endocrinol Metab (Seoul).
28:288–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mustafa T, Jiang SZ, Eiden AM, Weihe E,
Thistlethwaite I and Eiden LE: Impact of PACAP and PAC1 receptor
deficiency on the neurochemical and behavioral effects of acute and
chronic restraint stress in male C57BL/6 mice. Stress. 18:408–418.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cuadrado-Tejedor M, Ricobaraza A, Del Rio
J, Frechilla D, Franco R, Pérez-Mediavilla A and Garcia-Osta A:
Chronic mild stress in mice promotes cognitive impairment and
CDK5-dependent tau hyperphosphorylation. Behav Brain Res.
220:338–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Finsterwald C and Alberini CM: Stress and
glucocorticoid receptor-dependent mechanisms in long-term memory:
From adaptive responses to psychopathologies. Neurobiol Learn Mem.
112:17–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chakravarty S, Pathak SS, Maitra S,
Khandelwal N, Chandra KB and Kumar A: Epigenetic regulatory
mechanisms in stress-induced behavior. Int Rev Neurobiol.
115:117–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gutièrrez-Mecinas M, Trollope AF, Collins
A, Morfett H, Hesketh SA, Kersanté F and Reul JM: Long-lasting
behavioral responses to stress involve a direct interaction of
glucocorticoid receptors with ERK1/2-MSK1-Elk-1 signaling. Proc
Natl Acad Sci USA. 108:13806–13811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dombrowsky H and Uhlig S: Steroids and
histone deacetylase in ventilation-induced gene transcription. Eur
Respir J. 30:865–877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brownell JE and Allis CD: Special HATs for
special occasions: Linking histone acetylation to chromatin
assembly and gene activation. Curr Opin Genet Dev. 6:176–184. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shibasaki M, Mizuno K, Kurokawa K and
Ohkuma S: Enhancement of histone acetylation in midbrain of mice
with ethanol physical dependence and its withdrawal. Synapse.
65:1244–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J
and Ma L: Chronic cocaine-induced H3 acetylation and
transcriptional activation of CaMKIIalpha in the nucleus accumbens
is critical for motivation for drug reinforcement.
Neuropsychopharmacology. 35:913–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Kloet ER, Karst H and Joëls M:
Corticosteroid hormones in the central stress response:
Quick-and-slow. Front Neuroendocrinol. 29:268–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beato M and Sánchez-Pacheco A: Interaction
of steroid hormone receptors with the transcription initiation
complex. Endocr Rev. 17:587–609. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Revollo JR and Cidlowski JA: Mechanisms
generating diversity in glucocorticoid receptor signaling. Ann N Y
Acad Sci. 1179:167–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Y, Li J, Dunn S, Xiong S, Chen W,
Zhao Y, Chen BB, Mallampalli RK and Zou C: Histone deacetylase 2
(HDAC2) protein-dependent deacetylation of mortality factor 4-like
1 (MORF4L1) protein enhances its homodimerization. J Biol Chem.
289:7092–7098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hou N, Gong M, Bi Y, Zhang Y, Tan B, Liu
Y, Wei X, Chen J and Li T: Spatiotemporal expression of HDAC2
during the postnatal development of the rat hippocampus. Int J Med
Sci. 11:788–795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spencer JP: The impact of fruit flavonoids
on memory and cognition. Br J Nutr. 104 Suppl 3:40–47. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee CC, Huang CC and Hsu KS: Insulin
promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR
and Rac1 signaling pathways. Neuropharmacology. 61:867–879. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Hu J and Price SR: Inhibition of
PI3-kinase signaling by glucocorticoids results in increased
branched-chain amino acid degradation in renal epithelial cells. Am
J Physiol Cell Physiol. 292:C1874–C1879. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Noh JH, Bae HJ, Eun JW, Shen Q, Park SJ,
Kim HS, Nam B, Shin WC, Lee EK, Lee K, et al: HDAC2 provides a
critical support to malignant progression of hepatocellular
carcinoma through feedback control of mTORC1 and AKT. Cancer Res.
74:1728–1738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun C and Zhou J: Trichostatin A improves
insulin stimulated glucose utilization and insulin signaling
transduction through the repression of HDAC2. Biochem Pharmacol.
76:120–127. 2008. View Article : Google Scholar : PubMed/NCBI
|