Introduction
Multiple myeloma (MM) is an incurable B cell
malignancy characterized by the clonal expansion of plasma cells
within the bone marrow (BM). It is well established that the
transformation of myeloma is not only dependent on genetic and
epigenetic aberrations, but also on the interaction between the
malignant plasma cells and the BM microenvironment (1,2). The
BM microenvironment is composed of a noncellular compartment
including the extracellular matrix and the liquid milieu, and
cellular compartments of mesenchymal origin (3). Myeloma cells modify this
microenvironment through their production of regulatory factors and
direct cell-to-cell contact, to create a milieu that promotes
myeloma cell survival (4–6) and augments osteoclast (OC)
recruitment and OC mediated bone loss (7,8).
As an essential cell type in the BM
microenvironment, bone marrow mesenchymal stem cell (MSCs) is a
type of adult stem cell endowed with self-renew and differentiation
capacities (9). Abnormalities of
MSCs derived from MM patients (MM-MSCs) have been described in
previous studies (10–12). Compared with MSCs isolated from
healthy donors, increased expressions of cytokines and
extracellular matrix were detected in MM-MSCs (11,13,14).
Additionally, decreased osteogenic potentiality and reduced
efficiency of suppression of T-cell proliferation were also
described (15–17). As well, MM-MSCs exhibited an
abnormal upregulation of microRNA-135b and promoted MM tumor growth
(18,19). Moreover, MM-MSCs demonstrated
non-recurrent genomic imbalances and hypomethylation of some
genomic region resulted in upregulated several microRNAs, which are
involved in regulation of senescence status and cell cycle
characteristics of MM-MSCs (12,20).
Telomeres are composed of repetitive DNA sequences
and associated proteins at the end of the chromosomes. They serve a
dominant role in protecting the end of DNA from fusion,
recombination and degradation (21). Telomeric DNA shortens itself with
each cell division due to the end-replication limitation of
chromosomes (22). Correlation is
reported between telomere length and replicative capacity of human
fibroblasts (23). However, like
most normal somatic cells, telomerase activity has not been
detected in human MSCs (24).
Telomeres of MSCs shortens its length concomitantly with the
increased age or cell division (25,26).
Moreover, dysfunction of telomeres impairs function of MSCs and
alters their gene expressions (26,27).
In the current study, the authors compared the
telomere length (TL) in bone marrow MSCs from MM patients and
age-matched controls. The results demonstrated that the TL in
MM-MSCs was longer than that in controls, and MM-MSCs secreted
higher levels of interleukin (IL)-6 and macrophage inflammatory
protein (MIP)-1α than the controls. Moreover, the results indicated
that the telomere length of MM-MSCs was closely correlated with the
expressions of IL-6 or MIP-1α.
Materials and methods
Cell culture
The human myeloma cells RPMI-8226 were cultured in
RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) containing 10% fetal bovine serum with the addition of 2 mM
L-glutamine (both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and antibiotics (100 U/ml penicillin and 0.1
mg/ml streptomycin) in a humid atmosphere at 37°C and 5%
CO2. After cell culture for 24 h, the conditioned medium
of RPMI-8226 was collected and stored in −20°C.
Isolation and expansion of MSCs from
bone marrows
Bone marrow (BM) samples were obtained from patients
with MM or from controls (patients without a tumor, infection or
autoimmune diseases; Table I). The
current study was approved by the Ethics Committee of the Second
Hospital of Shandong University (Jinan, China) and informed consent
was obtained from all patients. Mononuclear cells (MNCs) were
obtained from BM samples by density gradient separetion using
Ficoll density gradient centrifugation. Finally, MNCs were cultured
in Dulbecco's modified Eagle's medium (Hyclone; GE Healthcare Life
Sciences) supplemented with 10% defined fetal bovine serum
(Shanghai ExCell Biology, Inc., Shanghai, China), growth factors
(R&D Systems, Inc., Minneapolis, MN, USA). At 72 or 96 h
incubation, non-adherent cells were carefully removed, whereas the
MSCs attached to the cell culture plate were defined as passage 0
(P0) MSCs. When the MSCs reached approximately 80% confluence, the
cells were passaged (P1) by digestion using 0.05% trypsin-EDTA
(Thermo Fisher Scientific, Inc.) The MSCs were collected near
confluency at P4 for subsequent experiments.
 | Table I.Clinical characteristics of patients
with multiple myeloma (n=12). |
Table I.
Clinical characteristics of patients
with multiple myeloma (n=12).
| Sample | Gender | Age (years) | Disease
stagea | β2-MG (mg/dl) | BM plasma cells
(%) | Bone lesions |
|---|
| 1 | F | 68 | Early | 3.31 | 58 | N |
| 2 | M | 65 | Early | 3.36 | 51 | N |
| 3 | F | 69 | Late | 11.5 | 67 | Y |
| 4 | F | 63 | Early | 3.74 | 51 | Y |
| 5 | M | 65 | Late | 12.4 | 65 | Y |
| 6 | M | 74 | Late | 6.72 | 48 | N |
| 7 | M | 58 | Late | 7.79 | 32 | Y |
| 8 | M | 57 | Late | 40.5 | 40 | Y |
| 9 | M | 59 | Late | 7.89 | 4 | Y |
| 10 | M | 70 | Late | 9 | 43 | Y |
| 11 | M | 58 | Late | 20.8 | 20 | N |
| 12 | F | 59 | Early | 4.25 | 32 | Y |
Osteoblastic differentiation of
MSCs
An osteocyte differentiation assay was performed
according to the instructions provided in the StemPro®
Osteogenesis Differentiation kit (cat no. A1007201; Thermo Fisher
Scientific, Inc.). Briefly, MSCs were seeded at
1×104/cm2 in 6-well culture plates in
osteoblast differentiation culture media for 21 days. Medium was
changed 2–3 times a week. The osteocytes were stained with Alizarin
red (cat no. A5533; Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany).
Immunophenotyping of bone marrow
MSCs
The bone marrow MSCs were labeled with
phycoerythrin-conjugated anti-CD73 (cat no. 550257), fluorescein
isothiocyanate (FITC)-conjugated anti-CD90 (cat no. 555595),
FITC-conjugated anti-CD105 (cat no. 561443) (all from BD
Biosciences, Franklin Lakes, NJ, USA) and human leukocyte antigen G
(HLA-G; cat no. 12-9957-73; eBioscience, Inc., San Diego, CA, USA).
The fluorescence of the above MSC surface markers was detected and
analyzed using flow cytometry with a BD FACSAria™ II system (BD
Biosciences). Data were analyzed using the FlowJo software version
7.6 (FlowJo, LLC, Ashland, Oregon, USA).
Quantitative polymerase chain reaction
(qPCR) and Flow-FISH for MSC telomere length assay
For qPCR detection, genomic DNA was isolated using
QiAamp DNA Blood Mini kit (Qiagen GmbH, Hilden, Germany). Telomere
length was determined by qPCR as described previously (28). The primer sequences for human
telomere (Tel) and for β-globin (HBG) are listed in Table II. The relative telomere length
was decided as the percentage of that in a MM-MSC. Flow-FISH of
MSCs was performed as described previously (29).
 | Table II.The primer sequences for quantitative
polymerase chain reaction and telomere length assessment. |
Table II.
The primer sequences for quantitative
polymerase chain reaction and telomere length assessment.
|
| Gene | Sequence
(5′-3′) |
|---|
| 1 | IL-6 forward |
GAGGCACTGGCAGAAAACAACC |
|
| IL-6 reverse |
CCTCAAACTCCAAAAGACCAGTGATG |
| 2 | MIP-1α forward |
GAATCATGCAGGTCTCCACTG |
|
| MIP-1α reverse |
CTCTAGGTCGCTGACATATTTC |
| 3 | IDO forward |
TGGGGCAAAGGTCATGGAG |
|
| IDO reverse |
TTTCTTGGAGAGTTGGCAGTAAG |
| 4 | hTERT forward |
CGGAAGAGTGTCTGGAGCAA |
|
| hTERT reverse | GGATGA
AGCGGAGTCTGGA |
| 5 | GAPDH forward |
GCACCGTCAAGGCTGAGAAC |
|
| GAPDH reverse |
TGGTGAAGACGCCAGTGGA |
| 6 | Tel1b |
CGGTTTGTTTGGGTTTGGGT-TTGGGTTTGGGTTTGGGTT |
|
| Tel 2b |
GGCTTGCCTTACCCTTACCCTTACCC-TTACCCTTACCCT |
| 7 | HBG3 |
TGTGCTGGCCCATCACTTTG |
|
| HBG4 |
ACCAGCCACCACTTTCTGATAGG |
Cell cycle analysis
Cell cycle analysis was performed using the Cell
Cycle and Apoptosis Analysis kit (cat no. C1052-1; Beyotime
Institute of Biotechnology, Haimen, China) and flow cytometry with
a BD FACSAria™ II system (BD Biosciences) was conducted following
the manufacturer's instructions. The percentage of each phase in
the cell cycle was calculated using cell cycle analysis software
Modfit version LT 3.1 (Verity Software House, Topsham, ME,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc.) following the manufacturer's instruction.
cDNA was synthesized using the M-MLV enzyme (Thermo Fisher
Scientific, Inc.) and an oligo-dT primer according to the protocol.
qPCR was performed using SYBR-Green kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) on an ABI 7700 detector (Applied
Biosystems; Thermo Fisher Scientific, Inc.). All sequences of
primers are listed in Table II.
Thermocycling conditions were as follows: At 50°C for 2 min, at
95°C for 10 min, followed by 45 cycles at 95°C for 15 sec and at
60°C for 1 min. The expression level of each gene was calculated
according to the comparative Cq method and normalized to GAPDH
expression (30).
Statistical analysis
The Student's t-test, paired t-test or Fisher's
exact test were used to compare differences between groups.
Pearson's correlation was applied to analysis for relationships
between variables. All the results were presented using GraphPad
Prism software (version, 5; GraphPad Software, Inc., La Jolla, CA,
USA). The data are shown as mean ± standard error of the mean
P<0.05 was considered to indicate a statistically significant
difference.
Results
The phenotype, growth characteristics
and osteoblastic differentiation of MSCs
As presented in Fig.
1, MSCs were cultured and analyzed of both MM patients
(Fig. 1A) and the control group
(Fig. 1B). The phenotypes were
analyzed using flow cytometry. As presented in Table III, MSCs from both MM patients
and controls were positive for CD73, CD90 and CD105, but negative
for HLA-G. There were no significant differences between MM
patients (6.08±0.69 days) and controls (8.62±1.33 days) on the days
for obtaining 80% confluent MSCs cultures. The osteoblastic
differentiation of MSCs was examined using Alizarin red staining.
As indicated in Fig. 2, MSCs from
three age-matched pairs of MM patients (Fig. 2A) and controls (Fig. 2B) displayed deposition of matrix
mineralization.
 | Table III.Flow cytometry analysis of
mesenchymal stem cells from control and MM patients. |
Table III.
Flow cytometry analysis of
mesenchymal stem cells from control and MM patients.
| Antigen
Expression | MM patients (MEAN ±
SEM) | Control group (MEAN
± SEM) |
P-valuea |
|---|
| CD73 (%) | 90.9±0.03 | 89.5±0.03 | 0.73 |
| CD90 (%) | 75.4±0.06 | 83.4±0.05 | 0.39 |
| CD105 (%) | 62.6±10.4 | 59±8 | 0.81 |
| HLA-G (%) | 1.1±4.4 | 0.9±0.5 | 0.77 |
The TL and cell cycle of MSCs from MM
patients and control group
The TL of the MSCs from 12 MM patients and 8
controls were compared by qPCR. As reported in Fig. 3A, the TL of MSCs from MM patients
was significantly longer (0.98±0.11) than the controls (0.65±0.07;
P=0.03). The authors further assessed the TL in MSCs using
Flow-FISH. Similarly, the TL of MSCs was longer in the MM patients
(10,860±807) than in the controls (5,227±1,143; P=0.015; Fig. 3B). Cell cycle characteristics of
MM-MSCs were determined using flow cytometry. As presented in
Fig. 3C, the percentage of MM-MSCs
in G0/G1 was increased compared with controls (77.33±1.83 vs.
61.87±2.51; P<0.01), whereas the MM-MSCs in the S phase and G2/M
phase fell to 14.36±1.36% (vs. 26.98±3.22; P<0.01) and
6.34±0.75% (vs. 10.95±1.14; P<0.01) respectively, when compared
to those of controls.
The human telomerase catalytic subunit gene
hTERT, which is the putative human telomerase catalytic
subunit gene serving as an indicator of telomerase activity
(31), were also measured using
RT-qPCR method in normal and MM-MSCs. However, hTERT
expression in those cells was not detected (data not shown). These
results indicated that both normal and MM-MSCs lacked telomerase
activity.
The expressions of IL-6, indoleamine
2,3-dioxygenase (IDO) and MIP-1α in MSCs from MM patients
The mRNA expressions of IL-6 and IDO in MSCs from MM
patients and controls were investigated using RT-qPCR. The
expression of IL-6 was significantly increased in MM-MSCs
(P<0.05), while the expression of IDO decreased obviously
compared with MSCs in control group (P<0.05; Fig. 3D). Moreover, the expression rate of
MIP-1α from MM-MSCs was significantly higher than that from MSCs in
control group (91.7 and 37.5% respectively, P<0.05).
The correlations between TL and IL-6,
MIP-1α and IDO
As presented in Fig.
4, the TL of MM-MSCs was correlated with their expression of
IL-6 (Fig. 4A) and MIP-1α
(Fig. 4B). However, no significant
correlation between the telomere length and the expression of IDO
in MM-MSCs was found.
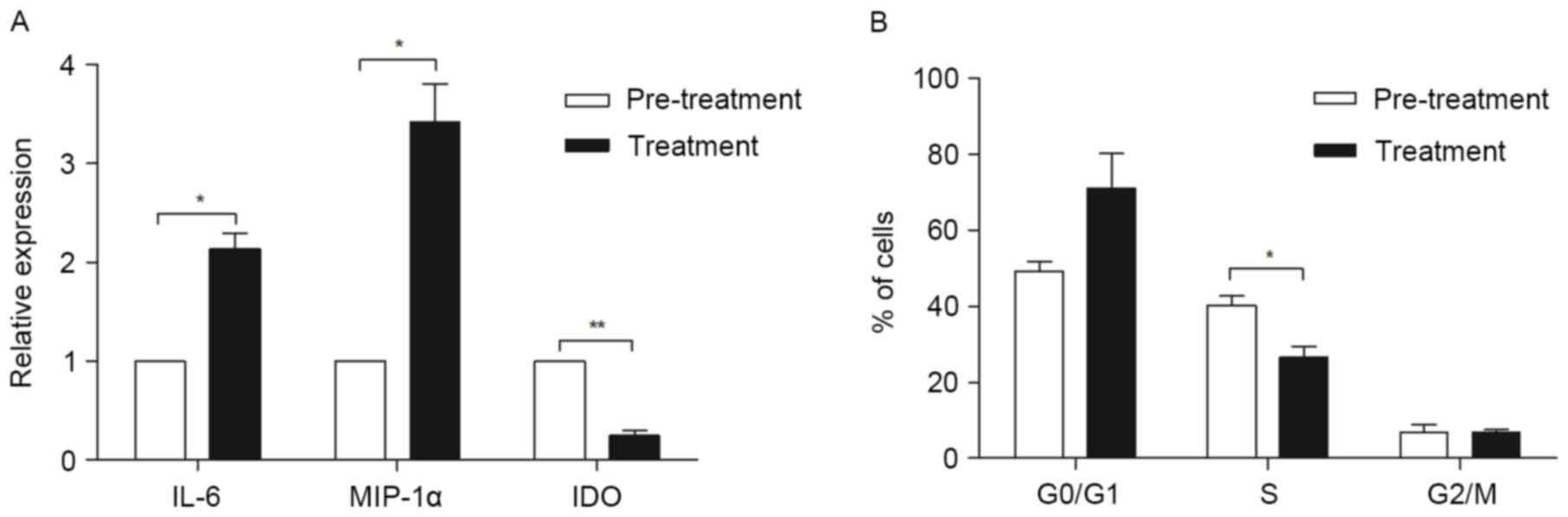
The conditioned medium of RPMI 8226
induces changes of gene expression and cell cycle in MSCs
MSCs isolated from three MM patients were cultured
in myeloma condition culture medium (MCCM) (a mixture of myeloma
cell line RPMI-8226 culture supernatant and MSCs complete medium)
for 24 h, then the expressions of IL-6, MIP-1α and IDO were
quantified using qPCR. When cultured in the MCCM, IL-6 (P<0.05)
and MIP-1α (P<0.01) were significantly increased, while IDO was
markedly downregulated (P<0.05), when compared with the MSCs
cultured in regular MSC medium (Fig.
5A).
To explore whether MCCM affects the cell cycle of
MM-MSCs, MSCs from three MM patients were cultured in MCCM. At 24 h
culture, MSCs reported an increase of cells in
G0/G1 phase and a decrease of the cells in S
phase compared to the controls (P<0.05; Fig. 5B).
The changes of MSCs were not
correlated with β2 microglobulin and bone marrow plasma cells
No significant correlations between TL and serum β2
microglobulin (β2-MG) (r=−0.24; P=0.45), and the percentage of
plasma cell in BM (r=0.55; P=0.07) was identified. Moreover, these
data did not present a correlation of IL-6 or MIP-1α expression
with serum β2-MG, as well as the percentage of plasma cells in BM
(data not shown).
Discussion
The direct and indirect interactions between BM-MSCs
and MM cells not only mediate MM cell growth and survival, but also
lead to the intrinsic abnormalities observed in MM-MSCs, such as
genomic imbalances and an overexpression of IL-6 (32). In the present study, the authors
indicated that bone marrow MSCs from MM are not different from
control group in terms of phenotypic markers (e.g., CD73 and CD90)
and capacity to differentiate into osteogenic tissues. However, the
TL and expressions of IL-6 and MIP-1α were significantly increased
in MM BM-MSCs than the controls. Moreover, the correlation between
TL and IL-6 as well as MIP-1α expression in MM-MSCs were
displayed.
The current results demonstrated that TL of MM-MSCs
was significantly longer than that of controls, indicating a tumor
or myeloma associated mechanism contributing to maintenance or
elongation of TL. It is known that TLs shorten accordingly when
cells divide. The authors' cell cycle analysis demonstrated that
the percentage of MSCs in the G0/G1 phase was
significantly increased in MM than in the controls, suggesting that
there are more MM-MSCs in stable or non-dividing phase, which may
in part explain why MM-MSCs present longer TL than the controls. In
line with a previous report (24),
these data suggested that MSCs from both controls and MM patients
lacked telomerase activity. To date, the underlying the mechanisms
of TL maintenance in MSCs is largely unknown. In MM cells lines,
IL-6 and IGF-1 was demonstrated to increase telomerase activity via
AKT-mediated phosphorylation of hTERT without alteration of the
expression of hTERT at the level of mRNA or protein (33), indicating that MSCs may also apply
this mechanism to maintain their TL in the microenvironment riched
in inflammatory cytokines, such as IL-6.
Moreover, reduced percent of MSCs in S phase during
cell cycle and increased in G0/G1 phase were
identified following conditional culture. This result is in
contrast with a recent report displaying that co-culture with MM
cells produced a marked increase in S/G2/M phases of the
cells (10). These differences may
be due to divergences in the in vitro culture system applied
or the myeloma condition medium, but not cell-cell direct contact
in our system.
In the present study, the authors also compared MSC
differentiation capacity between MM patients and controls, and did
not identify markedly difference in differentiation into bone
tissue between them. This result is controversial compared to the
previous reports that indicate that the differentiation capacity of
MSCs from MM patients is lower than those from the normal controls
(19,34). It may be speculated that the
primary reason causing the different results may be due to
different cell passages applied in our and other studies. Since
MSCs were used from passage 4, the in vitro culture or
expansion procedures may overcome subtle function abnormalities of
MM-MSCs. This issue will be addressed in future studies.
Changes in the expression of cytokines and growth
factors from MSCs serve a prominent role in the growth and survival
of multiple myeloma (1). High
expression of IL-6 from MM-MSCs was detected. MM-MSCs were
expressed a significantly higher level of IL-6 following culturing
with the supernatants from MM cultures. Our findings are consistent
with those previous reports that MM-MSCs overproduced IL-6
(35) and MM cells stimulated IL-6
expression in MSCs (4).
It has been demonstrated that MIP-1α contributes to
the development of bone disease in MM via tumor survival promotion
(36), osteoblast differentiation
(7,8) and osteoblast functional inhibition
(37). MIP-1α is indicated to be
primarily produced by myeloma cells (8). Interestingly, MIP-1α was shown to
also be expressed in MM-MSCs, and the expression of MIP-1α in
MM-MSCs was higher than that in MSCs from the control. Moreover,
MIP-1α expression in MM-MSCs was further increased under the
stimulation of the supernatants isolated from MM cell line culture.
Therefore, MIP-1α produced by MM-MSCs may also participate in the
development of MM.
Previous studies have illustrated that the
proliferative potential of human hematopoietic cells (38) and human fibroblasts (23) correlates with their telomere
lengths. Similarly, the correlation between growth of MSCs and
telomere length has also been suggested (39). Moreover, dysfunction of telomere
impairs the function of mesenchymal progenitor cells and affects
the expressions of various cytokines (27). Consistently, our findings of
positive correlation of IL-6 and MIP-1α with telomere lengths in
MM-MSCs highlight that telomere lengths affect cytokine expression
in MM-MSCs, by which MM-MSCs facilitate MM development.
IDO is an important immunoregulatory molecule
(40). Our results demonstrated
that IDO was significantly lower in MM-MSCs than controls.
Supernatants derived from MM cell line further downregulated IDO
expression. The IDO expression may result in impaired
immunomodulatory capacities of BM-MSCs in MM (15,17).
Undoubtedly, the significance of such decreased IDO expression in
MM-MSCs needs to be investigated in the future studies.
In summary, in the present study, the authors find
that MM-MSCs present longer telomere and higher IL-6 and MIP-1α
expression, and telomere lengths are positively correlated with
IL-6 and MIP-1α expression. These results indicated that MSCs in BM
may be involved in pathothegenesis of MM and MM related bone
diseases.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81372545 and
81600176), the Fundamental Research Funds of Shandong University
(grant no. 2014QY004-16 and 2015QY002-10), Youth Fund of Second
Hospital of Shandong University (grant no. Y2013010029), the
Natural Science Foundation of Shandong Province (grant no.
ZR2016HB71) and the Shenzhen Strategic Emerging Industry
Development Special Fund (grant no. JCYJ20150402105524048).
References
|
1
|
Bianchi G and Munshi NC: Pathogenesis
beyond the cancer clone(s) in multiple myeloma. Blood.
125:3049–3058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hideshima T, Mitsiades C, Tonon G,
Richardson PG and Anderson KC: Understanding multiple myeloma
pathogenesis in the bone marrow to identify new therapeutic
targets. Nat Rev Cancer. 7:585–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manier S, Sacco A, Leleu X, Ghobrial IM
and Roccaro AM: Bone marrow microenvironment in multiple myeloma
progression. J Biomed Biotechnol. 2012:1574962012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dankbar B, Padró T, Leo R, Feldmann B,
Kropff M, Mesters RM, Serve H, Berdel WE and Kienast J: Vascular
endothelial growth factor and interleukin-6 in paracrine
tumor-stromal cell interactions in multiple myeloma. Blood.
95:2630–2636. 2000.PubMed/NCBI
|
|
5
|
Gupta D, Treon SP, Shima Y, Hideshima T,
Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, et al:
Adherence of multiple myeloma cells to bone marrow stromal cells
upregulates vascular endothelial growth factor secretion:
Therapeutic applications. Leukemia. 15:1950–1961. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nefedova Y, Cheng P, Alsina M, Dalton WS
and Gabrilovich DI: Involvement of Notch-1 signaling in bone marrow
stroma-mediated de novo drug resistance of myeloma and other
malignant lymphoid cell lines. Blood. 103:3503–3510. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han JH, Choi SJ, Kurihara N, Koide M, Oba
Y and Roodman GD: Macrophage inflammatory protein-1alpha is an
osteoclastogenic factor in myeloma that is independent of receptor
activator of nuclear factor kappaB ligand. Blood. 97:3349–3353.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abe M, Hiura K, Wilde J, Moriyama K,
Hashimoto T, Ozaki S, Wakatsuki S, Kosaka M, Kido S, Inoue D and
Matsumoto T: Role for macrophage inflammatory protein (MIP)-1alpha
and MIP-1beta in the development of osteolytic lesions in multiple
myeloma. Blood. 100:2195–2202. 2002.PubMed/NCBI
|
|
9
|
Bergfeld SA and DeClerck YA: Bone
marrow-derived mesenchymal stem cells and the tumor
microenvironment. Cancer Metastasis Rev. 29:249–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kassen D, Moore S, Percy L, Herledan G,
Bounds D, Rodriguez-Justo M, Croucher P and Yong K: The bone marrow
stromal compartment in multiple myeloma patients retains capability
for osteogenic differentiation in vitro: Defining the stromal
defect in myeloma. Br J Haematol. 167:194–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noll JE, Williams SA, Tong CM, Wang H,
Quach JM, Purton LE, Pilkington K, To LB, Evdokiou A, Gronthos S
and Zannettino AC: Myeloma plasma cells alter the bone marrow
microenvironment by stimulating the proliferation of mesenchymal
stromal cells. Haematologica. 99:163–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berenstein R, Blau O, Nogai A, Waechter M,
Slonova E, Schmidt-Hieber M, Kunitz A, Pezzutto A, Doerken B and
Blau IW: Multiple myeloma cells alter the senescence phenotype of
bone marrow mesenchymal stromal cells under participation of the
DLK1-DIO3 genomic region. BMC Cancer. 15:682015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wallace SR, Oken MM, Lunetta KL,
Panoskaltsis-Mortari A and Masellis AM: Abnormalities of bone
marrow mesenchymal cells in multiple myeloma patients. Cancer.
91:1219–1230. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Corre J, Labat E, Espagnolle N, Hébraud B,
Avet-Loiseau H, Roussel M, Huynh A, Gadelorge M, Cordelier P, Klein
B, et al: Bioactivity and prognostic significance of growth
differentiation factor GDF15 secreted by bone marrow mesenchymal
stem cells in multiple myeloma. Cancer Res. 72:1395–1406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arnulf B, Lecourt S, Soulier J, Ternaux B,
Lacassagne MN, Crinquette A, Dessoly J, Sciaini AK, Benbunan M,
Chomienne C, et al: Phenotypic and functional characterization of
bone marrow mesenchymal stem cells derived from patients with
multiple myeloma. Leukemia. 21:158–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li B, Shi M, Li J, Zhang H, Chen B, Chen
L, Gao W, Giuliani N and Zhao RC: Elevated tumor necrosis
factor-alpha suppresses TAZ expression and impairs osteogenic
potential of Flk-1+ mesenchymal stem cells in patients
with multiple myeloma. Stem Cells Dev. 16:921–930. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
André T, Najar M, Stamatopoulos B, Pieters
K, Pradier O, Bron D, Meuleman N and Lagneaux L: Immune impairments
in multiple myeloma bone marrow mesenchymal stromal cells. Cancer
Immunol Immunother. 64:213–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai
YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, et al: BM
mesenchymal stromal cell-derived exosomes facilitate multiple
myeloma progression. J Clin Invest. 123:1542–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu S, Santini Cecilia G, De Veirman K,
Vande Broek I, Leleu X, De Becker A, Van Camp B, Vanderkerken K and
Van Riet I: Upregulation of miR-135b is involved in the impaired
osteogenic differentiation of mesenchymal stem cells derived from
multiple myeloma patients. PLoS One. 8:e797522013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garayoa M, Garcia JL, Santamaria C,
Garcia-Gomez A, Blanco JF, Pandiella A, Hernández JM, Sanchez-Guijo
FM, del Cañizo MC, Gutiérrez NC and Miguel San JF: Mesenchymal stem
cells from multiple myeloma patients display distinct genomic
profile as compared with those from normal donors. Leukemia.
23:1515–1527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blackburn EH: Structure and function of
telomeres. Nature. 350:569–573. 1991. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Allsopp RC, Vaziri H, Patterson C,
Goldstein S, Younglai EV, Futcher AB, Greider CW and Harley CB:
Telomere length predicts replicative capacity of human fibroblasts.
Proc Natl Acad Sci USA. 89:10114–10118. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zimmermann S, Voss M, Kaiser S, Kapp U,
Waller CF and Martens UM: Lack of telomerase activity in human
mesenchymal stem cells. Leukemia. 17:1146–1149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choumerianou DM, Martimianaki G, Stiakaki
E, Kalmanti L, Kalmanti M and Dimitriou H: Comparative study of
stemness characteristics of mesenchymal cells from bone marrow of
children and adults. Cytotherapy. 12:881–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Banfi A, Bianchi G, Notaro R, Luzzatto L,
Cancedda R and Quarto R: Replicative aging and gene expression in
long-term cultures of human bone marrow stromal cells. Tissue Eng.
8:901–910. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ju Z, Jiang H, Jaworski M, Rathinam C,
Gompf A, Klein C, Trumpp A and Rudolph KL: Telomere dysfunction
induces environmental alterations limiting hematopoietic stem cell
function and engraftment. Nat Med. 13:742–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cawthon RM: Telomere measurement by
quantitative PCR. Nucleic Acids Res. 30:e472002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ci X, Li B, Ma X, Kong F, Zheng C,
Björkholm M, Jia J and Xu D: Bortezomib-mediated down-regulation of
telomerase and disruption of telomere homeostasis contributes to
apoptosis of malignant cells. Oncotarget. 6:38079–38092. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meyerson M, Counter CM, Eaton EN, Ellisen
LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ,
Liu Q, et al: hEST2, the putative human telomerase catalytic
subunit gene, is up-regulated in tumor cells and during
immortalization. Cell. 90:785–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reagan MR and Ghobrial IM: Multiple
myeloma mesenchymal stem cells: Characterization, origin, and
tumor-promoting effects. Clin Cancer Res. 18:342–349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akiyama M, Hideshima T, Hayashi T, Tai YT,
Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC and
Anderson KC: Cytokines modulate telomerase activity in a human
multiple myeloma cell line. Cancer Res. 62:3876–3882.
2002.PubMed/NCBI
|
|
34
|
Bolzoni M, Donofrio G, Storti P, Guasco D,
Toscani D, Lazzaretti M, Bonomini S, Agnelli L, Capocefalo A, Dalla
Palma B, et al: Myeloma cells inhibit non-canonical wnt co-receptor
ror2 expression in human bone marrow osteoprogenitor cells: Effect
of wnt5a/ror2 pathway activation on the osteogenic differentiation
impairment induced by myeloma cells. Leukemia. 27:451–463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li B, Fu J, Chen P and Zhuang W:
Impairment in immunomodulatory function of mesenchymal stem cells
from multiple myeloma patients. Arch Med Res. 41:623–633. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lentzsch S, Gries M, Janz M, Bargou R,
Dörken B and Mapara MY: Macrophage inflammatory protein 1-alpha
(MIP-1 alpha) triggers migration and signaling cascades mediating
survival and proliferation in multiple myeloma (MM) cells. Blood.
101:3568–3573. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vallet S, Pozzi S, Patel K, Vaghela N,
Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT,
et al: A novel role for CCL3 (MIP-1α) in myeloma-induced bone
disease via osteocalcin downregulation and inhibition of osteoblast
function. Leukemia. 25:1174–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Ziffle JA, Baerlocher GM and Lansdorp
PM: Telomere length in subpopulations of human hematopoietic cells.
Stem Cells. 21:654–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Samsonraj RM, Raghunath M, Hui JH, Ling L,
Nurcombe V and Cool SM: Telomere length analysis of human
mesenchymal stem cells by quantitative PCR. Gene. 519:348–355.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ge W, Jiang J, Arp J, Liu W, Garcia B and
Wang H: Regulatory T-cell generation and kidney allograft tolerance
induced by mesenchymal stem cells associated with indoleamine
2,3-dioxygenase expression. Transplantation. 90:1312–1320. 2010.
View Article : Google Scholar : PubMed/NCBI
|