Introduction
Ginsenosides are a class of natural products
isolated from Panax ginseng and are responsible for the
majority of its pharmacological properties. Ginsenosides are
classified into 4 categories with regards to the chemical structure
of their aglycones: i) Protopanaxadiol (PPD)-; ii) protopanaxatriol
(PPT)-; iii) oleanolic acid; and iv) ocotillol-type ginsenosides
(1). Ginsenoside Rc (Rc; Fig. 1) is a PPD-type bioactive
ginsenoside, which are present at high concentrations in several
commercially available ginseng products (2).
Rc has been reported to interfere with the
intracellular production of reactive oxygen species (ROS); however,
its actions remain controversial and they may vary depending on
cell type (3,4). Under conditions of
tert-butylhydroperoxide-induced oxidative stress in HEK293 cells,
Rc was reported to attenuate ROS generation, through the
upregulation of catalase expression, a forkhead box protein O1
(FOXO1)-targeting gene (3). In
addition, Rc was demonstrated to directly scavenge superoxide free
radicals in HEK293 cells (3).
Antidiabetic properties have also been reported for Rc, as it was
revealed to potentiate glucose uptake via increasing ROS production
and activating 5′ adenosine monophosphate-activated protein kinase
and p38 mitogen-activated protein kinase, in an insulin-independent
pathway (4). Therefore, the
present study aimed to evaluate the putative antioxidative
properties of Rc in skin cells.
UVB radiation has been identified as a major cause
of photoaging, as it initiates photooxidative reactions that
disrupt the redox balance of skin cells and increase intracellular
ROS levels, thus leading to oxidative stress (5). Matrix metalloproteinases (MMPs) are
responsible for the degradation of extracellular matrix (ECM)
components that constitute the dermal connective tissue (6). A UVB-induced increase in ROS
production may stimulate MMPs through redox-regulated transcription
factors (7). Photoaging is the
result of the degradation of dermal ECM due to enhanced MMP
activation and suppressed collagen biosynthesis under conditions of
oxidative stress (8). The
UV-induced gelatinolytic activity of MMP-2 and MMP-9 is involved in
the development of UV-induced skin damage, including skin
thickening and wrinkle formation (9). UVB irradiation has previously been
demonstrated to increase the expression of MMP-2 and MMP-9 and
potentiate their gelatinolytic activity in human HaCaT
keratinocytes (8).
Filament aggregating protein (filaggrin) is an
important component of the cornified cell envelope (CE) of the
epidermal stratum corneum (SC), and is initially synthesized
as profilaggrin, a 500-kDa highly phosphorylated His-rich protein
containing 10–12 tandemly arranged filaggrin repeats in its central
region (10). Monomeric filaggrins
are generated by proteolytic cleavage and dephosphorylation and
trigger the aggregation of keratin filaments. Filaggrin has an
important function during water retention in SC, whereas its
mutations have been associated with natural moisturizing factor
(NMF) deficiency in the SC, leading to skin barrier dysfunction
(11). A previous study has also
reported that silencing of filaggrin expression may impair the skin
barrier functions of normal human epidermal keratinocytes,
primarily via targeting the CE and triggering immune responses
(12). The downregulation or
complete loss of filaggrin expression has been previously reported
to disturb skin barrier function and enhance the percutaneous
transfer of allergens, thus suggesting that filaggrin may have a
protective function against the entry of foreign environmental
substances (13).
Caspase-14 is a Cys-specific proteinase localized in
stratified epithelia, including the skin, and is involved in the
production of filaggrin monomers and the synthesis of NMFs in the
skin (14). Caspase-14 is
activated only in terminally differentiated keratinocytes, and its
downregulation has been reported to lead to impairments in skin
barrier function (15).
The findings of the present study suggested that Rc
may possess protective properties against UVB-induced
photooxidative damage and may exert anti-photoaging and barrier
function-protective effects in keratinocytes.
Materials and methods
Chemicals
Rc (purity, ≥98%) was obtained from Ambo Institute
(Daejeon, Korea; www.ambo.co.kr). Bradford reagent, MTT solution,
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA),
dihydrorhodamine 123 (DHR-123), dihydroethidium (DHE),
5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), glutathione reductase
(GR) and nicotinamide-adenine dinucleotide phosphate (NADPH) were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Ac-Trp-Glu-His-Asp-7-amino-4-methylcoumarin (Ac-WEHD-MCA) was
purchased from Peptide Institute, Inc. (Osaka, Japan). Cell lysis
buffer was obtained from Promega Corporation (Madison, WI,
USA).
Cell culture
The human HaCaT keratinocyte cell line was purchased
from American Type Culture Collection (Manassas, VA, USA). Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone;
GE Healthcare Life Science, Logan, UT, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; HyClone; GE Healthcare
Life Sciences), 100 U/ml penicillin and 100 µg/ml streptomycin, and
maintained at 37°C in a humidified atmosphere with 5%
CO2.
UVB irradiation
A VL-6 M ultraviolet lamp (peak, 312 nm; Vilber
Lourmat, Marne-la-Vallée, France) was the source of UVB radiation,
and was used with a VLX-3 W radiometer (Vilber Lourmat) equipped
with a CX-312 sensor (bandwidth, 280–320 nm; Vilber Lourmat). Prior
to the irradiation, 1×105 cells, grown overnight and
washed twice with 1 ml PBS, were resuspended in 1 ml FBS-free DMEM.
HaCaT keratinocytes at 25°C were irradiated with solar simulated
UVB radiation at 70 mJ/cm2 for 2 min, an intensity
estimated to induce oxidative stress in preliminary experiments
(data not shown).
Preparation of cell lysates
Adherent cells were harvested using a cell scraper,
washed twice with PBS and maintained on ice for 5 min. Following
centrifugation at 3,000 × g for 10 min at room temperature, the
cell pellets were dissolved in cell lysis buffer containing 50 mM
HEPES (pH 7.5), 10% sucrose and 0.1% Triton X-100, and maintained
on ice for 30 min. Following centrifugation at 10,000 × g for 15
min at 4°C, the supernatants were collected. Protein concentration
in cell lysates was determined by the Bradford protein assay as
previously described (16), using
bovine serum albumin (Sigma-Aldrich; Merck KGaA) as the
standard.
Intracellular ROS production
The ROS-sensitive fluorescent probe DCFH-DA produces
2′,7′-dichlorofluorescein (λexcitation, 485 nm; λemission, 530 nm)
upon enzymatic reduction and oxidation by ROS (17). DHR-123 and DHE are additional ROS
probes which produce rhodamine 123 (λexcitation, 500 nm; λemission,
535 nm) and 2-hydroxyethidium (λexcitation, 480 nm; λemission, 525
nm), respectively, upon reaction with ROS (18). Prior to the treatment,
1×105 cells, grown overnight and washed twice with 1 ml
PBS, were resuspended in 1 ml FBS-free DMEM. HaCaT keratinocytes
were incubated with Rc (0, 5, 12 and 30 µM) and 20 µM DCFH-DA, 5 µM
DHR-123 or 5 µM DHE for 30 min at 37°C. Then, the cells were washed
twice with 1 ml FBS-free DMEM, dissolved in 1 ml FBS-free DMEM and
irradiated with 70 mJ/cm2 UVB radiation, if required.
The appropriate control cells did not receive Rc and were treated
with or without UVB irradiation. Intracellular ROS levels were
determined via quantification of the fluorescence of the samples
using the Synergy HTX Multi-Mode microplate reader (BioTek
Instruments Inc., Winooski, VT, USA).
Cell viability assay
In order to investigate the cytotoxic effects of UVB
irradiation on HaCaT keratinocytes, and the putative cytoprotective
properties of Rc, cell viability was evaluated using an MTT assay,
which reflects cellular metabolic activity (19). A total of 1×105 HaCaT
keratinocytes, grown overnight and washed twice with 1 ml PBS, were
resuspended in FBS-free DMEM and treated with Rc for 1 h. If
necessary, the cells were irradiated following the Rc treatment.
The quantity of formazan dissolved in dimethyl sulfoxide, generated
from the reduction of MTT by the mitochondria of viable cells, was
determined by the absorbance at 540 nm using the Synergy HTX
Multi-Mode microplate reader.
Gelatin zymography
The proteolytic activity of MMP-2 and MMP-9 in
conditioned media, obtained through centrifugation at 10,000 × g
for 10 min at 4°C, was assessed using gelatin zymographic analysis
(20) with a slight modification.
Briefly, following staining of the SDS-polyacrylamide gel with 0.1%
Coomassie Brilliant Blue R-250, the areas of gelatinolytic activity
were identified as clear white bands against a darkly stained
background. Molecular mass markers were used to verify the MMP-2
and MMP-9 activity bands at 72 and 92 kDa, respectively. The band
strength was determined with densitometry using ImageJ 1.48
software (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Western blot analysis was used to assess the protein
expression levels of MMP-2, MMP-9 and filaggrin in keratinocyte
lysates. The following primary antibodies were used: Anti-MMP-2
(cat. no. ALX-210-753; Enzo Life Sciences Inc., Farmingdale, NY,
USA), anti-MMP-9 (cat. no. 3852S; Cell Signaling Technology Inc.,
Danvers, MA, USA), anti-filaggrin (cat. no. SC-30229; Santa Cruz
Biotechnology Inc., Dallas, TX, USA) and anti-GAPDH (cat. no.
LF-PA0212; Young In Frontier Co., Ltd., Seoul, Korea). Cellular
lysates (protein content, 10 µg/lane) were separated using SDS-PAGE
on a 10% (w/v) gel and electrotransferred to a polyvinylidene
fluoride membrane (Sigma-Aldrich; Merck KGaA). The membranes, after
blocking with 2% BSA (Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature, were probed with the primary antibodies at a 1:1,000
dilution at 4°C overnight, and subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit immunoglobulin G; 1:1,000; cat. no. ADI-SAB-300; Enzo
Life Sciences Inc.) at room temperature for 1 h. Protein bands were
visualized by enhanced chemiluminescence using the WESTSAVE Femto
detection kit (cat. no. LF-QC0109; Young In Frontier Co., Ltd.).
GAPDH was used as the loading control. Densities of the protein
bands were determined using ImageJ software.
Total glutathione (GSH) contents
Total GSH contents in keratinocyte lysates were
evaluated using a GR-coupled enzymatic recycling assay, as
previously described (21).
Lysates were incubated at 25°C for 5 min in 200 µl reaction
mixture, which contained 175 mM KH2PO4, 6.3
mM EDTA, 0.21 mM NADPH, 0.6 mM DTNB, and 0.5 U/ml GR. The
absorbance of the samples at 412 nm was determined using a
microplate reader. Total GSH contents, expressed as µg/mg protein,
were determined using a GSH standard curve and normalized to the
total protein content of cell lysates.
Superoxide dismutase (SOD) activity
assay
Total SOD activity in keratinocyte lysates was
determined based upon the reduction of cytochrome c with a
xanthine/xanthine oxidase system, as previously described (22). The reaction mixture (200 µl)
contained 50 mM PB (pH 7.4), 0.01 U/ml xanthine oxidase, 0.1 mM
EDTA, 1 µM catalase, 0.05 mM xanthine, 20 µM cytochrome c
and the cell lysate. The mixture was incubated at 25°C for 10 min.
The absorbance of each sample was measured at 550 nm using a
microplate reader. SOD activity was normalized to total protein
contents of the lysates, and expressed as ΔA550/min/mg protein.
Caspase-14 activity assay
Caspase-14 activity in keratinocyte lysates was
assessed using Ac-WEHD-MCA (Peptide Institute Inc.) as a
fluorogenic substrate, as previously described (15). The reaction mixture (95 µl)
contained 0.1 M HEPES buffer (pH 7.5), 0.06 M NaCl, 0.01% CHAPS
detergent, 5 mM dithiothreitol, 1.3 M sodium citrate, and 10 µM
Ac-WEHD-MCA. Cell lysate (5 µl) was added to the mixture and
incubated at room temperature for 30 min. The intensity of
fluorescence (λexcitation, 355 nm; λemission, 460 nm) was measured
using the Synergy HTX Multi-Mode microplate reader. Caspase-14
activity was normalized to the protein contents of the lysates.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The statistical significance of the differences between groups was
assessed using one-way analysis of variance followed by a post hoc
Tukey's honest significant difference test for multiple
comparisons. SPSS version 16.0 for Windows was used to perform the
statistical analyses (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Rc treatment suppresses UVB-induced
ROS production
HaCaT keratinocytes were treated with various
concentrations (0, 5, 12 or 30 µM) of Rc prior to UVB irradiation
and intracellular ROS generation was assessed using ROS-sensitive
probes. The DCFH-DA assay demonstrated that UVB irradiation led to
a ~8.4-fold elevation in intracellular ROS levels compared with
non-irradiated control cells (Fig.
2A). Rc was revealed to prevent UVB-induced increase in ROS
production, as ROS levels were reduced to 65.7, 45.6 and 34.6% of
UVB-irradiated cells following treatment with 5, 12 and 30 µM Rc,
respectively (Fig. 2A). The
DHR-123 assay indicated that UVB induced a ~6.0-fold increase in
ROS production compared with non-irradiated control cells, whereas
Rc attenuated the UVB-induced elevation in ROS levels in a
dose-dependent manner (Fig. 2B).
Furthermore, DHE was also used as a ROS probe, and similar results
were obtained, as Rc was revealed to suppress UVB-induced ROS
production (Fig. 2C). The present
findings suggested that Rc may suppress intracellular ROS
generation following exposure to UVB radiation in human
keratinocytes.
 | Figure 2.Rc treatment suppresses UVB-induced
ROS generation in human HaCaT keratinocytes. HaCaT cells were
pretreated with 0, 5, 12 and 30 µM Rc for 30 min prior to UVB
irradiation. ROS levels were quantified using (A) DCFH-DA, (B)
DHR-123 and (C) DHE and expressed as relative (A) DCF, (B) RH-123
and (C) 2-HE fluorescence. Data are expressed as the mean ±
standard deviation of three independent experiments.
###P<0.001 vs. non-irradiated untreated cells.
*P<0.05, **P<0.01, ***P<0.001 vs. untreated irradiated
cells. Rc, ginsenoside Rc; ROS, reactive oxygen species; DCFH-DA,
2′,7′-dichlorodihydrofluorescein diacetate; DHR-123,
dihydrorhodamine 123; DHE, dihydroethidium; DCF,
2′,7′-dichlorofluorescein; RH-123, rhodamine 123; 2-HE,
2-hydroxyethidium. |
Rc treatment does not exert cytotoxic
effects in keratinocytes
An MTT assay was used to investigate the effects of
UVB exposure and treatment with Rc on the viability of HaCaT
keratinocytes. The present findings demonstrated that Rc did not
exert cytotoxic actions on HaCaT cells in the concentration range
that was used (Fig. 3A). In
addition, UVB irradiation did not affect the viability of
keratinocytes (Fig. 3B), and Rc
did not appear to influence the viability of UVB-irradiated cells
(Fig. 3B). The present findings
suggested that UVB radiation, at the conditions used in the present
study, and Rc do not have a toxic effect on HaCaT
keratinocytes.
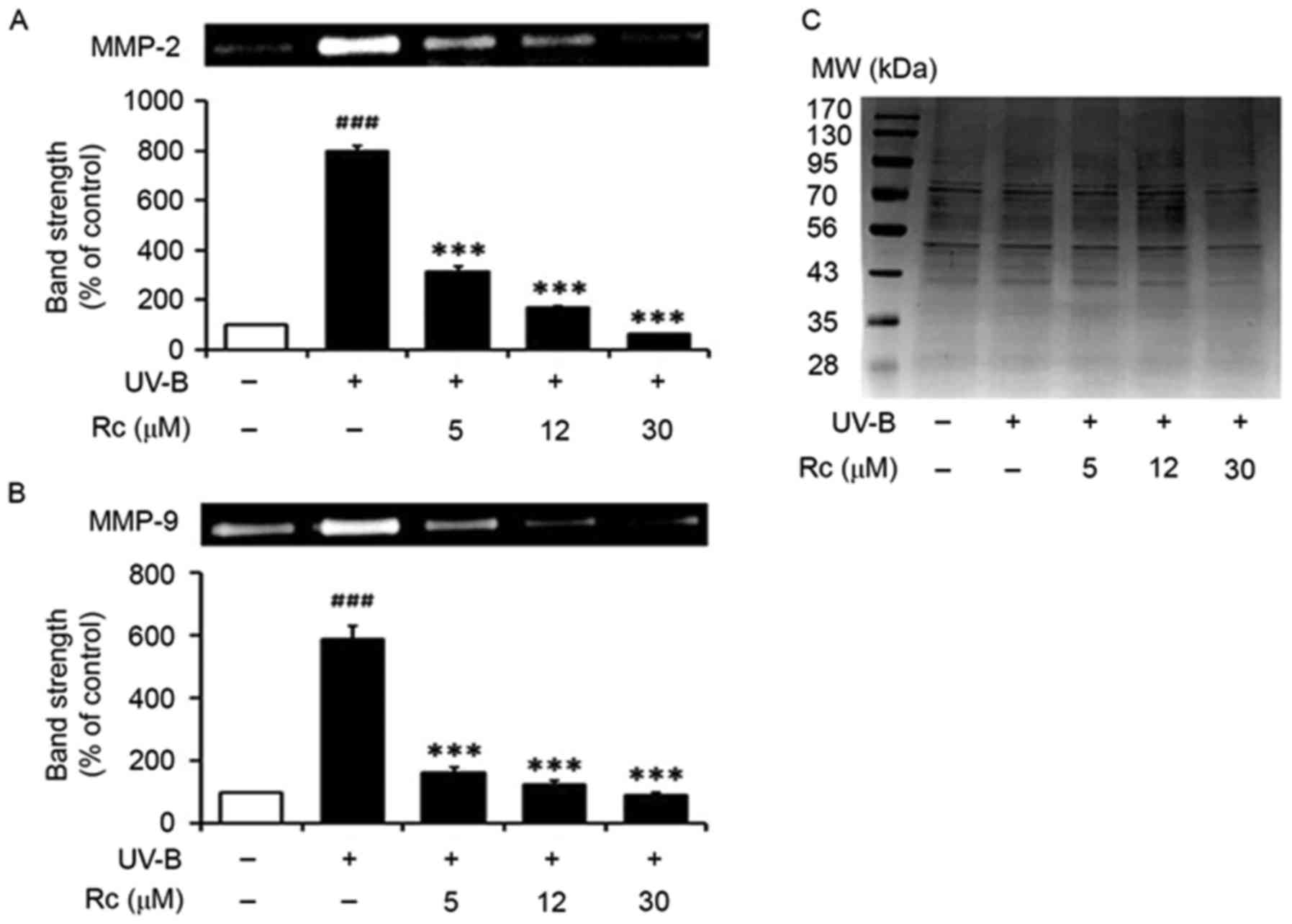
Rc treatment suppresses MMP-2 and
MMP-9 activity following UVB irradiation
In accordance with a previous study (8), UVB irradiation potentiated the
proteolytic activity of MMP-2 in conditioned media (Fig. 4A). Notably, treatment of
keratinocytes with Rc attenuated the UVB-induced increase in MMP-2
activity, as 5, 12 and 30 µM Rc were revealed to reduce MMP-2
activity to 39.3, 21.0 and 8.0% of that in untreated irradiated
cells, respectively (Fig. 4A).
Similarly, keratinocytes exposed to UVB radiation exhibited
significantly increased MMP-9 gelatinolytic activity (Fig. 4B), whereas Rc counteracted this
effect, as 5, 12 and 30 µM Rc were demonstrated to suppress MMP-9
activity to 27.8, 21.1 and 15.4% compared with untreated irradiated
cells, respectively. The present findings suggested that treatment
of keratinocytes with Rc may counteract the increase in proteolytic
activity following UVB exposure in vitro.
Rc treatment prevents the UVB-induced
upregulation of MMP-2 and MMP-9 protein expression
Western blot analysis demonstrated that following
exposure to UVB radiation, HaCaT keratinocytes exhibited
significantly increased MMP-2 protein expression levels (3.7-fold
in non-irradiated control cells; Fig.
5A). Notably, treatment with 5, 12 and 30 µM Rc reduced MMP-2
protein expression levels in UVB-treated cells to 57.2, 40.1 and
11.7% of those in untreated irradiated cells, respectively
(Fig. 5A). Similarly, UVB
irradiation led to a significant potentiation of MMP-9 protein
expression, whereas Rc was demonstrated to attenuate the
UVB-induced MMP-9 upregulation in a dose-dependent manner (Fig. 5B). The present findings suggested
that treatment with Rc may reduce the UVB-induced upregulation of
MMP-2 and MMP-9 protein expression in human keratinocytes.
Rc treatment prevents UVB-induced GSH
depletion
The present study demonstrated that total GSH
contents were significantly reduced in UVB-irradiated HaCaT
keratinocytes (Fig. 6A), which was
in accordance with a previous study (23). Notably, following treatment with 5,
12 and 30 µM Rc, total GSH levels were increased by 1.9-, 2.0- and
2.3-fold, respectively, compared with untreated irradiated cells
(Fig. 6A). These findings
suggested that the increased GSH contents may be implicated in the
molecular mechanisms underlying the effects of Rc against
UVB-induced keratinocyte damage.
Rc treatment prevents the UVB-induced
suppression of SOD activity
UVB irradiation was revealed to significantly
suppress total SOD activity in HaCaT keratinocytes (Fig. 6B). However, following treatment
with 5, 12 and 30 µM Rc, total SOD activity was potentiated by
2.0-, 2.9- and 3.7-fold, respectively, compared with untreated
irradiated cells (Fig. 6B). The
present findings suggested that Rc may counteract the UVB-induced
impairments in SOD activity in keratinocytes in vitro.
Rc treatment enhances caspase-14
activity and upregulates filaggrin protein expression
Following exposure to UVB radiation, the activity of
caspase-14 was significantly reduced (75.0% of that in
non-irradiated control cells; Fig.
7A). Notably, treatment with 5, 12 and 30 µM Rc was
demonstrated to potentiate the activity of caspase-14 by 2.2-. 2.5-
and 2.8-fold, respectively, compared with untreated irradiated
keratinocytes (Fig. 7A).
The protein expression levels of filaggrin were
revealed to be significantly reduced in UVB-irradiated HaCaT cells
compared with non-irradiated control cells (66.7% of those in
non-irradiated cells; Fig. 7B).
Following treatment with 5, 12 and 30 µM Rc, filaggrin protein
expression was significantly upregulated by 4.5-, 5.4- and
7.8-fold, respectively, compared with untreated irradiated
keratinocytes (Fig. 7B). The
present findings suggested that treatment with Rc may counteract
UVB-induced impairments in skin barrier function under conditions
of photooxidative stress.
Discussion
The present study aimed to evaluate the putative
antioxidative properties of Rc in UVB-exposed human keratinocytes
in vitro. DCFH-DA, DHR-123 and DHE are fluorescent ROS
probes and were used to investigate the effects of Rc treatment on
UVB-induced ROS production. DCFH-DA, originally used as a specific
probe for hydrogen peroxide, also reacts with other ROS, including
hydroxyl and peroxy radicals (18). DHR-123 reacts with hydrogen
peroxide in the presence of peroxidases and may be oxidized by
other oxidants, including peroxynitrite anions and hypochlorous
acid (18). DHE is used as a probe
for the detection of superoxide radicals, whereas it is also
oxidized by hydrogen peroxide via non-specific peroxidase catalysis
(18). The present study
demonstrated that UVB irradiation led to increased ROS levels in
HaCaT keratinocytes. These findings are in accordance with a
previous study (24), which
reported that UVB radiation induced the production of superoxide
radicals in epidermal keratinocytes, which are subsequently
converted to other ROS species, including hydrogen peroxide and
hydroxyl radicals. Notably, treatment with Rc was revealed to
attenuate the UVB-induced increase in ROS production, thus
suggesting that Rc may exert antioxidative effects against
UVB-induced photooxidative stress.
MMPs have a key role during collagen degradation and
have been implicated in skin photoaging (25). Enhanced ROS levels following UV
irradiation have been reported to promote the production and
secretion of MMPs, including MMP-2 and MMP-9, in dermal and
epidermal tissue, thus leading to skin damage and photoaging
(26). In the present study, Rc
treatment was revealed to attenuate the UVB-induced upregulation in
MMP-2 and MMP-9 protein expression in human keratinocytes. In
addition, their proteolytic activity was similarly suppressed.
These findings suggested that MMP-2 and MMP-9 protein expression
may be regulated by a common mechanism in keratinocytes.
Natural antioxidants have been previously reported
to exert their physiological functions through the downregulation
of MMP-2 and MMP-9 via various mechanisms (27–29).
Photodynamic therapy has been demonstrated to suppress the
migration and invasion ability of laryngeal squamous carcinoma
cells in vitro, via downregulation of MMP-2 and MMP-9
expression levels through the ROS-mediated inhibition of the
mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase signaling pathway (27). Polysaccharides derived from
Inonotus obliquus have been revealed to suppress the
migration and invasion ability of highly metastatic melanoma cells
in vitro by decreasing the expression and activity levels of
MMP-2 and MMP-9 via the inhibition of MAPK, cyclooxygenase-2 and
nuclear factor-κB signaling pathways (28). Amsacrine has been previously
reported to downregulate MMP-2 and MMP-9 expression by suppression
of gene transcription and promotion of mRNA decay in human leukemia
cells (29). The findings of the
present study suggested that Rc may simultaneously downregulate the
expression of MMP-2 and MMP-9 in keratinocytes under conditions of
photooxidative stress; however, additional studies are required to
fully elucidate the underlying molecular mechanisms.
The present study revealed that Rc counteracted the
UVB-induced depletion of GSH contents and the suppression of SOD
activity. These findings suggested that the modulation of
endogenous antioxidants may be implicated in the antioxidative
properties of Rc; however, additional studies are required to
determine the molecular mechanisms that are involved. Numerous
antioxidants exert their biological roles through the upregulation
of the endogenous antioxidative mechanisms of cells. Fucoxanthin is
a natural antioxidant carotenoid, which is abundant in seaweed, and
has been previously reported to increase GSH levels by upregulating
mRNA and protein expression levels of γ-glutamylcysteine synthetase
and glutathione synthetase in keratinocytes, via promoting the
nuclear translocation and phosphorylation of nuclear factor
(erythroid-derived 2)-like 2 (30). The essential oil of Pogostemon
cablin, which is a Chinese herb traditionally used for the
treatment of skin disorders, has been reported to reduce wrinkle
formation and increase skin elasticity and collagen content,
possibly due to its antioxidative properties by suppressing lipid
peroxidation through the potentiation of SOD, glutathione
peroxidase and catalase activity (31). Coenzyme Q has also been revealed to
prevent UVB-induced photooxidative stress by increasing SOD and
glutathione peroxidase activity reduced by UVB radiation in mice
(32).
In the present study, exposure to UVB radiation led
to the downregulation of filaggrin expression and reduced
caspase-14 activity in keratinocytes, thus suggesting the
dysfunction of the skin barrier. Rc treatment was revealed to
increase filaggrin expression and potentiate caspase-14 activity,
thus counteracting the UVB-induced skin barrier impairments. These
findings suggested that the antioxidative properties of Rc may be
associated with its functions in preserving skin barrier
functionality; however, additional studies are required to
elucidate the underlying molecular mechanisms.
Previous studies (33–38)
have assessed the pharmacological actions of purified ginsenosides,
including their anti-photoaging, anti-inflammatory, barrier
function-preserving and antioxidative functions, using skin cell
lines. Similar to Rc, PPD-type ginsenosides, such as Rb1 (33), Rb2 (34,35)
and Rb3 (36), used as enantiomer
mixtures, were reported to exhibit anti-photoaging properties in
keratinocytes and fibroblasts. The PPD-type ginsenoside Rg3
exhibited a stereoselective anti-photoaging action, as only the
S-enantiomer was revealed to have a ROS-scavenging and MMP-2
inhibitory effect in keratinocytes (37). In UVB-irradiated keratinocytes, the
PPD-type ginsenoside 20(S)-Rh2 reduced ROS generation and
MMP-2 expression levels, whereas 20(R)-Rh2 downregulated
MMP-2, but did not affect ROS production, thus suggesting that the
two enantiomers may exert anti-photoaging effects that involve
different molecular mechanisms (38). In addition, 20(R)-Rh2
demonstrated an anti-inflammatory effect in
lipopolysaccharide-stimulated macrophages, by downregulating nitric
oxide, prostaglandin E2, ROS and MMP-9 levels (39). Notably, 20(S)-, but not
20(R)-PPD, was reported to prevent the UVB-induced
upregulation in ROS and MMP-2 levels in keratinocytes, thus
suggesting the anti-photoaging potential of 20(S)-PPD-type
ginsenosides (40). Furthermore,
the PPT-type ginsenoside Rg2 exhibited stereospecific protective
properties, as only the 20(S)-enantiomer was revealed to
prevent UVB-induced photoaging, via upregulation of endogenous
antioxidants, such as GSH and SOD (41). Similarly, 20(S)-PPT was
previously reported to exert anti-photoaging effects in
UVB-irradiated keratinocytes (42). Re, which is a PPT-type ginsenoside,
was demonstrated to preserve skin barrier functionality, via
enhancing CE formation, and potentiating filaggrin and caspase-14
activity in skin cells under physiological conditions (43). Ro, an oleanolic acid-type
ginsenoside, also exhibited anti-photoaging potential by
counteracting the UVB-induced decrease in fibroblast GSH contents
(44). These previous findings
suggested that ginsenosides may exert beneficial actions on human
skin; however, the mechanisms underlying their effects may vary
depending on the type of ginsenoside and their
stereospecificity.
In conclusion, the present study investigated the
putative anti-photoaging and barrier function-preserving properties
of Rc in UVB-irradiated human HaCaT keratinocytes. Rc treatment was
demonstrated to prevent UVB-induced ROS generation and suppress the
expression and activity of MMP-2 and MMP-9 and counteract the
UVB-induced GSH depletion and suppression of SOD activity. In
addition, Rc was revealed to attenuate the UVB-induced
downregulation in filaggrin expression and caspase-14 activity. The
findings of the present study suggested that ginsenoside Rc may be
a potential target for the development of novel strategies based on
natural compounds for skin protection with fewer adverse
effects.
Acknowledgements
The present study was supported by the Ministry of
Trade, Industry and Energy, Korea Institute for Advancement of
Technology, through the Encouragement Program for the Industries of
Economic Cooperation Region (grant no. R0005382).
References
|
1
|
Shi Y, Sun C, Zheng B, Li Y and Wang Y:
Simultaneous determination of nineginsenosides in functional foods
by high performance liquid chromatography with diode array detector
detection. Food Chem. 123:1322–1327. 2010. View Article : Google Scholar
|
|
2
|
Harkey MR, Henderson GL, Gershwin ME,
Stern JS and Hackman RM: Variability in commercial ginseng
products: An analysis of 25 preparations. Am J Clin Nutr.
73:1101–1106. 2001.PubMed/NCBI
|
|
3
|
Kim DH, Park CH, Park D, Choi YJ, Park MH,
Chung KW, Kim SR, Lee JS and Chung HY: Ginsenoside Rc modulates
Akt/FoxO1 pathways and suppresses oxidative stress. Arch Pharm Res.
37:813–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee MS, Hwang JT, Kim SH, Yoon S, Kim MS,
Yang HJ and Kwon DY: Ginsenoside Rc, an active component of
Panax ginseng, stimulates glucose uptake in C2C12 myotubes
through an AMPK-dependent mechanism. J Ethnopharmacol. 127:771–776.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peres PS, Terra VA, Guarnier FA, Cecchini
R and Cecchini AL: Photoaging and chronological aging profile:
Understanding oxidation of the skin. J Photochem Photobiol B.
103:93–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Curran S and Murray GI: Matrix
metalloproteinases in tumour invasion and metastasis. J Pathol.
189:300–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masaki H, Atsumi T and Sakurai H:
Detection of hydrogen peroxide and hydroxyl radicals in murine skin
fibroblasts under UVB irradiation. Biochem Biophys Res Commun.
206:474–479. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MS, Oh GH, Kim MJ and Hwang JK:
Fucosterol inhibits matrix metalloproteinase expression and
promotes type-1 procollagen production in UVB-induced HaCaT cells.
Photochem Photobiol. 89:911–918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inomata S, Matsunaga Y, Amano S, Takada K,
Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y and Fukuda M:
Possible involvement of gelatinases in basement membrane damage and
wrinkle formation in chronically ultraviolet B-exposed hairless
mouse. J Invest Dermatol. 120:128–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGrath JA and Uitto J: The filaggrin
story: Novel insights into skin-barrier function and disease.
Trends Mol Med. 14:20–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kezic S, O'Regan GM, Lutter R, Jakasa I,
Koster ES, Saunders S, Caspers P, Kemperman PM, Puppels GJ,
Sandilands A, et al: Filaggrin loss-of-function mutations are
associated with enhanced expression of IL-1 cytokines in the
stratum corneum of patients with atopic dermatitis and in a murine
model of filaggrin deficiency. J Allergy Clin Immunol.
129:1031–1039.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dang NN, Pang SG, Song HY, An LG and Ma
XL: Filaggrin silencing by shRNA directly impairs the skin barrier
function of normal human epidermal keratinocytes and then induces
an immune response. Braz J Med Biol Res. 48:39–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sandilands A, Sutherland C, Irvine AD and
McLean WH: Filaggrin in the frontline: Role in skin barrier
function and disease. J Cell Sci. 122:1285–1294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hvid M, Johansen C, Deleuran B, Kemp K,
Deleuran M and Vestergaard C: Regulation of caspase 14 expression
in keratinocytes by inflammatory cytokines-a possible link between
reduced skin barrier function and inflammation? Exp Dermatol.
20:633–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hibino T, Fujita E, Tsuji Y, Nakanishi J,
Iwaki H, Katagiri C and Momoi T: Purification and characterization
of active caspase-14 from human epidermis and development of the
cleavage site-directed antibody. J Cell Biochem. 109:487–497.
2010.PubMed/NCBI
|
|
16
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Royall JA and Ischiropoulos H: Evaluation
of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent
probes for intracellular H2O2 in cultured
endothelial cells. Arch Biochem Biophys. 302:348–355. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gomes A, Fernandes E and Lima JL:
Fluorescence probes used for detection of reactive oxygen species.
J Biochem Biophys Methods. 65:45–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Freshney RI: Culture of Animal Cells: A
Manual of Basic Technique. 4th. Wiley-Liss Press; New York:
1994
|
|
20
|
Kleiner DE and Stetler-Stevenson WG:
Quantitative zymography: Detection of picogram quantities of
gelatinases. Anal Biochem. 218:325–329. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakagawa K, Saijo N, Tsuchida S, Sakai M,
Tsunokawa Y, Yokota J, Muramatsu M, Sato K, Terada M and Tew KD:
Glutathione-S-transferase pi as a determinant of drug resistance in
transfectant cell lines. J Biol Chem. 265:4296–4301.
1990.PubMed/NCBI
|
|
22
|
Lee YY, Kim HG, Jung HI, Shin YH, Hong SM,
Park EH, Sa JH and Lim CJ: Activities of antioxidant and redox
enzymes in human normal hepatic and hepatoma cell lines. Mol Cells.
14:305–311. 2002.PubMed/NCBI
|
|
23
|
Zhu M and Bowden GT: Molecular
mechanism(s) for UV-B irradiation-induced glutathione depletion in
cultured human keratinocytes. Photochem Photobiol. 80:191–196.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aitken GR, Henderson JR, Chang SC, McNeil
CJ and Birch-Machin MA: Direct monitoring of UV-induced free
radical generation in HaCaT keratinocytes. Clin Exp Dermatol.
32:722–727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YM, Kang SM, Lee SR, Kong KH, Lee JY,
Kim EJ and Chung JH: Inhibitory effects of TRPV1 blocker on
UV-induced responses in the hairless mice. Arch Dermatol Res.
303:727–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Shen B, Swinarska JT, Li W, Xiao
K and He P: 9-Hydroxypheophorbide α-mediated photodynamic therapy
induces matrix metalloproteinase-2 (MMP-2) and MMP-9
down-regulation in Hep-2 cells via ROS-mediated suppression of the
ERK pathway. Photodiagnosis Photodyn Ther. 11:55–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee KR, Lee JS, Kim YR, Song IG and Hong
EK: Polysaccharide from Inonotus obliquus inhibits migration
and invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via
downregulation of NF-κB signaling pathway. Oncol Rep. 31:2447–2453.
2014.PubMed/NCBI
|
|
29
|
Liu WH, Chen YJ, Chien JH and Chang LS:
Amsacrine suppresses matrix metalloproteinase-2 (MMP-2)/MMP-9
expression in human leukemia cells. J Cell Physiol. 229:588–598.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng W, Zhang Y, Ma D, Shi Y, Liu C and
Wang P: (±)Equol inhibits invasion in prostate cancer DU145 cells
possibly via down-regulation of matrix metalloproteinase-9, matrix
metalloproteinase-2 and urokinase-type plasminogen activator by
antioxidant activity. J Clin Biochem Nutr. 51:61–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin RF, Feng XX, Li CW, Zhang XJ, Yu XT,
Zhou JY, Zhang X, Xie YL, Su ZR and Zhan JY: Prevention of UV
radiation-induced cutaneous photoaging in mice by topical
administration of patchouli oil. J Ethnopharmacol. 154:408–418.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim DW, Hwang IK, Kim DW, Yoo KY, Won CK,
Moon WK and Won MH: Coenzyme Q_{10} effects on manganese superoxide
dismutase and glutathione peroxidase in the hairless mouse skin
induced by ultraviolet B irradiation. Biofactors. 30:139–147. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oh SJ, Kim K and Lim CJ: Protective
properties of ginsenoside Rb1 against UV-B radiation-induced
oxidative stress in human dermal keratinocytes. Pharmazie.
70:381–387. 2015.PubMed/NCBI
|
|
34
|
Oh SJ, Kim K and Lim CJ: Ginsenoside Rb2
attenuates UV-B radiation-induced reactive oxygen species and
matrix metalloproteinase-2 through upregulation of antioxidant
components in human dermal fibroblasts. Pharmacology. 96:32–40.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oh SJ, Kim K and Lim CJ: Suppressive
properties of ginsenoside Rb2, a protopanaxadiol-type ginseng
saponin, on reactive oxygen species and matrix metalloproteinase-2
in UV-B-irradiated human dermal keratinocytes. Biosci Biotechnol
Biochem. 79:1075–1081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oh SJ, Oh Y, Ryu IW, Kim K and Lim CJ:
Protective properties of ginsenoside Rb3 against UV-B
radiation-induced oxidative stress in HaCaT keratinocytes. Biosci
Biotechnol Biochem. 80:95–103. 2015.PubMed/NCBI
|
|
37
|
Lim CJ, Choi WY and Jung HJ:
Stereoselective skin anti-photoaging properties of ginsenoside Rg3
in UV-B-irradiated keratinocytes. Biol Pharm Bull. 37:1583–1590.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oh SJ, Lee S, Choi WY and Lim CJ: Skin
anti-photoaging properties of ginsenoside Rh2 epimers in
UV-B-irradiated human keratinocyte cells. J Biosci. 39:673–682.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi WY, Lim HW and Lim CJ:
Anti-inflammatory, antioxidative and matrix metalloproteinase
inhibitory properties of 20(R)-ginsenoside Rh2 in cultured
macrophages and keratinocytes. J Pharm Pharmacol. 65:310–316. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oh SJ, Lee S, Kho YE, Kim K, Jin CD and
Lim CJ: Stereoselective suppressive effects of protopanaxadiol
epimers on UV-B-induced reactive oxygen species and matrix
metalloproteinase-2 in human dermal keratinocytes. Can J Physiol
Pharmacol. 93:91–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang HJ, Huang YH, Lim HW, Shin D, Jang K,
Lee Y, Kim K and Lim CJ: Stereospecificity of ginsenoside Rg2
epimers in the protective response against UV-B radiation-induced
oxidative stress in human epidermal keratinocytes. J Photochem
Photobiol B. 165:232–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oh SJ, Kim K and Lim CJ: Photoprotective
properties of 20(S)-protopanaxatriol, an aglycone of ginseng
saponins: Protection from ultraviolet-B radiation-induced oxidative
stress in human epidermal keratinocytes. Mol Med Rep. 14:2839–2845.
2016.PubMed/NCBI
|
|
43
|
Oh Y, Lim HW, Kim K and Lim CJ:
Ginsenoside Re improves skin barrier function in HaCaT
keratinocytes under normal growth conditions. Biosci Biotechnol
Biochem. 13:1–3. 2016.
|
|
44
|
Kang HJ, Oh Y, Lee S, Ryu IW, Kim K and
Lim CJ: Antioxidative properties of ginsenoside Ro against
UV-B-induced oxidative stress in human dermal fibroblasts. Biosci
Biotechnol Biochem. 79:2018–2021. 2015. View Article : Google Scholar : PubMed/NCBI
|