Introduction
Gliomas account for approximately 30% of all brain
and central nervous system tumors, and 80% of all malignant brain
tumors (1). Although conventional
treatments for gliomas are effective in controlling the disease,
the median survival time of patients with gliomas is only 15 months
(2). Exceptional invasiveness is
one of the major reasons for treatment failure. Therefore, it is
necessary to elucidate the molecular mechanisms for glioma
invasion, and to find novel targets for the treatment of
gliomas.
Recently, the association between microRNAs (miRNAs)
and tumorigenesis has become a hot topic in the field of cancer
research (3–5). miRNAs are small, non-coding RNAs
which regulate a wide range of cellular processes, including cell
survival, death, differentiation, and motility (6,7).
miRNAs act as tumor oncogenes or suppressors in various types of
cancer (8–11), including gliomas (12–16).
miR-140-5p has been reported to be a tumor suppressor in
hepatocellular carcinoma (17),
tongue squamous cell carcinoma (18), non-small cell lung cancer (19), and colorectal cancer (20). Recent research has demonstrated
that the gene expression of hsa-miR-140-5p correlates with several
selected magnetic resonance imaging features of patients with
glioblastoma multiforme (21).
However, little is known about the expression and the function of
miR-140-5p in human gliomas.
The Notch signaling pathway participates in various
aspects of tumor biology, and it has attracted increasing attention
as a therapeutic target for cancer (22). Jagged1 (JAG1) is one of the
canonical ligands for Notch receptors. It binds to Notch receptors
and triggers Notch activation (23). Previous studies have demonstrated
that JAG1 acts as an oncogene in brain tumors (24–26).
JAG1 is overexpressed in glioblastoma blood vessels, and it
promotes tumor growth as well as maintains cancer stem-like cells
(24,25,27).
In the present study, the expression of miR-140-5p
was detected in human glioma tissues and cell lines and compared to
that of normal tissue. In vitro gain-of-function and
loss-of-function experiments were then performed to investigate the
effect of miR-140-5p on glioma cell growth, invasion and adhesion.
Furthermore, the hypothesis that JAG1 may be a target of miR-140-5p
was tested. The present study may contribute to developing novel
therapeutic strategies for gliomas.
Materials and methods
Tissue samples
The study was approved by the Ethics Committee of
Weinan Central Hospital (Weinan, China). A total of 48 glioma
tissue samples and 48 adjacent normal tissue samples were collected
from 48 patients with glioma who underwent surgery at Weinan
Central Hospital between March 2012 and July 2015, including 21
patients who had low-grade (grades I–II) and 27 patients who had
high-grade (grades III–IV) glioma. Among these patients, 23 were
male and 25 were female, with the age range 30–69 years. All
patients signed informed consent prior to enrollment in the
study.
Cell culture
The human glioma cell lines (U-87, LN-18, U-118,
U-138 and SW1783) and the human embryonic kidney HEK293 cell line
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The normal human astrocytes were purchased from ScienCell
Research Laboratories, Inc. (Carlsbad, CA, USA). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) in a humidified
atmosphere of 5% CO2 at 37°C.
Cell transfection
The miR-control (5′-UUGUACUACACAAAAGUACUG-3′),
miR-140-5p mimic (sense 5′-CAGUGGUUUUACCCUAUGGUAG-3′; antisense
5′-ACCAUAGGGUAAAACCACUGUU-3′), miR-140-5p inhibitor (antisense
5′-CUACCAUAGGGUAAAACCACUG-3′), control empty vector (cat. no.
v855-20; Invitrogen; Thermo Fisher Scientific, Inc.), JAG1
overexpression plasmid (JAG1-pcDNA3.1), JAG1 small interfering (si)
RNA (sense 5′-GAUCUAAAAGGGAAUAAAAGGTT-3′; antisense
5′-CCUUUUAUUCCCUUUUAGAUCTT-3′) and scramble siRNA (sense
5′-UUCUCCGAACGUGUCACGUTT-3′; antisense 5′-ACGUGACACGUUCGGAGAATT-3′)
were synthesized from Sangon Biotech Co., Ltd. (Shanghai, China).
JAG1 overexpression plasmid was synthesized by ligating the cDNA of
JAG1 into pcDNA3.1 vector. They were transfected into the cells
using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. Briefly, 50 nM RNA
oligonucleotides or 100 ng plasmids, and 10 µl lipofectamine were
diluted in 250 µl Opti-MEM (Thermo Fisher Scientific, Inc.). Then
they were mixed and incubated at room temperature for 20 min to
form a complex. Cells (1×105) were incubated with the
complex for 6 h and then the cells were maintained in fresh medium
for at least 24 h prior to analyses.
Luciferase reporter assay
TargetScan 7.1 software (http://www.targetscan.org/vert_71/) was used to
predict the targets of miR-140-5p, and JAG1 was found to be a
target gene. The wild type and mutant 3′-untranslated region (UTR)
of JAG1 were cloned from JAG1 cDNA (accession number: NM_000214.2)
and inserted into the pmirGLO luciferase reporter vector (Promega
Corporation, Madison, WI, USA) in the XhoI/XbaI
sites. Primers used in this study were: wild type, forward (F)
5′-CCGCTCGAGAACCAGCAACGATCACAA-3′ and reverse (R)
5′-TCTAGAGCAAAGCCGGTAGAACTACG-3′; mutant, F
5′-CCGCTCGAGCATAATACTGTTACTACTGTAGATTTGA-3′ and R
5′-TCTAGAGCAAAGCCGGTAGAACTACG-3′. The conditions for PCR
amplification were: 95°C for 5 min, followed by 32 cycles of 95°C
for 20 sec, 56°C for 30 sec, and 72°C for 70 sec. For the
luciferase assay, the HEK293 cells were transfected with the JAG1
3′UTR-pmirGLO plasmid and miR-140-5p mimic or miR-control, using
Lipofectamine 2000. The pRL-TK Renilla luciferase reporter
vector (Promega Corporation) was used as an internal control for
normalization. Luciferase activity in the cells was determined
using a Luciferase Reporter Assay system (Promega Corporation).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MTT assay was used to determine cell growth by
measuring the numbers of live cells over 96 h. Cells were plated at
a density of 1×104 cells/well, and the cells were
allowed to grow for 24, 48, 72 and 96 h in 96-well plates, and then
incubated with MTT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
at 37°C, according to the manufacturer's instructions. Following 4
h, dimethyl sulfoxide was added to solubilize the formazan
crystals. Absorbance at 570 nm was measured using a Multiskan
Ascent plate reader (Thermo Fisher Scientific, Inc.). Results were
calculated relative to optical density of blank cells at 570 nm at
24 h.
Transwell invasion assay
Transwell invasion assays were used to measure cell
invasion ability. The Transwell inserts (Corning, Inc., Corning,
NY, USA) were precoated with Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA) at 37°C for 30 min. The resuspended cells (2 ml) at
the final concentration of 5×104 cells/ml in serum-free
medium were added to the upper chambers, and cell culture medium
containing 10% FBS was added to the lower chambers. Following
incubation at 37°C for 12 h, a cotton swab was used to gently
remove the non-invasive cells which remained on the upper surface
of the membrane. The invasive cells which migrated on the lower
surface of the membrane were fixed with 95% ethanol for 30 min, and
then stained with hematoxylin (Beyotime Institute of Biotechnology,
Shanghai, China) for 10 min at room temperature. The number of
invaded cells was counted in 10 randomly selected fields using an
inverted light microscope (37XC; Shanghai Optical Instrument Co.,
Ltd., Shanghai, China). Results are presented as the mean number of
cells per field.
Cell adhesion assay
Cell adhesion was examined using 96-well plates
coated with fibronectin (Sigma-Aldrich; Merck KGaA). The plates
were blocked with 1% bovine serum albumin (BSA; Sigma-Aldrich;
Merck KGaA) at room temperature for 2 h. The cells were suspended
to the density of 3×105 cells/ml, and 200 µl cell
suspension were cultured in serum-free medium at 37°C for 2 h.
After washing with PBS, the adhesive cells were fixed with 4%
paraformaldehyde (Sangon Biotech Co., Ltd.) for 15 min and then
stained with 0.1% crystal violet (Sangon Biotech Co., Ltd.) for 20
min. 12% SDS was added to dissolve the crystals and the absorbance
at 570 nm was measured using a microplate reader. Results were
calculated relative to optical density at 570 nm of blank
cells.
Western blot analysis
The proteins were extracted from the cells using
Western and Immunoprecipitation Cell Lysis buffer (Sangon Biotech
Co., Ltd.). Briefly, 5×105 cells were incubated with 50
µl cell lysis buffer at 4°C for 20 min. and protein concentrations
were determined using a bicinchoninic acid protein assay kit
(Sangon Biotech Co., Ltd.). Equal amounts of total protein (20 µg)
were separated on 8% SDS polyacrylamide gel electrophoresis, and
then transferred to nitrocellulose membranes (Merck KGaA).
Following blocking in tris-buffered saline containing 3% BSA at 4°C
overnight, the membranes were incubated with rabbit polyclonal
antibody to JAG1 (1:500 dilution; cat no. ab7771; Abcam, Cambridge,
MA, USA) and rabbit polyclonal antibody to β-actin (1:1,000
dilution; cat no. ab8227; Abcam) at 4°C overnight. Subsequently,
the membranes were incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:2,000 dilution; cat no.
ab205718; Abcam) at room temperature for 2 h. The signals were
detected using a chemiluminescence detection kit (Pierce; Thermo
Fisher Scientific, Inc.), and band intensities were quantified
using ImageJ version 1.46 (National Institutes of Health, Bethesda,
MD, USA) (28).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.), and miRNAs were isolated
from tissues and cells using the miRNeasy Mini kit (Qiagen GmbH,
Hilden, Germany), as per the manufacturer's instructions. Total RNA
(1 µg) was converted into cDNA using a RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.), as per the
manufacturer's instructions. Dilute the cDNA samples at 1:5 in
RNAse free water. Subsequently, the qPCR reactions were carried out
using a SYBR-Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in a 7900 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primers used in
this study were: miR-140-5p, F 5′-CAGTGGTTTTACCCTATGGTAG-3′ and R
5′-TGGTGTCGTGGAGTCG-3′; U6, F 5′-CTCGCTTCGGCAGCACA-3′ and R
5′AACGCTTCACGAATTTGCGT-3′; JAG1, F 5′-GACTCATCAGCCGTGTCTCA-3′ and R
5′-TGGGGAACACTCACACTCAA-3′; β-actin, F 5′-GGACTTCGAGCAAGAGATGG-3′
and R 5′-AGCACTGTGTTGGCGTACAG-3′. The conditions for PCR
amplification were: 95°C for 5 min, followed by 40 cycles of 95°C
for 20 sec, 60°C for 30 sec, and 72°C for 30 sec. The U6
spliceosomal RNA gene or the β-actin gene were used as internal
controls for miRNA and mRNA expression respectively. The relative
mRNA expression levels were calculated in accordance with the
2−∆∆Cq method (29).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical analysis was
performed using SPSS 19.0 statistical software (IBM SPSS, Armonk,
NY, USA). Student's t-test or one-way analysis of variance followed
by least significant difference test were used to analyze the
statistical difference between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
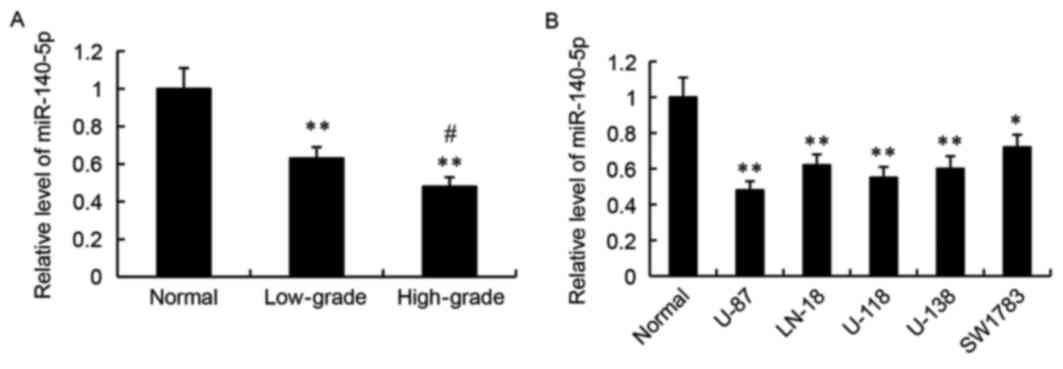
Expression of miR-140-5p in human
glioma tissues and cell lines
RT-qPCR was used to detect the relative expression
levels of miR-140-5p in human glioma tissues and cell lines.
Compared with adjacent normal tissues, the relative expression
levels of miR-140-5p were significantly decreased in the low-grade
and high-grade human glioma tissues (P<0.01; Fig. 1A). Furthermore, miR-140-5p
expression was significantly lower in the high-grade gliomas
compared with the low-grade gliomas (P<0.05; Fig. 1A). Next, miR-140-5p expression was
examined in normal human astrocytes and in five glioma cell lines,
U-87, LN-18, U-118, U-138 and SW1783. As demonstrated in Fig. 1B, the relative expression levels of
miR-140-5p was significantly lower in the glioma cell lines
compared with the normal human astrocytes.
Effect of miR-140-5p on glioma cell
growth, invasion and adhesion
A miR-140-5p mimic or a miR-140-5p inhibitor was
transfected into the SW1783 glioma cells in vitro, in order
to overexpress or suppress miR-140-5p expression respectively. As
demonstrated by RT-qPCR analysis, transfection with a miR-control
did not alter miR-140-5p expression compared with the untransfected
blank group (Fig. 2A). However,
the relative expression levels of miR-140-5p were significantly
increased in the mimic group and significantly decreased in the
inhibitor group, compared with the miR-control group (P<0.01;
Fig. 2A).
The effect of miR-140-5p on glioma cell growth was
determined by MTT assay. The results demonstrated that transfection
with the miR-140-5p mimic significantly decreased the number of
live cells over 96 h, while transfection with the miR-140-5p
inhibitor significantly increased the number of live cells,
compared with the miR-control group (Fig. 2B).
Transwell invasion assay was used to examine the
ability of the cells to invade through matrigel. The results
demonstrated that, compared with the control, miR-140-5p mimic
transfection resulted in decreased numbers of invaded cells, while
miR-140-5p inhibitor transfection had the opposite effect
(P<0.05; Fig. 2C).
Finally, a cell adhesion assay revealed that,
compared with the cells transfected with the miR-control, the cells
transfected with the miR-140-5p mimic exhibited decreased cell
adhesive ability (P<0.01; Fig.
2D). By contrast, the cell adhesive ability was significantly
increased in the cells transfected with the miR-140-5p inhibitor
compared with the miR-control group (P<0.05; Fig. 2D).
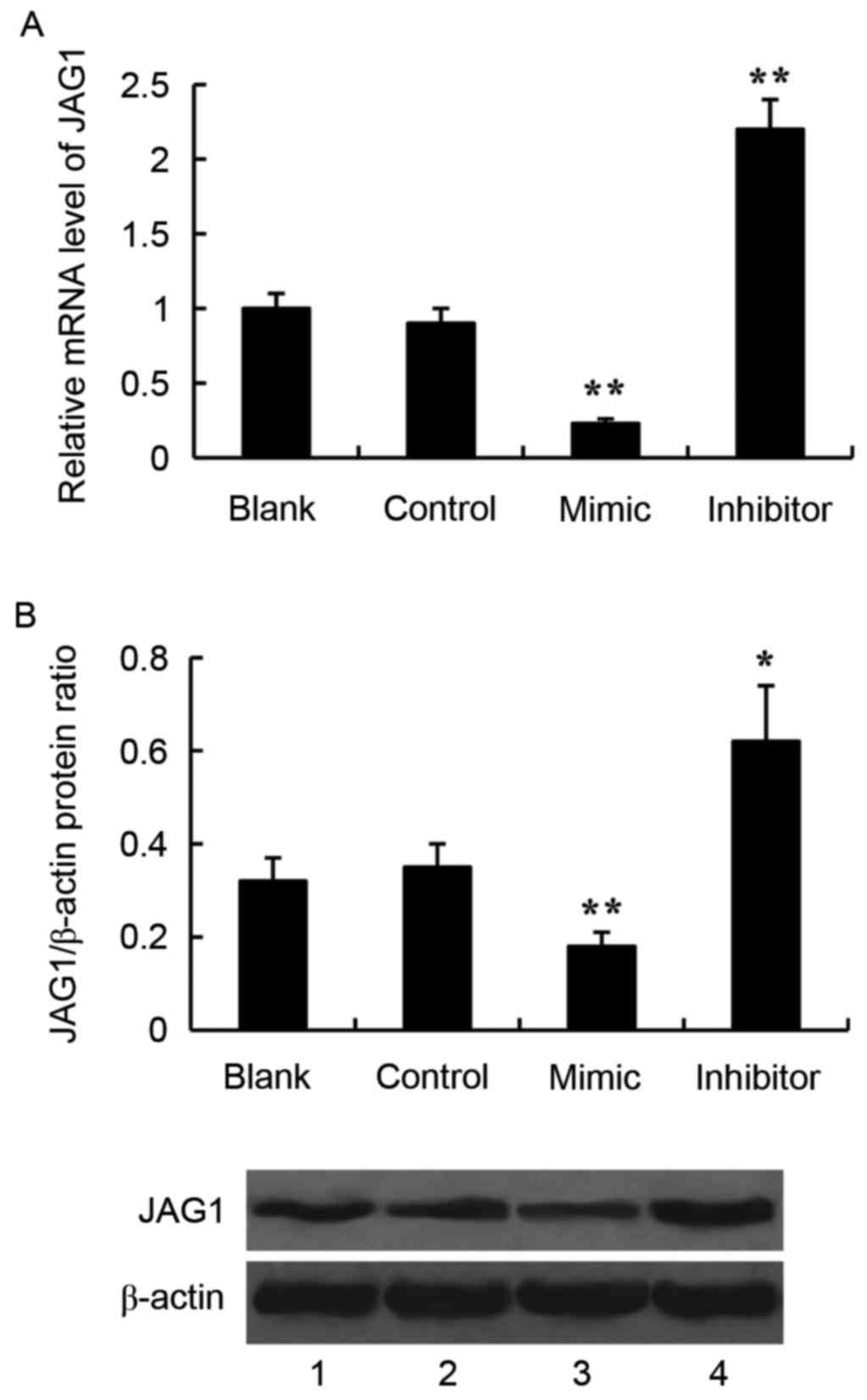
Effect of miR-140-5p on JAG1
expression in glioma cells
JAG1 was predicted to be a target gene of miR-140-5p
by using TargetScan 7.1 software (http://www.targetscan.org/vert_71/). To verify the
effect of miR-140-5p on JAG1 expression, the miR-140-5p mimic or
the miR-140-5p inhibitor was transfected into the SW1783 glioma
cells, and expression of JAG1 was examined by RT-qPCR and western
blotting. Transfection with the miR-140-5p mimic significantly
inhibited JAG1 expression compared with control, both at the mRNA
and protein level (Fig. 3). By
contrast, transfection with the miR-140-5p inhibitor significantly
increased JAG1 mRNA and protein expression levels, compared with
control (Fig. 3).
JAG1 is a direct target of
miR-140-5p
A dual-luciferase reporter assay was used in order
to determine whether JAG1 was a direct target of miR-140-5p. The
wild-type or mutant JAG1 3′UTR was cloned into the pmirGLO vector,
and co-transfected into HEK293 cells with either the miR-140-5p
mimic or the miR-control. The results revealed that, compared with
control, transfection of the miR-140-5p mimic significantly
repressed the luciferase activity of wild-type JAG1 3′UTR
(P<0.01; Fig. 4). By contrast,
transfection of the miR-140-5p mimic had no effect on the mutant
JAG1 3′UTR luciferase activity compared with control (P>0.05;
Fig. 4).
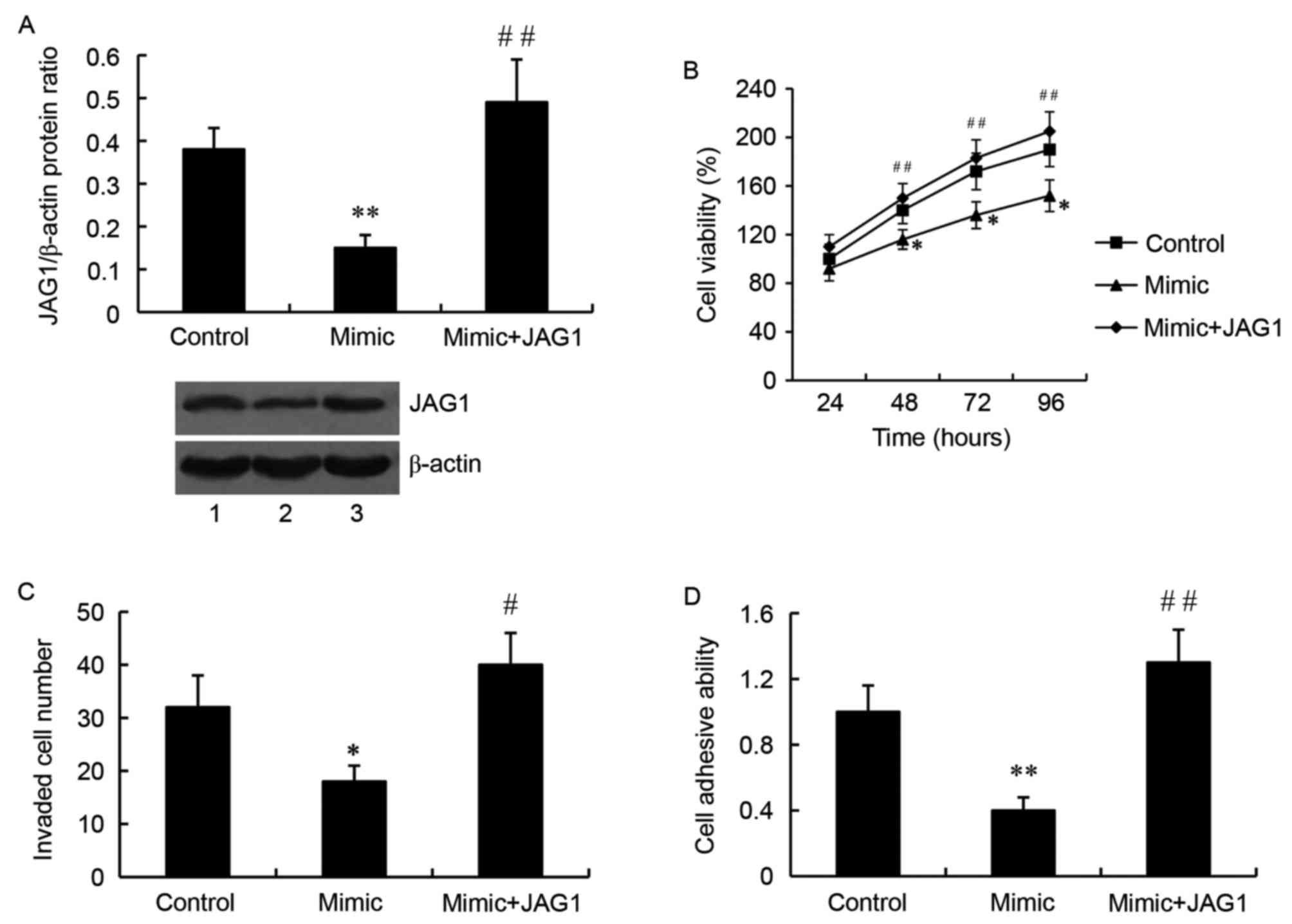
JAG1 mediates the effect of miR-140-5p
on glioma cell growth, invasion and adhesion
JAG1 overexpression plasmid or JAG1 siRNA was used
to overexpress or silence JAG1 respectively, and they were
transfected into SW1783 cells together with the miR-140-5p mimic or
inhibitor. As demonstrated by western blot analysis, the relative
protein level of JAG1 was significantly higher in the cells
transfected with the miR-140-5p mimic and the JAG1 overexpression
plasmid, compared with the cells transfected with the mimic alone
(P<0.01; Fig. 5A). Importantly,
JAG1 overexpression attenuated the inhibitory effect of miR-140-5p
on glioma cell growth (Fig. 5B),
invasion (Fig. 5C) and adhesion
(Fig. 5D).
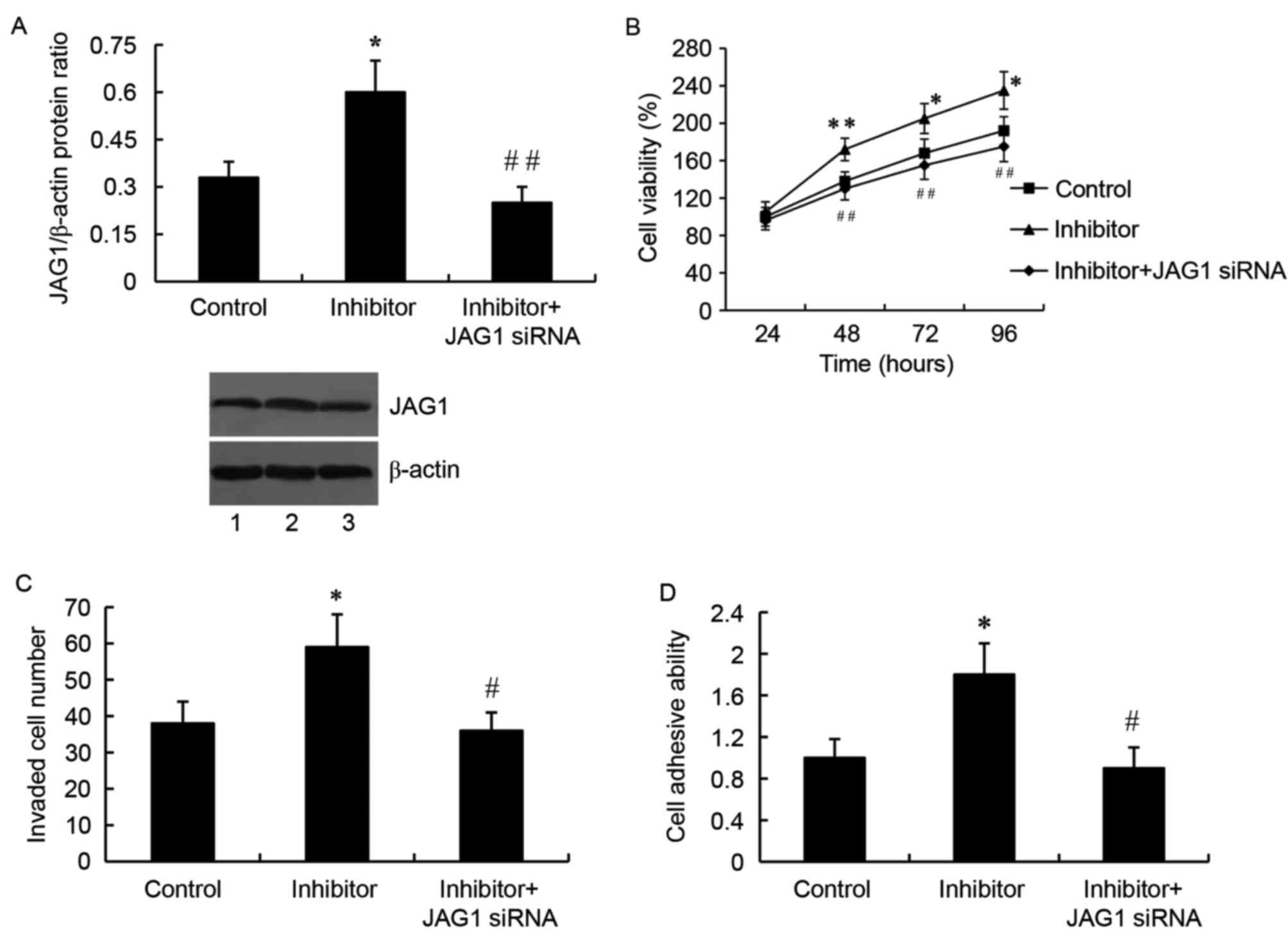
By contrast, cells transfected with the miR-140-5p
inhibitor and the JAG1 siRNA exhibited a significantly
downregulated JAG1 protein expression compared with cells
transfected with the inhibitor only (P<0.01; Fig. 6A). As expected, JAG1 silencing by
siRNA reversed the miR-140-5p inhibitor-mediated increased cell
growth (Fig. 6B), invasion
(Fig. 6C) and adhesion (Fig. 6D) in SW1783 glioma cells.
Discussion
Abnormal expression of miR-140-5p is implicated in
several types of cancer (17,30).
miR-140-5p has been reported to suppress the growth and metastasis
of hepatocellular carcinoma, and its expression levels are
correlated with various clinical characteristics of hepatocellular
carcinoma, including vein invasion, multiple nodules, capsular
formation, as well as overall and disease-free survival (30). miR-140-5p affects the proliferation
of lung cancer cells by regulating mitogen-activated protein kinase
1 signaling (19). Upregulation of
miR-140-5p suppresses migration and invasion of FaDu hypopharyngeal
carcinoma cells by inhibiting the ADAM metallopeptidase domain
10-mediated Notch1 signaling pathway, suggesting that miR-140-5p
may have potential therapeutic applications in hypopharyngeal
squamous cell carcinoma (31).
Consistent with previous studies, the present study demonstrated
that miR-140-5p was significantly downregulated in human glioma
tissues and cell lines compared with normal tissues, indicating
that miR-140-5p may be a tumor suppressor in human gliomas. In
addition, miR-140-5p expression was significantly correlated with
the grade of gliomas, suggesting that miR-140-5p may be important
in glioma progression. Subsequently, in vitro
gain-of-function and loss-of-function experiments were performed to
investigate the effect of miR-140-5p on glioma cell growth,
invasion and adhesion. The present results revealed that miR-140-5p
suppressed cell growth, invasion and adhesion in SW1783 glioma
cells, suggesting that miR-140-5p may have an inhibitory effect on
glioma growth and metastasis. Consistent with the role of
miR-140-5p in other types of cancer, this study demonstrated for
the first time that miR-140-5p acts as a tumor suppressor in human
gliomas.
JAG1 mediates multiple signaling pathways, and is
important in both physiological and pathological conditions
(32–37). It has been demonstrated that JAG1
is overexpressed in various types of cancer, and it is involved in
several aspects of tumor biology, including cell growth, apoptosis,
cancer stem cell maintenance, epithelial-mesenchymal transition and
metastasis (38). Fiaschetti et
al (39) revealed that JAG1 is
overexpressed in the majority of medulloblastoma (MB), and JAG1 is
crucial in maintaining Notch-related pro-survival functions in MB
cells (39). In the present study,
JAG1 expression was demonstrated to be regulated by miR-140-5p in
glioma cells. JAG1 was predicted to be a target gene of miR-140-5p
by using TargetScan 7.1 software (http://www.targetscan.org/vert_71/). Using a
dual-luciferase reporter assay, the present study confirmed that
miR-140-5p inhibited JAG1 expression by directly targeting the
3′UTR of JAG1. Furthermore, the effect of miR-140-5p on glioma cell
proliferation, invasion and adhesion was reversed by JAG1
overexpression, suggesting that JAG1 mediated the effect of
miR-140-5p on glioma cell growth and invasion.
In conclusion, the present study revealed that
miR-140-5p was downregulated in human glioma tissues and cell lines
compared with normal tissues. miR-140-5p exerted its inhibitory
effect on glioma growth and invasion at least partly by suppressing
JAG1 expression. The present study revealed a novel molecular
mechanism underlying human glioma growth and invasion, and provided
a potential novel target for the treatment of gliomas.
References
|
1
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: Primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14:(Suppl 5). v1–v49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chi Y and Zhou D: MicroRNAs in colorectal
carcinoma-from pathogenesis to therapy. J Exp Clin Cancer Res.
35:432016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y and Sarkar FH: MicroRNA targeted
therapeutic approach for pancreatic cancer. Int J Biol Sci.
12:326–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuninty PR, Schnittert J, Storm G and
Prakash J: MicroRNA targeting to modulate tumor microenvironment.
Front Oncol. 6:32016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun W, Li YS Julie, Huang HD, Shyy JY and
Chien S: microRNA: A master regulator of cellular processes for
bioengineering systems. Annu Rev Biomed Eng. 12:1–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hurst DR, Edmonds MD and Welch DR:
Metastamir: The field of metastasis-regulatory microRNA is
spreading. Cancer Res. 69:7495–7498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manrique-Guzmán S: Biomarkers in
high-grade gliomas: A systematic review. Gac Med Mex. 152:87–93.
2016.(In Spanish). PubMed/NCBI
|
|
13
|
Silber J, James CD and Hodgson JG:
microRNAs in gliomas: Small regulators of a big problem.
Neuromolecular Med. 11:208–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong JW: MicroRNA-induced silencing of
glioma progression. J Neurosci. 30:3868–3869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang HW, Xing H and Johnson MD: A major
role for microRNAs in glioblastoma cancer stem-like cells. Arch
Pharm Res. 38:423–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Costa PM, Cardoso AL, Mano M and de Lima
MC: MicroRNAs in glioblastoma: Role in pathogenesis and
opportunities for targeted therapies. CNS Neurol Disord Drug
Targets. 14:222–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Fang F, Chang R and Yang L:
MicroRNA-140-5p suppresses tumor growth and metastasis by targeting
transforming growth factor β receptor 1 and fibroblast growth
factor 9 in hepatocellular carcinoma. Hepatology. 58:205–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kai Y, Peng W, Ling W, Jiebing H and Zhuan
B: Reciprocal effects between microRNA-140-5p and ADAM10 suppress
migration and invasion of human tongue cancer cells. Biochem
Biophys Res Commun. 448:308–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li W and He F: Monocyte to macrophage
differentiation-associated (MMD) targeted by miR-140-5p regulates
tumor growth in non-small cell lung cancer. Biochem Biophys Res
Commun. 450:844–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhai H, Fesler A, Ba Y, Wu S and Ju J:
Inhibition of colorectal cancer stem cell survival and invasive
potential by hsa-miR-140-5p mediated suppression of Smad2 and
autophagy. Oncotarget. 6:19735–19746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li WB, Chen HY, Zhang W, Yan W, Shi R, Li
SW and Jiang T: Relationship between magnetic resonance imaging
features and miRNA gene expression in patients with glioblastoma
multiforme. Chin Med J (Engl). 126:2881–2885. 2013.PubMed/NCBI
|
|
22
|
Xiao YF, Yong X, Tang B, Qin Y, Zhang JW,
Zhang D, Xie R and Yang SM: Notch and Wnt signaling pathway in
cancer: Crucial role and potential therapeutic targets (Review).
Int J Oncol. 48:437–449. 2016.PubMed/NCBI
|
|
23
|
Shimizu K, Chiba S, Saito T, Kumano K and
Hirai H: Physical interaction of Delta1, Jagged1, and Jagged2 with
Notch1 and Notch3 receptors. Biochem Biophys Res Commun.
276:385–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu TS, Costello MA, Talsma CE, Flack CG,
Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM,
et al: Endothelial cells create a stem cell niche in glioblastoma
by providing NOTCH ligands that nurture self-renewal of cancer
stem-like cells. Cancer Res. 71:6061–6072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeon HM, Kim SH, Jin X, Park JB, Kim SH,
Joshi K, Nakano I and Kim H: Crosstalk between glioma-initiating
cells and endothelial cells drives tumor progression. Cancer Res.
74:4482–4492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jubb AM, Browning L, Campo L, Turley H,
Steers G, Thurston G, Harris AL and Ansorge O: Expression of
vascular Notch ligands Delta-like 4 and Jagged-1 in glioblastoma.
Histopathology. 60:740–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Zou C, Pan L, Xu Y, Qi W, Ma G,
Hou Y and Jiang P: MicroRNA-140-5p inhibits the progression of
colorectal cancer by targeting VEGFA. Cell Physiol Biochem.
37:1123–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jing P, Sa N, Liu X, Liu X and Xu W:
MicroR-140-5p suppresses tumor cell migration and invasion by
targeting ADAM10-mediated Notch1 signaling pathway in
hypopharyngeal squamous cell carcinoma. Exp Mol Pathol.
100:132–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Stoeck A, Lee SJ, Shih IeM, Wang
MM and Wang TL: Jagged1 expression regulated by Notch3 and
Wnt/β-catenin signaling pathways in ovarian cancer. Oncotarget.
1:210–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto M, Taguchi Y, Ito-Kureha T, Semba
K, Yamaguchi N and Inoue J: NF-κB non-cell-autonomously regulates
cancer stem cell populations in the basal-like breast cancer
subtype. Nat Commun. 4:22992013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi K, Ahn YH, Gibbons DL, Tran HT,
Creighton CJ, Girard L, Minna JD, Qin FX and Kurie JM: Distinct
biological roles for the notch ligands Jagged-1 and Jagged-2. J
Biol Chem. 284:17766–17774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Z, Li Y, Banerjee S, Kong D, Ahmad A,
Nogueira V, Hay N and Sarkar FH: Down-regulation of Notch-1 and
Jagged-1 inhibits prostate cancer cell growth, migration and
invasion, and induces apoptosis via inactivation of Akt, mTOR and
NF-kappaB signaling pathways. J Cell Biochem. 109:726–736.
2010.PubMed/NCBI
|
|
36
|
Zeng Q, Li S, Chepeha DB, Giordano TJ, Li
J, Zhang H, Polverini PJ, Nor J, Kitajewski J and Wang CY:
Crosstalk between tumor and endothelial cells promotes tumor
angiogenesis by MAPK activation of Notch signaling. Cancer Cell.
8:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li D, Masiero M, Banham AH and Harris AL:
The notch ligand JAGGED1 as a target for anti-tumor therapy. Front
Oncol. 4:2542014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fiaschetti G, Schroeder C, Castelletti D,
Arcaro A, Westermann F, Baumgartner M, Shalaby T and Grotzer MA:
NOTCH ligands JAG1 and JAG2 as critical pro-survival factors in
childhood medulloblastoma. Acta Neuropathol Commun. 2:392014.
View Article : Google Scholar : PubMed/NCBI
|