Introduction
Esophageal cancer is a common type of digestive
tract cancer, and the province of Jiangsu is a high incidence area
(1–3). Esophageal squamous cell carcinoma
(ESCC), esophageal adenocarcinoma (EA) and small cell carcinoma of
the esophagus are the most common pathological types of esophageal
cancer. The high incidence of ESCC in China is significantly
different from that of the European and American countries
(4,5). Surgical resection is the first choice
of treatment for patients with early esophageal cancer, but the
majority of patients experience recurrence or metastasis following
surgery; therefore, it is of great significance to investigate the
relevant factors that affect the prognosis of postoperative
survival (6–8).
Secreted protein acidic and rich in cysteine (SPARC)
is a small protein rich in cysteine, which is also known as
basement-membrane protein 40 (9–11).
As a non structural matrix glycoprotein its function is very
complex, and it is involved in many physiological and pathological
processes (12,13). In addition, it is significant in
the microenvironment of tumor cell activity and tumor growth
(14). It was observed that the
SPARC protein was highly expressed in the fibrous cells and
endothelial cells associated with invasive malignant tumors. The
expression level of SPARC was closely associated with the
occurrence, development and prognosis of tumors (13,15,16).
To investigate the association between the
expression of SPARC and the prognosis of postoperative patients
with ESCC, immunohistochemistry and reverse
transcription-polymerase chain reaction (RT-PCR) were employed to
measure SPARC protein expression levels in cases with ESCC, and in
healthy esophageal mucosa samples, which served as the control. In
addition, the underlying mechanism of the formation of ESCC was
evaluated in an attempt to establish a novel method for its early
diagnosis.
Patients and methods
From January 2013 to January 2016, samples of ESCC
were collected from 89 patients who underwent surgical resection at
the First People's Hospital of Yancheng City (Yancheng, China) who
had been diagnosed by clinical pathology. Each case had detailed
clinical and pathological data and none had received preoperative
chemotherapy or radiotherapy. The ESCC patients included 45 males
and 44 females (aged 36–73 years; mean age, 53.9±11.6 years). A
total of 100 cases with heathy esophageal mucosa were selected from
the First People's Hospital of Yancheng City (Yancheng, China) and
served as a control group. These included 55 males and 45 females
(aged 35–69 years; mean age, 49.5±10.4 years).
No statistically significant differences were
detected in age between the ESCC group and the healthy esophageal
mucosa group. All specimens were obtained following receipt of
informed consent with approval by the Ethics Committee of the First
People's Hospital of Yancheng City (Yancheng, China) [ID no. HMU
(Ethics) 20131103].
Immunohistochemical staining
techniques
The immunohistochemical staining method from Agilent
Technologies, Inc. (Santa Clara, CA, USA) was used to detect the
distribution of SPARC. Immunohistochemical procedures were
performed in strict accordance with the manufacturer's
instructions. The EnVision and DAB chromogenic reagent kit (Agilent
Technologies, Inc., Santa Clara, CA, USA) were used for
immunohistochemical staining. All staining was performed under the
same conditions; the tissue samples were sliced to a thickness of
2–3 µm, dehydrated in 80, 90, 95 and 100% ethanol, dewaxed and
antigen repair was performed using 0.01 mol/l citric acid (pH 6.0).
Normal goat serum (Toyobo Co., Ltd., Osaka, China) was dropped onto
the slide and incubated for 10 min at room temperature.
Subsequently, the corresponding specific antibody (mouse
anti-osteonectin/SPARC; (1:1,000; catalog no. 5420; Cell Signaling
Technologies Inc., Danvers, MA, USA) was added to the slide and
incubated for 1.5 h at room temperature. The slides were washed
with phosphate-buffered saline (PBS) for 3 min three times. The
secondary antibody (1:1,000; catalog no. 341200; Cell Signaling
Technologies Inc.) was added and incubated for 30 min at room
temperature. The slide was stained with DAB, and the nucleus was
stained with hematoxylin, dehydrated using a gradient of ethanol,
cleared with xylene and sealed using natural gum. SPARC (mouse
anti-osteonectin/SPARC; (1:1,000; catalog no. 5420; Cell Signaling
Technologies Inc., Danvers, MA, USA) immunoreactivity in the blood
vessel walls of ESCC tissues served as a positive control, and the
specific antibodies were replaced with PBS to serve as the negative
control.
The immunohistochemical results were determined by
three pathologists, who observed the positive granule-stained cells
in the esophageal cancer tissue samples and the adjacent healthy
esophageal mucosa using a BH-2 light microscope (Olympus
Corporation, Tokyo, Japcan). The staining score criteria were as
follows: 0, 0–15%; 1, >15–30%; 2, >30–45%; 3, >45%.
According to the staining intensity for semi-quantitative
determination, colorless was 0 and 3 (strong staining) was brown.
The final staining score of a sample was determined as the product
of the positive cell percentage score and the staining intensity
score. Staining score <2, negative (−); staining score 2–4
points, weakly positive (+); staining score, 4–6 points, positive
(+ +); staining score ≥6 points, strong positive (+ + +). For the
convenience of statistical analysis of the data, the (−) group was
defined as the negative expression group (−), and the (+), (+ +)
and (+ + +) groups were designated as the positive expression group
(+).
Detecting the expression level of
SPARC mRNA using RT-PCR
Total RNA was isolated from the tissue samples using
TRIzol (Sangon Biotech Co., Ltd., Shanghai, China) and quantified
using a Nandrop spectrophotometer. RNA (2 ug) was reverse
transcribed to cDNA according to the Titanium® One-Step
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China), and was
amplified by semi-quantitative PCR with β-actin serving as the
reference. The primer sequences (Sangon Biotech Co., Ltd.) are
presented in Table I. The thermal
cycling conditions were as follows: Predenaturation at 94°C for 4
min; 30 cycles of 94°C for 10 sec, 55°C for 30 sec and 72°C for 60
sec.
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction analysis.
| Primer | Primer sense | Primer sequences
5′-3′ | Product size
(bp) |
|---|
| Secreted protein
acidic and rich in cysteine | Forward |
CTGCTGGCAGACAACAGGTA | 344 |
|
| Reverse |
CTGTTTGCTGCTGTGGAAAA |
|
| β-actin | Forward |
TGACGTGGACATCCGCAAAG | 231 |
|
| Reverse |
CTGGAAGGTGGACAGCGAGG |
|
Amplification of SPARC by PCR was examined by
agarose gel electrophoresis and analyzed using Quantity One version
3 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
absorbance value of the belt and the reference were read, and the
results were expressed as a ratio (sample value/reference value).
When the ratio of the ESCC value and reference value was greater
than the β-actin reference value, it was expressed positively.
Otherwise, it was considered to be negative.
Statistical analysis
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The χ2 test
was performed to compare the distribution of SPARC expression
levels between the healthy and ESCC tissue samples. Kaplan-Meier
survival analysis with the log-rank test was performed to analyze
the association between the protein expression levels in the cancer
tissue samples, and multi factor survival stage and independent
factor survival stage were used for the other clinicopathologic
characteristics and the survival rate of the patients. The hazard
ratios were determined using SPSS software version 13.0 (SPSS,
Inc., Chicago, IL, USA) and the 95% confidence intervals (CI) were
computed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between the expression
level of SPARC and the overall survival of postoperative patients
with ESCC
The overall survival of patients who were positive
for the SPARC protein was 60.92±3.45 months, after a median
follow-up time of 61.5 months (6.1–77.3 months). The overall
survival of SPARC protein-negative patients was 55.68±5.65 months.
Kaplan-Meier survival analysis indicated that there was no
significant difference between SPARC-positive and SPARC-negative
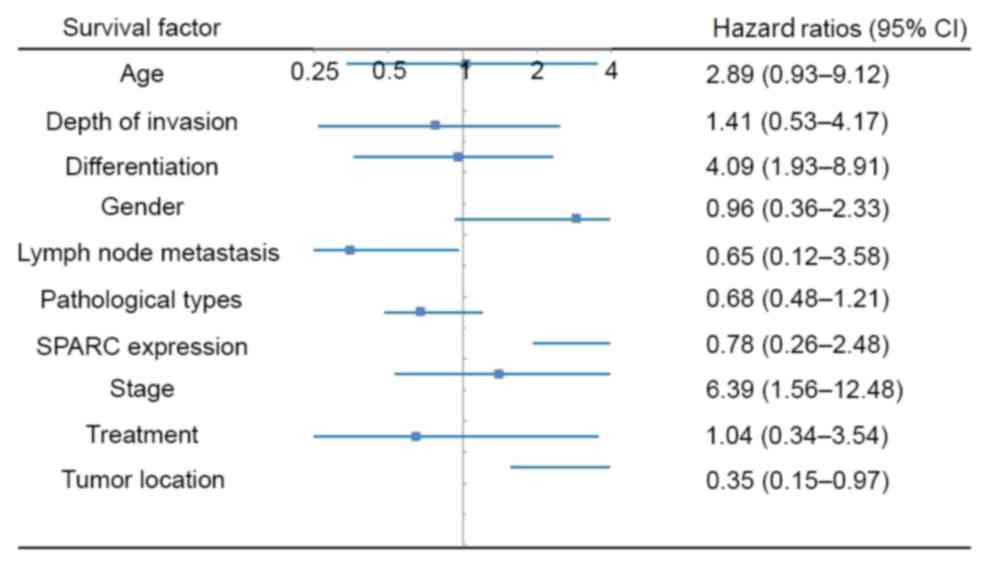
patients (P>0.05). Multi factor survival stage indicated that
the tumor location (upper, middle and lower segment), tumor
differentiation (high, moderate and poor) and tumor stage (I, II
and III) were independent factors affecting the overall survival of
the postoperative patients. Additionally, adjuvant therapy, gender,
age, gross morphology, tumor invasion depth and lymph node
metastasis were not identified as independent factors affecting the
overall survival of postoperative patients (Fig. 1).
SPARC mRNA expression in ESCC and
healthy esophageal mucosa tissue samples
RT-PCR demonstrated the expression level of SPARC
mRNA in ESCC and healthy esophageal mucosa tissue samples. The
positive rate of SPARC mRNA in ESCC was 71.91% (64/89), which was
significantly higher than that in the healthy esophageal mucosa
15.00% (15/100; P<0.05) (Fig.
2).
Expression levels of SPARC protein in
ESCC and healthy esophageal mucosa tissue samples
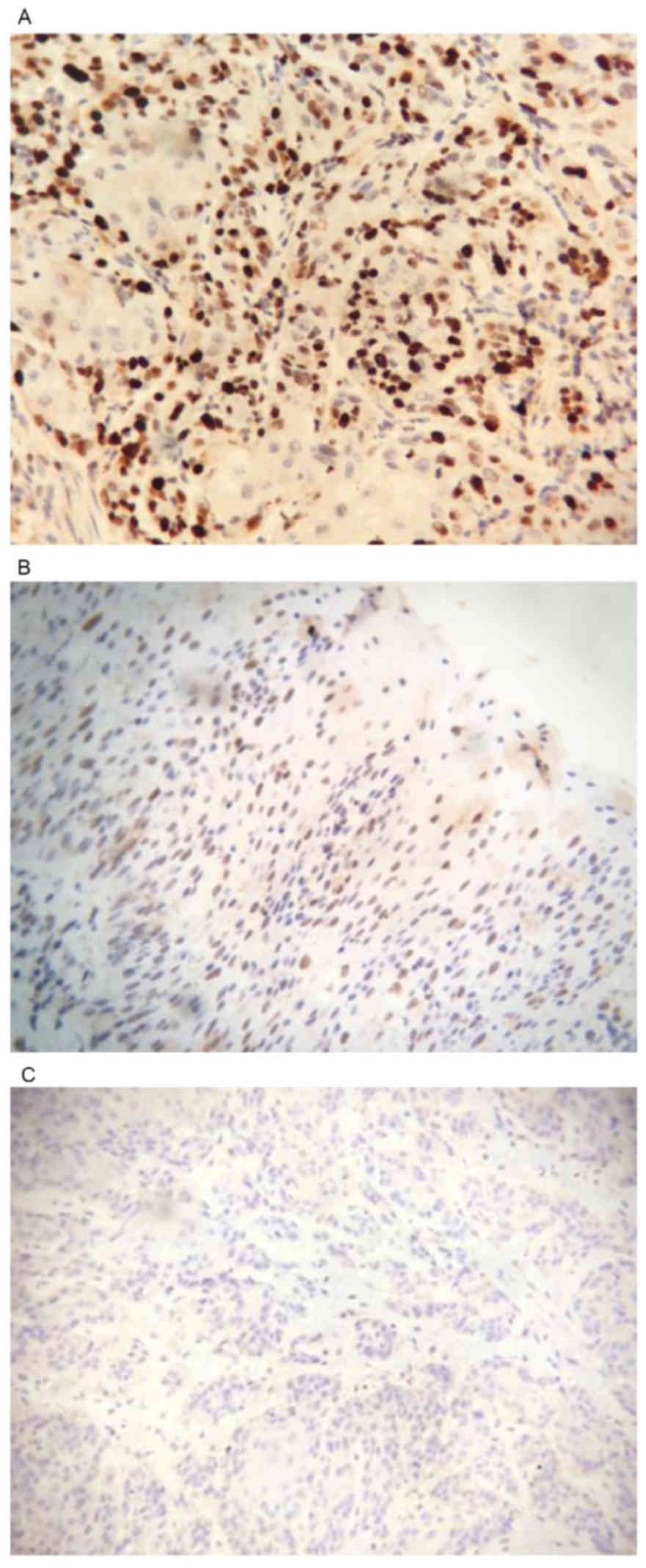
The positive expression rate of SPARC protein in
ESCC was 65.17% (58/89) and the positive rate was 8% (8/100) in the
normal esophageal mucosa. The expression level of SPARC protein in
the ESCC tissue samples was significantly higher than that in the
healthy esophageal mucosa samples (P<0.05; Fig. 3).
Association between the expression
levels of SPARC mRNA and protein in different pathological types of
ESCC
The expression levels of SPARC mRNA and protein in
ESCC were consistent. SPARC was highly expressed in ESCC tissue
samples, and was not associated with sex, age, tumor size,
pathologic type or the degree of tumor differentiation, but was
associated with staging and metastasis (Table II).
 | Table II.Correlation of SPARC mRNA and protein
expression levels with clinicopathological features in
osteosarcoma. |
Table II.
Correlation of SPARC mRNA and protein
expression levels with clinicopathological features in
osteosarcoma.
| Characteristic | n | SPARC protein
positive rate, n (%) | χ2 | P-value | SPARC mRNA positive
rate, n (%) | χ2 | P-value |
|---|
| Gender |
| Male | 45 | 30 (66.7) | 0.190 | 0.663 | 33 (73.3) | 0.008 | 0.927 |
|
Female | 44 | 28 (63.6) |
|
| 31 (70.5) |
|
|
| Age (years) |
|
<40 | 46 | 31 (67.4) | 0.121 | 0.728 | 34 (73.9) | 0.005 | 0.945 |
| ≥40 | 43 | 27 (62.8) |
|
| 30 (70.0) |
|
|
| Tumor diameter
(cm) |
| ≥10 | 35 | 23 (65.7) | 0.351 | 0.553 | 26 (74.3) | 0.072 | 0.789 |
|
<10 | 54 | 35 (64.8) |
|
| 38 (70.4) |
|
|
| Lymph node
metastasis |
| Yes | 37 | 33 (89.2) | 7.601 | 0.006 | 35 (94.6) | 7.411 | 0.008 |
| No | 52 | 25 (48.1) |
|
| 29 (55.8) |
|
|
| Pathologic type |
|
Ulcer | 52 | 32 (61.54) | 0.323 | 0.125 | 37 (71.15) | 0.332 | 0.119 |
|
Medullary | 19 | 13 (68.42) |
|
| 14 (73.68) |
|
|
|
Mushroom | 11 | 8
(72.72) |
|
| 8
(72.72) |
|
|
|
Coarctation | 7 | 5
(71.43) |
|
| 5
(71.43) |
|
|
| Degree of tumor
differentiation |
|
High | 19 | 11 (57.89) | 0.234 | 0.512 | 13 (68.42) | 0.276 | 0.565 |
|
Moderate | 44 | 29 (65.91) |
|
| 32 (72.73) |
|
|
|
Poor | 26 | 18 (69.23) |
|
| 19 (73.08) |
|
|
| Tumor stage |
| I | 43 | 33 (76.74) | 7.231 | 0.005 | 38 (88.37) | 7.012 | 0.002 |
| II | 28 | 16 (57.14) |
|
| 16 (57.14) |
|
|
|
III | 18 | 9
(50.00) |
|
| 10 (55.56) |
|
|
A total of 89 cases of patients with ESCC (according
to the pathological morphology) were divided into 52 cases of ulcer
type, 19 cases of medullary type, mushroom type in 11 cases and 7
cases of coarctation. The positive expression rates of SPARC
protein were as follows: Ulcer type, 61.54% (32/52); medullary
type, 68.42% (13/19); mushroom type, 72.72% (8/11); and coarctation
type, 71.43% (5/7). Although the results showed that the positive
rate of mushroom type was highest, the difference was not
statistically significant (P>0.05).
The positive expression rates of SPARC mRNA were as
follows: Ulcer type, 71.15% (37/52); medullary type, 73.68%
(14/19); mushroom type, 72.72% (8/11); and coarctation type, 71.43%
(5/7). Although the results showed that the positive rate of
mushroom type was highest, the difference was not statistically
significant (P>0.05).
According to the degree of tumor differentiation,
the 89 cases of ESCC were divided into 19 cases of high, 44 cases
of moderate and 26 cases of poor differentiation. The positive
expression rate of SPARC protein was not statistically significant
between differentiated samples (P>0.05): High differentiation,
57.89% (11/19); moderate differentiation, 65.91% (29/44); and poor
differentiation, 69.23% (18/26).
The positive expression rate of SPARC mRNA was not
statistically significant (P>0.05): High differentiation, 68.42%
(13/19); moderate differentiation, 72.73% (32/44); and poor
differentiation, 73.08% (19/26).
Single factor analysis indicate that tumor stage and
lymph node metastasis were negatively associate with SPARC protein
and SPARC mRNA expression levels (P<0.05). The SPARC protein and
SPARC mRNA expression levels were relatively large in patients with
early stage of tumors and no lymph node metastasis. Multi-factor
analysis indicated that only lymph node metastasis was negatively
correlated with SPARC protein and SPARC mRNA expression levels
(P<0.05).
Discussion
Recent studies have demonstrated the particularly
complicated processes involved in the occurrence and development of
tumors (17,18). It may be caused by the regulation
of cell growth and proliferation (19). In addition, abnormal expression of
tumor-associated genes and aberrant activation of cell signal
transduction may also be involved (20–21).
Cell growth and proliferation in the human body are affected and
controlled by numerous factors (22,23).
Notably, cell signaling proteins, growth factors and their
receptors, apoptotic proteins and transcription factors, and the
changes of these factors are closely associated with the occurrence
and development of tumors (24).
Previous studies have reported high expression
levels of SPARC protein in ESCC (25). Tumor cells that express SPARC in
the nucleus are associated with a higher degree of malignancy
(26). The present study
demonstrated that the SPARC protein was localized in the tumor
stroma, which is consistent with the high expression levels of the
SPARC protein in fibroblasts and endothelial cells during tissue
repair and in aggressive malignant tumors.
The SPARC protein is an important molecule in
locally advanced esophageal carcinoma; however, its association
with the clinical prognosis of esophageal cancer invasion remains
unclear (27,28). The results of the present study
indicated that SPARC protein expression in the tumor stroma aided
the development of esophageal cancer. A study revealed that SPARC
protein expression was not associated with tumor differentiation
and the depth of invasion, but was positively correlated with lymph
node metastasis, and is associated with poor prognosis (29). Porte et al (30) and other studies (31) revealed that the SPARC protein was
not associated with tumor size, lymph node status, tumor adjacent
tissue invasion, disease recurrence and overall survival. The
current study demonstrated that SPARC protein expression in ESCC
was not associated with the degree of differentiation and invasion
depth, and was not linked to tumor location, gross morphology, sex
and age. In contrast to other studies, the current study identified
that the SPARC protein was associated with lymph node metastasis
and tumor stage in patients with ESCC, but it was negatively
correlated. Expression of the SPARC protein in early stage ESCC is
highly expressed, and is not associated with lymph node metastasis.
This inconsistent result reflects the heterogeneity of patients
with ESCC and reveals the complex role of the SPARC protein in the
development of ESCC.
Studies have identified that the high expression
level of SPARC protein in melanoma and prostate cancer promotes
tumor growth and metastasis (32).
However, the SPARC protein may act as an antitumor factor in
pancreatic and colorectal cancer, resulting in anti-angiogenesis,
apoptosis, inhibition of cell proliferation and cell cycle arrest,
thus inhibiting tumor growth (33). In the present study, SPARC protein
expression in patients with ESCC was associated with the survival
prognosis, and the clinical features of the tumor were
significantly associated with survival, differentiation and
staging.
A limitation of the current study was the relatively
small sample size. However, this is one of the larger studies
addressing SPARC protein expression in ESCC. The results of the
current study demonstrated that the expression levels of SPARC in
ESCC tissue samples were significantly higher than those in healthy
esophageal mucosa tissue samples, which may indicate the
association between the occurrence and development of tumors, and
the high expression of SPARC.
In conclusion, the results indicate the potential
role of SPARC in the progression of ESCC. Further research on SPARC
is required to aid the development of novel therapeutic strategies
for ESCC.
Acknowledgements
The present study was supported by the Jiangsu
Pharmaceutical Association (grant no. 201542) and the Science and
Technology commission of Yancheng City (grant no. YK2015002) and
the Youth Medical Talent of Jiangsu Province (grant no.
QNRC2016475).
References
|
1
|
Tustumi F, Takeda FR, Kimura CM, Sallum
RA, Ribeiro U Junior and Cecconello I: Esophageal carcinoma: Is
squamous cell carcinoma different disease compared to
adenocarcinoma? A transversal study in a quaternary high volume
hospital in Brazil. Arq Gastroenterol. 53:44–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu YM, Zhang H, Ni S, Wang J, Li DZ and
Liu SY: Multi-disciplinary treatment increases the survival rate of
late stage pharyngeal, laryngeal or cervical esophageal cancers
treated by free jejunal flap reconstruction after cancer resection.
Zhonghua Zhong Liu Za Zhi. 38:389–394. 2016.(In Chinese).
PubMed/NCBI
|
|
3
|
Huang XE, Wang L, Ji ZQ, Liu MY, Qian T
and Li L: Safety of lienal polypeptide injection combined with
chemotherapy in treating patients with advanced cancer. Asian Pac J
Cancer Prev. 16:7837–7841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jia X, Liu P, Zhang M, Feng T, Tang H,
Tang Z, Zhao H and Jin T: Genetic variants at 6p21, 10q23, 16q21
and 22q12 are associated with esophageal cancer risk in a Chinese
Han population. Int J Clin Exp Med. 8:19381–19387. 2015.PubMed/NCBI
|
|
5
|
Zhang J, Jiang Y, Wu C, Cai S, Wang R,
Zhen Y, Chen S, Zhao K, Huang Y, Luketich J and Chen H: Comparison
of clinicopathologic features and survival between eastern and
western population with esophageal squamous cell carcinoma. J
Thorac Dis. 7:1780–1786. 2015.PubMed/NCBI
|
|
6
|
Nakamura R, Omori T, Takeuchi H, Kawakubo
H, Takahashi T, Wada N, Saikawa Y and Kitagawa Y: Salvage
endoscopic resection as a treatment for locoregional failure or
recurrence following chemoradiotherapy or radiotherapy for
esophageal cancer. Oncol Lett. 11:3631–3636. 2016.PubMed/NCBI
|
|
7
|
Gamboa AM, Kim S, Force SD, Staley CA,
Woods KE, Kooby DA, Maithel SK, Luke JA, Shaffer KM, Dacha S, et
al: Treatment allocation in patients with early-stage esophageal
adenocarcinoma: Prevalence and predictors of lymph node
involvement. Cancer. 122:2150–2157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho JW: The role of endosonography in the
staging of gastrointestinal cancers. Clin Endosc. 48:297–301. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anandarajah EM, Ditgen D, Hansmann J,
Erttmann KD, Liebau E and Brattig NW: SPARC (secreted protein
acidic and rich in cysteine) of the intestinal nematode
Strongyloides ratti is involved in mucosa-associated parasite-host
interaction. Mol Biochem Parasitol. 207:75–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi D, Jiang K, Fu Y, Fang R, Liu XI and
Chen J: Overexpression of SPARC correlates with poor prognosis in
patients with cervical carcinoma and regulates cancer cell
epithelial-mesenchymal transition. Oncol Lett. 11:3251–3258.
2016.PubMed/NCBI
|
|
11
|
Rossi MK, Gnanamony M and Gondi CS: The
‘SPARC’ of life: Analysis of the role of osteonectin/SPARC in
pancreatic cancer (Review). Int J Oncol. 48:1765–7871.
2016.PubMed/NCBI
|
|
12
|
Rosset EM and Bradshaw AD:
SPARC/osteonectin in mineralized tissue. Matrix Biol. 52–54:78–87.
2016. View Article : Google Scholar
|
|
13
|
Notaro A, Sabella S, Pellerito O, Vento R,
Calvaruso G and Giuliano M: The secreted protein acidic and rich in
cysteine is a critical mediator of cell death program induced by
WIN/TRAIL combined treatment in osteosarcoma cells. Int J Oncol.
48:1039–1044. 2016.PubMed/NCBI
|
|
14
|
Tseng C and Kolonin MG: Proteolytic
Isoforms of SPARC induce adipose stromal cell mobilization in
obesity. Stem Cells. 34:174–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim H, Samuel S, Lopez-Casas P, Grizzle W,
Hidalgo M, Kovar J, Oelschlager D, Zinn K, Warram J and Buchsbaum
D: SPARC-independent delivery of Nab-Paclitaxel without depleting
tumor stroma in patient-derived pancreatic cancer xenografts. Mol
Cancer Ther. 15:680–688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaz J, Ansari D, Sasor A and Andersson R:
SPARC: A potential prognostic and therapeutic target in pancreatic
cancer. Pancreas. 44:1024–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mattina J, MacKinnon N, Henderson VC,
Fergusson D and Kimmelman J: Design and reporting of targeted
anticancer preclinical studies: A meta-analysis of animal studies
investigating sorafenib antitumor efficacy. Cancer Res.
76:4627–4636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huo Y, Su T, Cai Q and Macara IG: An in
vivo gain-of-function screen identifies the Williams-Beuren
Syndrome Gene GTF2IRD1 as a mammary tumor promoter. Cell Rep.
15:2089–2096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee J, Katzenmaier EM, Kopitz J and Gebert
J: Reconstitution of TGFBR2 in HCT116 colorectal cancer cells
causes increased LFNG expression and enhanced
N-acetyl-d-glucosamine incorporation into Notch1. Cell Signal.
28:1105–1113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao R, Ma LQ, Du X, Zhang TT, Zhao L, Liu
L, Liu JC, Guo F, Cheng Z and Huang H: Rnf25/AO7 positively
regulates wnt signaling via disrupting Nkd1-Axin inhibitory complex
independent of its ubiquitin ligase activity. Oncotarget.
7:23850–23859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang M, Yuan J, Peng C and Li Y: Collagen
as a double-edged sword in tumor progression. Tumour Biol.
35:2871–2882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai J, Zhang X, Hu K, Liu B, Wang H, Li A,
Lin F, Zhang L, Sun X, Du Z and Song J: Silencing DNA
methyltransferase 1 (DNMT1) inhibits proliferation, metastasis and
invasion in ESCC by suppressing methylation of RASSF1A and DAPK.
Oncotarget. 7:44129–44141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu JY, Lu JB and Xu Y: MicroRNA-153
inhibits the proliferation and invasion of human laryngeal squamous
cell carcinoma by targeting KLF5. Exp Ther Med. 11:2503–2508.
2016.PubMed/NCBI
|
|
24
|
Lin W, Zhong M, Yin H, Chen Y, Cao Q, Wang
C and Ling C: Emodin induces hepatocellular carcinoma cell
apoptosis through MAPK and PI3K/AKT signaling pathways in vitro and
in vivo. Oncol Rep. 36:961–967. 2016.PubMed/NCBI
|
|
25
|
Che Y, Luo A, Wang H, Qi J, Guo J and Liu
Z: The differential expression of SPARC in esophageal squamous cell
carcinoma. Int J Mol Med. 17:1027–1033. 2006.PubMed/NCBI
|
|
26
|
Xue LY, Zou SM, Zheng S, Liu XY, Wen P,
Yuan YL, Lin DM and Lu N: Expressions of the γ2 chain of laminin-5
and secreted protein acidic and rich in cysteine in esophageal
squamous cell carcinoma and their relation to prognosis. Chin J
Cancer. 30:69–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagaraju GP and Sharma D: Anti-cancer role
of SPARC, an inhibitor of adipogenesis. Cancer Treat Rev.
37:559–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zinovyeva MV, Monastyrskaya GS, Kopantzev
EP, Vinogradova TV, Kostina MB, Sass AV, Filyukova OB, Uspenskaya
NY, Sukhikh GT and Sverdlov ED: Identification of some human genes
oppositely regulated during esophageal squamous cell carcinoma
formation and human embryonic esophagus development. Dis Esophagus.
23:260–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jakharia A, Borkakoty B and Singh S:
Expression of SPARC like protein 1 (SPARCL1), extracellular
matrix-associated protein is down regulated in gastric
adenocarcinoma. J Gastrointest Oncol. 7:278–283. 2016.PubMed/NCBI
|
|
30
|
Porte H, Triboulet JP, Kotelevets L,
Carrat F, Prévot S, Nordlinger B, DiGioia Y, Wurtz A, Comoglio P,
Gespach C and Chastre E: Overexpression of stromelysin-3,
BM-40/SPARC, and MET genes in human esophageal carcinoma:
Implications for prognosis. Clin Cancer Res. 4:1375–1382.
1998.PubMed/NCBI
|
|
31
|
Hong Y, Zhang J, Zhang H, Li X, Qu J, Zhai
J, Zhang L, Chen F and Li T: Heterozygous PTCH1 mutations impact
the bone metabolism in patients with nevoid basal cell carcinoma
syndrome likely by regulating SPARC expression. J Bone Miner Res.
31:1413–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kao SC, Kirschner MB, Cooper WA, Tran T,
Burgers S, Wright C, Korse T, van den Broek D, Edelman J, Vallely
M, et al: A proteomics-based approach identifies secreted protein
acidic and rich in cysteine as a prognostic biomarker in malignant
pleural mesothelioma. Br J Cancer. 114:524–531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slusser-Nore A, Larson-Casey JL, Zhang R,
Zhou XD, Somji S, Garrett SH, Sens DA and Dunlevy JR: SPARC
expression is selectively suppressed in tumor initiating urospheres
isolated from As+3- and Cd+2-transformed human urothelial cells
(UROtsa) stably transfected with SPARC. PLoS One. 11:e01473622016.
View Article : Google Scholar : PubMed/NCBI
|