Introduction
Comitant strabismus (CS) is a common form of
strabismus characterized ocular motility disorders, which may lead
to compromised binocular vision and amblyopia (1,2). The
birth occurrence of infantile esotropia is ~25 per 10,000 in the
United States (3). Strabismus is
characterized by a constant angle deviation in many directions of
the gaze. Clinically, CS is also associated with stereopsis
impairment (4).
Previous research revealed the abnormal brain
function in strabismus patients. A study exhibited that there had
reduced functional connectivity between the V1 and V2 areas in
monkeys with strabismic amblyopia (5). Another study reported that there is
increased mean diffusivity of the occipital tracts in patients with
strabismic amblyopia (6).
Additionally, a previous study demonstrated that patients with
strabismus were associated with suppression in the primary visual
cortex (7). Although the
abovementioned studies have demonstrated functional changes in the
neurons of patients with strabismus, the changes in brain
anatomical morphology in CS remain unknown.
Voxel-based morphometry (VBM) is a whole-brain
measurement method that compares voxel-wise between groups
morphological differences in the brain (8). The VBM method has been successfully
used to assess neural mechanisms of nervous and mental diseases
such as Alzheimer's disease (9),
optic neuritis (10) and
schizophrenia (11). Using the VBM
method, a previous study indicated that the lower GMV values were
located in the brain regions of the occipital eye field and
parietal eye field in patients with strabismus amblyopia (12). In the present study, the authors
demonstrated that there were many brain regions with a dysfunction
in neural activity in patients with CS using regional homogeneity
methods. However, there were far less evidence for
neuromorphological changes. To the best of the authors' knowledge,
the current study is the first to use the VBM approach to detect
changes in volumes of the GMV and WMV of CS patients.
Materials and methods
Subjects
A total of 20 patients with CS (10 males and 10
females) were recruited from the First Affiliated Hospital of
Nanchang University Hospital (Nanchang, China). The criteria of the
study on CS were as follows: i) Strabismus at birth; ii) strabismus
with impairment to stereopsis and visual fusion; iii) equal
binocular best corrected vision; iv) the a range of squint angle is
50–60 delta.
Patients with following conditions were excluded
from the study: i) Acquired strabismus, incomitant strabismus; ii)
conditions due to eye diseases (infection, inflammation, ischemic
diseases), iii) patients with eye surgery; iv) psychiatric
disorders cardiovascular diseases, cerebral infarction diseases
such as systemic disorders; v) alcohol or drug addiction.
A total of 20 HCs (10 males and 10 females) with
matched age, sex, education status were also recruited for the
study. All HCs met the following criteria: i) No abnormalities in
brain parenchyma with head magnetic resonance imaging (MRI); ii)
without any eye diseases and the corrected visual acuity (VA)
>1.0; iii) no nervous system diseases; iv) no external
accessories that interfere with the magnetic resonance imaging
signal (such as cardiac pacemaker or metal device).
All the research contents and methods followed the
Declaration of Helsinki. All volunteers were informed of the
purposes, methods and potential risks before signing an informed
consent form. The study was approved by the medical ethics
committee of the First Affiliated Hospital of Nanchang University
Hospital (Nanchang, China).
MRI parameters
All subjects were performed with a 3-Tesla MR
scanner (Siemens AG, Munich, Germany) with an 8-channel.
High-resolution T1-weighted images were obtained with a
magnetization-prepared rapid gradient echo (MP-RAGE) sequence. The
details of scanning parameters are as follows: Slices=176; section
thickness=1. 0 mm; echo time=2.26 msec; repetition time=1,900 msec;
field of view=215×230 mm.
VBM analysis
Structural images were classified by MRIcro software
(version, 1.40; build, 1; www.MRIcro.com) to eliminate incomplete data, and then
processed with the voxel-based morphometry toolbox (VBM8)
(dbm.neuro.uni-jena.de/vbm8) implemented in Statistical Parametric
Mapping software (version, 8.0; Wellcome Trust Centre for
Neuroimaging, London, UK). All procedures were performed with
MATLAB (version, 7.9.0; The Mathworks, Inc. Natick, MA, USA).
Individual brain images was segregated into gray matter, white
matter and cerebrospinal fluid based on the VBM8 toolbox. More
details are presented in a previous study of the authors (10).
Statistical analysis
General linear model analysis was performed with the
SPM8 toolkit to investigate the group differences in GMV and WMV
between CS groups and HCs. P<0.05 was considered to indicate a
statistically significant difference. Voxel threshold was set to 20
neighboring voxels to analysis the area of gray matter changes in
CS.
Brain-behavior correlation
analysis
With the VBM findings, different brain regions were
classified as regions of interests (ROIs) using REST software
(version, 1.8; www.resting-fmri.Sourceforge.net). For each ROI, the
mean GMV or WMV value was extracted by averaging the GMV or WMV
values over all voxels. Finally, the relationship between the mean
GMV value in different brain regions in the CS group and the
clinical manifestations were investigated using correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Clinical data analysis
All clinical data of the CS patients were collected,
including the onset of CS disease, and best-corrected VA.
Results
General data analysis
Compared with HCs and strabismus groups, the authors
did not find marked differences in weight (P=0.918), age (P=0.344),
best-corrected VA-Right (P=0.814) and best-corrected VA-Left
(P=0.903; Table I).
 | Table I.Demographic information and clinical
measurements for CS and HCs. |
Table I.
Demographic information and clinical
measurements for CS and HCs.
|
| CS | HCs | t-value | P-values |
|---|
| Male/Female | 10/10 | 10/10 | N/A | >0.99 |
| Age (years) | 30.40±10.44 | 27.45±8.99 | 0.957 | 0.344 |
| Weight (kg) | 60.25±6.46 | 60.05±5.77 | 0.103 | 0.918 |
| Handedness (n,
hand) | 20, right | 20, right | N/A | >0.99 |
| Exotropic and
esotropic (n) | 5/15 | N/A | N/A | N/A |
| Duration of
strabismus (years) | 26.95±9.05 | N/A | N/A | N/A |
| Best-corrected
VA-right | 1.07±0.18 | 1.09±0.22 | −0.237 | 0.814 |
| Best-corrected
VA-left | 1.02±0.09 | 1.03±0.16 | −0.123 | 0.903 |
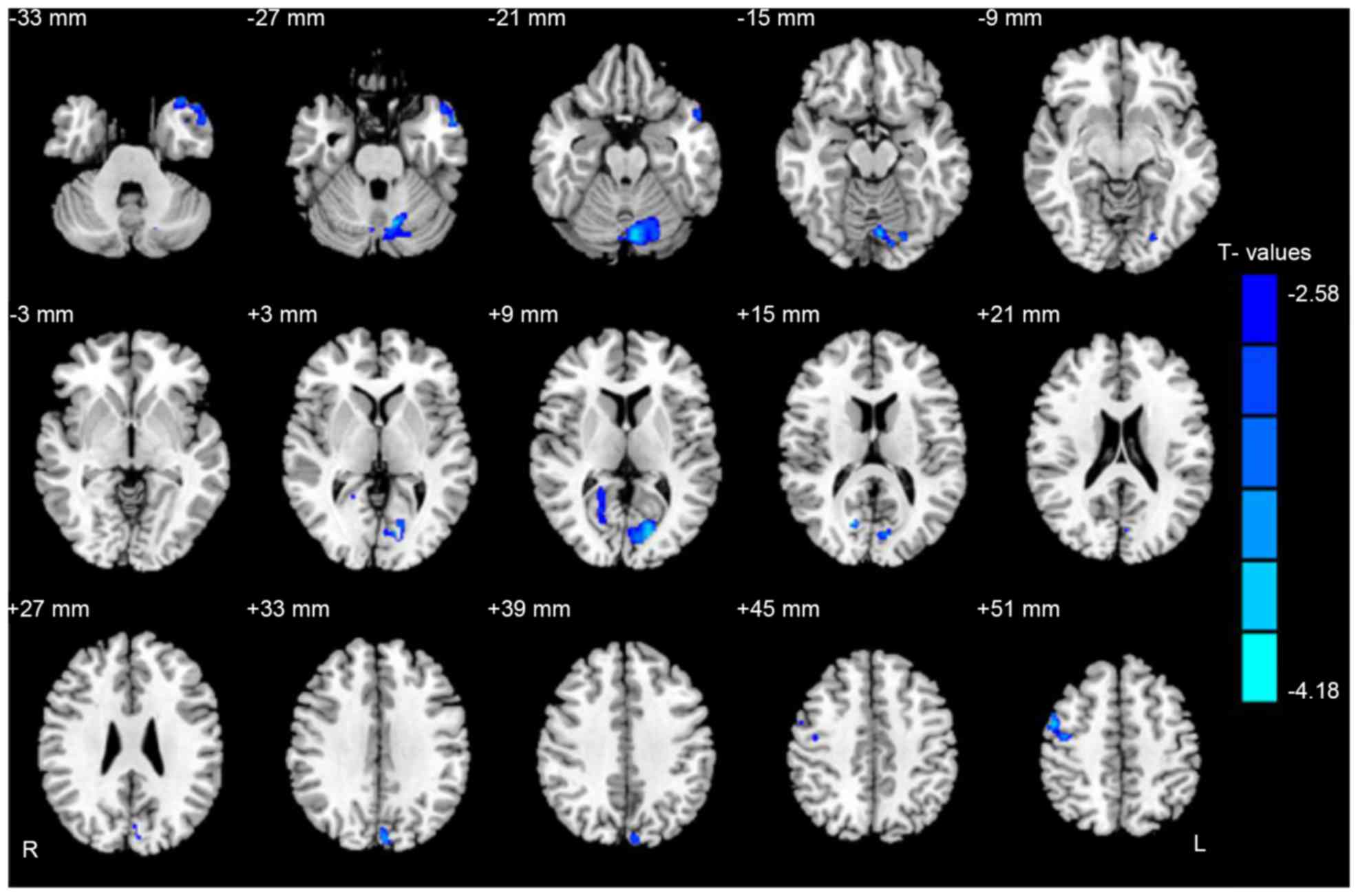
Gray and white matter differences
Compared with HCs, CS groups had significantly lower
GMV in the brain regions of the left middle temporal pole, left
cerebellum posterior lobe, right posterior cingulate cortex, left
cuneus and right premotor cortex (Fig.
1 and Table II).
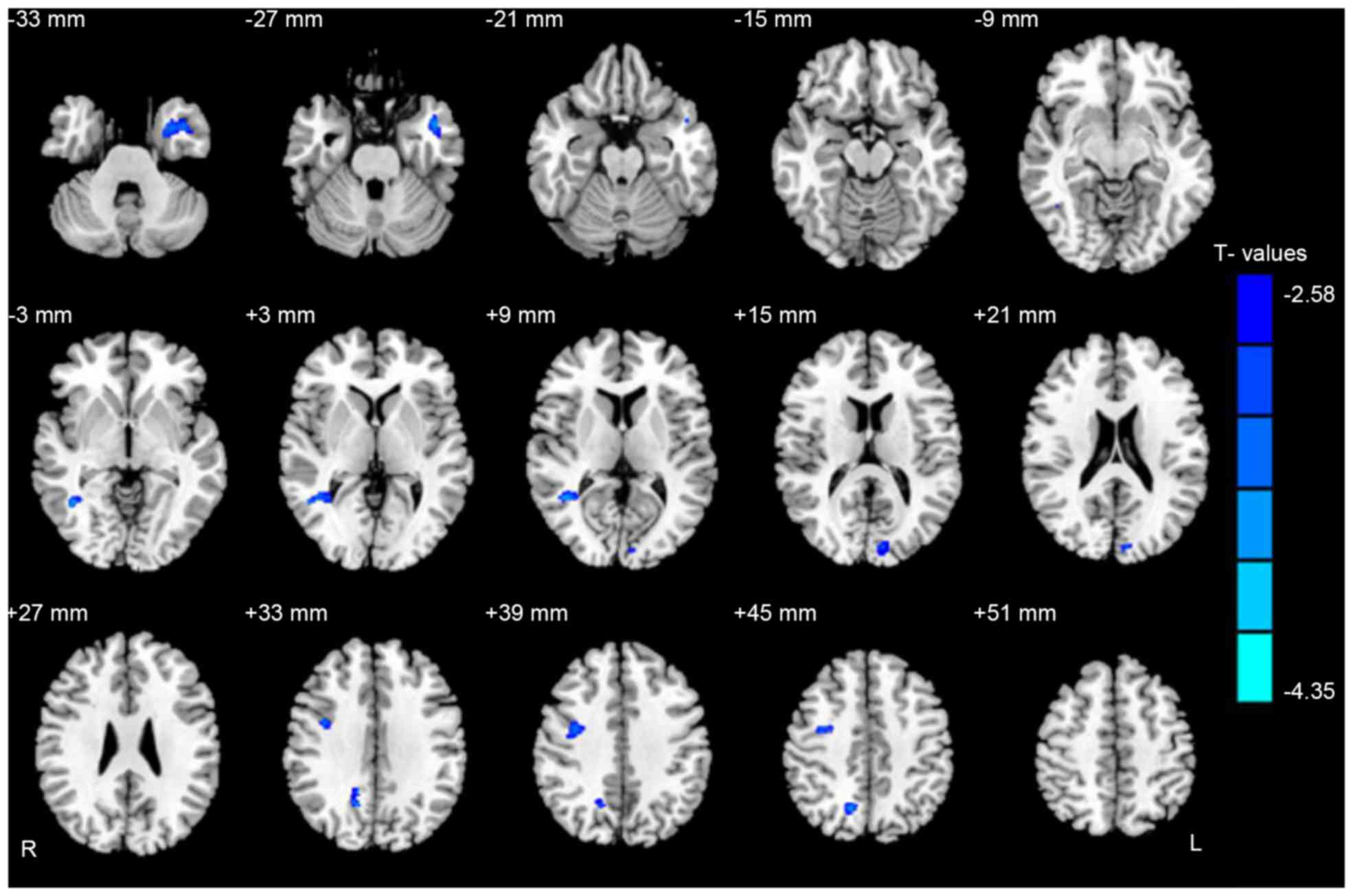
Additionally, CS patients had significantly lower WMV in the brain
regions of the left middle temporal gyrus, right middle temporal
gyrus, right precuneus and right premotor cortex (Fig. 2 and Table III). Moreover, the authors
demonstrated the mean of altered GMV and WMV between the two groups
(Fig. 3).
 | Table II.Brain regions with significant
differences in GMV between CS group and HCs. |
Table II.
Brain regions with significant
differences in GMV between CS group and HCs.
|
| CS group and healthy
controls | MNI coordinates |
|---|
|
|
|
|
|---|
| GMV | Brain areas | BA | Peak T values | x | y | z |
|---|
| CS< HC |
|
|
|
|
|
|
|
| 1. | Left middle
temporal pole | 21,38 | −4.179 | 1,077 | −33 | 12 | −37.5 |
| 2. | Left cerebellum
posterior lobe | – | −4.204 | 1,461 | −4.5 | −76.5 | −18 |
| 3. | Right posterior
cingulate cortex | 18,30 | −3.692 |
342 | 16.5 | −72 | 15 |
| 4. | Left cuneus | 17,18,19 | −3.871 | 1,034 | −13.5 | −78 | 6 |
| 5. | Right premotor
cortex | 6 | −3.752 |
348 | 3 | −42 | 48 |
 | Table III.Brain regions with significant
differences in WMV between CS group and HCs. |
Table III.
Brain regions with significant
differences in WMV between CS group and HCs.
|
| CS group and
healthy controls | MNI
coordinates |
|---|
|
|
|
|
|---|
| WMV | Brain areas | BA | Peak T values | x | y | z |
|---|
| CS<HC |
|
|
|
|
|
|
|
| 1. | Left middle
temporal gyrus 1 | 21,38 | −3.569 |
657 | −43.5 | 4.5 | −25.5 |
| 2. | Right middle
temporal gyrus | 39 | −4.345 |
424 | 42 | −52.5 | 7.5 |
| 3. | Left middle
temporal gyrus 2 | 18 | −3.420 |
222 | −7.5 | −90 | 12 |
| 4. | Right
precuneus | 7 | −3.871 | 1,034 | −13.5 | −78 | 6 |
| 5. | Right premotor
cortex | 6 | −3.752 |
348 | 3 | −42 | 48 |
Correlation analysis
In the CS groups, it was observed that the duration
of comitant strabismus negatively correlated with the GMV values of
the left middle temporal pole (r=−0.486, P=0.030; Fig. 4).
Receiver operating characteristic
(ROC) curve
The authors assumed that the differences of the WMV
and GMV values in two groups may be useful diagnostic markers. The
mean values of the WMV and GMV in different brain regions were
extracted and used to analyze ROC curves. The areas under the ROC
for GMV values were: The left middle temporal pole (0.795), the
left cerebellum posterior lobe (0.835), the right posterior
cingulate cortex (0.790), the left cuneus (0.870) and the right
premotor cortex (0.830; Fig. 5A).
The AUCs for WMV values were: The left middle temporal gyrus 1
(0.810), the right middle temporal gyrus (0.818), the left middle
temporal gyrus 2 (0.823), the right precuneus (0.800) and the right
premotor cortex (0.835; Fig.
5B).
 | Figure 5.ROC curve analysis of the mean GMV and
WMV values for altered brain regions. Note: (A) The areas under the
ROC curve (AUCs) for GMV values: Left MTP, 0.795; left CP, 0.835;
right PCC, 0.790; left CN, 0.870; right PC, 0.830. (B) The areas
under the ROC curve (AUCs) for WMV values were: Left MT1, 0.810;
right MT, 0.818; left MT2, 0.823; right P, 0.800; right PC, 0.835.
ROC, receiver operating characteristic; GMV, grey matter volume;
WMV, white matter volume; MTP, middle temporal pole; CP, cerebellum
posterior lobe; PCC, posterior cingulate cortex; CN, cuneus; PC,
premotor cortex; MT, middle temporal gyrus; P, precuneus. |
Discussion
To the best of the authors' knowledge, the current
study is the first to evaluate changes in WMV and GMV in patients
with CS using a VBM approach. They identified a remarkable decrease
in GMV values in the brain regions of the left middle temporal
pole, left cerebellum posterior lobe, right posterior cingulate
cortex, left cuneus and right premotor cortex in patients with CS.
Meanwhile, WMV values in the brain regions of the left middle
temporal gyrus, right middle temporal gyrus, right precuneus and
right premotor cortex were also significantly reduced in CS
patients. Furthermore, the authors observed that the duration of
comitant strabismus demonstrated a negative correlation with the
GMV values of the left middle temporal pole.
The middle temporal gyrus is located between the
superior temporal gyrus and inferior temporal gyrus, a region
responsible for three-dimensional surface orientation and retinal
image velocities (13). A previous
study reported that the middle temporal gyrus contains a
rudimentary representation of three-dimensional surface orientation
(14). Additionally, the middle
temporal gyrus has been suggested to encode three-dimensional
motion (15). It has been well
known that patients with CS often are accompanied with dysfunction
of fusion and stereopsis (16).
Yan et al (17) observed
that patients with there had decreased WMV in the right inferior
temporal gyrus in comitant exotropia. Duan et al (18) found that strabismic amblyopia
indeed affected mean diffusion, not only in the occipital tracts
but also the association tracts connecting the visual cortex to the
frontal and temporal lobes. In agreement with these findings, the
authors identified significantly decreased GMV values in the brain
regions of the left middle temporal pole. Furthermore, the mean
area of the GMV of the left middle temporal pole was 0.795. There
were significantly reduced WMV values in the bilateral middle
temporal gyrus in the patients with CS, whereas that of the WMV of
the left middle temporal gyrus 1 was 0.810, the right middle
temporal gyrus was 0.818 and the left middle temporal gyrus 2 was
0.823. It was demonstrated that there was both white matter and
gray matter atrophy in CS patients. Therefore, the authors
speculated that the CS possibly led to the atrophy of the temporal
gyrus, which may reflect the impairment of the visual fusion in CS
patients. Furthermore, the duration of CS presented a negative
correlation with the GMV values of the left middle temporal pole
(r=−0.486, P=0.030). This suggests that a significant atrophy of
the left middle temporal pole occur in the lingual gyrus during the
early phase in CS.
The cerebellum is involved in the execution of
accurate eye movements (19). A
previous study reported that the cerebellum is responsible for the
execution of eye movements (20).
Other research reported that cerebellar vermis activation is
related to visually guided saccades (21). Joshi et al (22) reported that the posterior
interposed nucleus in the cerebellum is involved in eye movements
in strabismic monkeys (22).
Consistent with these findings, CS patients were observed to have
significantly decreased GMV in the left cerebellum posterior lobe.
The area under ROC curves of GMV of the left cerebellum posterior
lobe was 0.835. The authors therefore concluded that the CS may
associate with deficits in the left cerebellum posterior lobe,
reflecting eye movement damages.
Primary motor cortex, located in the primate frontal
cortex (23), has been strongly
implicated in motor processing (24). Previous studies have demonstrated
the relationship between premotor cortex and the ocular movement
(25,26). As reported, the FEF is located in
the posterior part of the middle frontal gyrus (27). A previous study demonstrated that
the FEF is involved in the saccade selection and execution
(28). Additionally, the FEF was
also involved in sustained attention (29). Yan et al (17) found that WMV values were reduced in
the right frontal lobe/sub-gyral in patients with comitant
exotropia. In support of these findings, the authors also reported
a significantly decreased WMV in the brain regions of the premotor
cortex in CS patients. Furthermore, the CS patients presented a
significantly decreased GMV in the regions of the premotor cortex.
They came to the conclusion that the CS may lead to the atrophy of
the premotor cortex, which may reflect the impaired oculomotor in
the CS patients.
The precuneus is located in the rear region between
the somatosensory cortex and forward of the cuneus between the two
cerebral hemispheres. The precuneus is not only involved in the
interwoven network of the self-consciousness (30), but also involved in different
aspects of visuospatial mental operations (31). A previous study suggested that the
bold signal was increased in the left cingulate gyrus, bilateral
precuneus and left angular gyrus in the patients with infantile
esotropia (32). In the present
study, significantly decreased GMV values were observed in the
brain regions of the left cuneus in CS patients. Meanwhile, CS
patients had decreased WMV values in the brain regions of the right
precuneus. The authors speculated that the CS may lead to the
dysfunction of the right precuneus and the left cuneus.
The present study indicated that CS patients had
decreased GMV and WMV in the different regions of the brain, which
may give much important information to explain the underlying
neural mechanisms of the fusion defects and ocular motility
disorders in CS patients.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81160118, 81460092
and 81400372), the Jiangxi Province Voyage Project (grant no.
2014022), the Natural Science Key Project of Jiangxi Province
(grant no. 20161ACB21017), the Youth Science Foundation of Jiangxi
Province (grant no. 20151BAB215016), the Technology and Science
Foundation of Jiangxi Province (grant no. 20151BBG70223).
References
|
1
|
Adams DL, Economides JR and Horton JC:
Contrasting effects of strabismic amblyopia on metabolic activity
in superficial and deep layers of striate cortex. J Neurophysiol.
113:3337–3344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louwagie CR, Diehl NN, Greenberg AE and
Mohney BG: Is the incidence of infantile esotropia declining?: A
population-based study from Olmsted County, Minnesota, 1965 to
1994. Arch Ophthalmol. 127:200–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koc F, Erten Y and Yurdakul NS: Does
restoration of binocular vision make any difference in the quality
of life in adult strabismus. Br J Ophthalmol. 97:1425–1430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iordanous Y, Mao A and Makar I:
Preoperative factors affecting stereopsis after surgical alignment
of acquired partially accommodative esotropia. Strabismus.
23:151–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bi H, Zhang B, Tao X, Harwerth RS, Smith
EL 3rd and Chino YM: Neuronal responses in visual area V2 (V2) of
macaque monkeys with strabismic amblyopia. Cereb Cortex.
21:2033–2045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan Y, Norcia AM, Yeatman JD and Mezer A:
The Structural properties of major white matter tracts in
strabismic amblyopia. Invest Ophthalmol Vis Sci. 56:5152–5160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen VJ and Tarczy-Hornoch K: Functional
magnetic resonance imaging of binocular interactions in visual
cortex in strabismus. J Pediatr Ophthalmol Strabismus. 48:366–374.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashburner J and Friston KJ: Voxel-based
morphometry-the methods. Neuroimage. 11:805–821. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimoda K, Kimura M, Yokota M and Okubo Y:
Comparison of regional gray matter volume abnormalities in
Alzheimer's disease and late life depression with hippocampal
atrophy using VSRAD analysis: A voxel-based morphometry study.
Psychiatry Res. 232:71–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang X, Zhang Q, Hu PH, Zhong YL, Zhang
Y, Wei R, Xu TT, Shao Y, et al: Oculopathy fMRI study group: White
and gray matter volume changes and correlation with visual evoked
potential in patients with optic Neuritis: A voxel-based
morphometry study. Med Sci Monit. 22:1115–1123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim GW and Jeong GW: White matter volume
change and its correlation with symptom severity in patients with
schizophrenia: A VBM-DARTEL study. Neuroreport. 26:1095–1100.
2015.PubMed/NCBI
|
|
12
|
Chan ST, Tang KW, Lam KC, Chan LK, Mendola
JD and Kwong KK: Neuroanatomy of adult strabismus: A voxel-based
morphometric analysis of magnetic resonance structural scans.
Neuroimage. 22:986–994. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyenkim JD and DeAngelis GC:
Disparity-based coding of three-dimensional surface orientation by
macaque middle temporal neurons. J Neurosci. 23:7117–7128.
2003.PubMed/NCBI
|
|
14
|
Sanada TM, Nguyenkim JD and Deangelis GC:
Representation of 3-D surface orientation by velocity and disparity
gradient cues in area MT. J Neurophysiol. 107:2109–2122. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Czuba TB, Huk AC, Cormack LK and Kohn A:
Area MT encodes three-dimensional motion. J Neurosci.
34:15522–15533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng X, Zhang X and Jia Y: Improvement in
fusion and stereopsis following surgery for intermittent exotropia.
J Pediatr Ophthalmol Strabismus. 52:52–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan X, Lin X, Wang Q, Zhang Y, Chen Y,
Song S and Jiang T: Dorsal visual pathway changes in patients with
comitant extropia. PLoS One. 5:e109312010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duan Y, Norcia AM, Yeatman JD and Mezer A:
The structural properties of major white matter tracts in
strabismic amblyopia. Invest Ophthalmol Vis Sci. 56:5152–5160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herzfeld DJ, Kojima Y, Soetedjo R and
Shadmehr R: Encoding of action by the purkinje cells of the
cerebellum. Nature. 526:439–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nitschke MF, Arp T, Stavrou G, Erdmann C
and Heide W: The cerebellum in the cerebro-cerebellar network for
the control of eye and hand movements-an fMRI study. Prog Brain
Res. 148:151–164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayakawa Y, Nakajima T, Takagi M, Fukuhara
N and Abe H: Human cerebellar activation in relation to saccadic
eye movements: A functional magnetic resonance imaging study.
Ophthalmologica. 216:399–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joshi AC and Das VE: Muscimol inactivation
of caudal fastigial nucleus and posterior interposed nucleus in
monkeys with strabismus. J Neurophysiol. 110:1882–1891. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boussaoud D and Wise SP: Primate frontal
cortex: Neuronal activity following attentional versus intentional
cues. Exp Brain Res. 95:15–27. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen L and Alexander GE: Neural correlates
of a spatial sensory-to-motor transformation in primary motor
cortex. J Neurophysiol. 77:1171–1194. 1997.PubMed/NCBI
|
|
25
|
Halsband U, Matsuzaka Y and Tanji J:
Neuronal activity in the primate supplementary, pre-supplementary
and premotor cortex during externally and internally instructed
sequential movements. Neurosci Res. 20:149–155. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boussaoud D: Primate premotor cortex:
Modulation of preparatory neuronal activity by gaze angle. J
Neurophysiol. 73:886–890. 1995.PubMed/NCBI
|
|
27
|
Blanke O, Spinelli L, Thut G, Michel CM,
Perrig S, Landis T and Seeck M: Location of the human frontal eye
field as defined by electrical cortical stimulation: Anatomical,
functional and electrophysiological characteristics. Neuroreport.
11:1907–1913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fernandes HL, Stevenson IH, Phillips AN,
Segraves MA and Kording KP: Saliency and saccade encoding in the
frontal eye field during natural scene search. Cereb Cortex.
24:3232–3245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esterman M, Liu G, Okabe H, Reagan A, Thai
M and DeGutis J: Frontal eye field involvement in sustaining visual
attention: Evidence from transcranial magnetic stimulation.
Neuroimage. 111:542–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cavanna AE and Trimble MR: The precuneus:
A review of its functional anatomy and behavioural correlates.
Brain. 129:564–583. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oshio R, Tanaka S, Sadato N, Sokabe M,
Hanakawa T and Honda M: Differential effect of double-pulse TMS
applied to dorsal premotor cortex and precuneus during internal
operationof visuospatial information. Neuroimage. 49:1108–1115.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang X, Zhang J, Lang L, Gong Q and Liu L:
Assessment of cortical dysfunction in infantile esotropia using
fMRI. Eur J Ophthalmol. 24:409–416. 2014. View Article : Google Scholar : PubMed/NCBI
|