Introduction
Pulmonary hypertension (PH) is defined as a mean
pulmonary artery pressure (mPAP) ≥25 mmHg, measured via right heart
catheter at rest. PH may be fatal, and the natural course of
idiopathic pulmonary artery hypertension (PAH) is 2.5–3.4 years
following diagnosis (1). The
pathogenesis of PAH is not completely clear, and appears to be
primarily associated with heredity and immune-based pulmonary
vascular remodeling (PVR) (2,3).
Accepted therapeutic approaches for PAH primarily target
vasoconstriction, intimal injury or cardiac dysfunction. However,
PVR must be reversed in order to cure PAH.
Stimuli which may lead to vascular remodeling
include transmural pressure, mechanical stretching or hypoxia, and
repeated insults from these damaging factors cause a state of
oxidative stress; the production of reactive oxygen species (ROS)
is increased and ROS clearance is decreased, thereby inducing
oxidative injury (4). reduced
nicotinamide adenine dinucleotide phosphate oxidase (Nox) is one of
the primary sources of ROS, and alterations in its expression have
been demonstrated to be associated with pulmonary vascular disease
and the survival of patients with PAH (2). The experimental model of
hypoxia-induced pulmonary hypertension has been widely-used, but
few studies have used the monocrotaline-induced pulmonary
hypertension (MCT-PH) model to study Nox-derived ROS (4). The PAH-associated Nox family of
proteins includes Nox1 and Nox4, although conflicting reports exist
on the roles of Nox1 and Nox4 in the occurrence and development of
PAH (5,6). Previous studies have used different
cell and tissue samples; therefore, the present study analyzed the
Nox1 and Nox4 expression levels in lung tissue homogenate and
pulmonary artery smooth muscle cells (PASMCs), aiming to
investigate whether the differences observed in regulatory
mechanisms were caused by different samples, modeling methods or
pathogenic mechanisms.
Previous studies into PAH have focused on the
antioxidant enzyme superoxide dismutase (SOD), the role of which is
to catalyze the conversion of O2− to
O2 and H2O. During this process, two
molecules of glutathione (GSH) are oxidized (7). N-acetylcysteine (NAC) is a synthetic
precursor of GSH, which exhibits antioxidant properties and has
been clinically applied for the treatment of idiopathic pulmonary
fibrosis and ischemia reperfusion injury (8–10). A
previous study demonstrated that NAC was able to improve PH and
right ventricular function via immune regulatory mechanisms
(11). Therefore, it was
hypothesized that NAC may protect against oxidation, thereby
alleviating MCT-induced pulmonary vascular and right heart
diseases. The possible mechanisms underlying the role of NAC in
treating PAH were investigated in the present study.
Materials and methods
Grouping and establishment of PH rat
model
A total of 18 8-week-old male Wistar rats (Central
Laboratory, Affiliated Hospital of Qingdao University, Qingdao,
China), weighing 150–180 g, were randomly divided into three groups
(n=6): Rats in the control group received one intraperitoneal
injected with 60 mg kg−1 saline; those in the MCT group
were intraperitoneally injected once with 60 mg kg−1 MCT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); and those in the
NAC group intraperitoneally injected once with 60 mg
kg−1 MCT, and orally administered 500 mg kg−1
d−1 NAC (Sigma-Aldrich; Merck KGaA). The control and MCT
groups were orally administered 500 mg kg−1
d−1 saline. All of the rats were fed under the same
conditions (temperature 22±2°C, 12-h light/dark cycle and food and
water ad libitum) and medicated for 6 weeks. The right
ventricular systolic pressure (RVSP) and mPAP of each rat was
subsequently measured using a right heart catheterized polygraph
(AD Instruments Pty., Ltd., Bella Vista, Australia). The present
study was performed in accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health (Bethesda, MA, USA). The animal use protocol
was reviewed and approved by the Institutional Animal Care and Use
Committee of Qingdao University.
Tissue and cell preparation
The rats were sacrificed, and the pulmonary arteries
were isolated. Following rinsing with saline at 37°C, the upper
left lung lobe was fixed for 12 h in 10% neutral formalin, followed
by paraffin-embedding and slicing; PASMCs were isolated from the
lower left lung lobe using D-Hank's solution (Hefei Bomei
Biotechnology Co., Ltd., Hefei, China). The right lung was rapidly
frozen in liquid nitrogen prior to extraction of RNA and proteins.
The heart was removed, and the right ventricle (RV) and left
ventricle plus septum (LV + S) were separated for weighing.
Following rinsing in PBS, the distal pulmonary
artery was separated from the lower left lung lobe and the PASMCs
were purified to produce a cell suspension. The first generation
was identified using immunofluorescence staining of smooth muscle
actin with polyclonal anti-α-SMA antibody (A03744; 1:1,000; Wuhan
Boster Biological Technology, Wuhan, China) incubated overnight at
4°C, and the second generation was used for subsequent experiments.
The obtained PASMCs was divided into three groups: Group C,
isolated from the saline-treatment group; Group M, isolated from
the MCT-treatment group; and group N, isolated from the
NAC-treatment group. Groups C and M were sub-divided: Subgroups C1
and M1 (control), pretreated with an equal volume of PBS solution
for 24 h; subgroups C2 and M2, pretreated with 100 µmol
l−1 ML171 (Merck KGaA) for 24 h. Group N was not
subdivided (blank control).
Dihydroethidium (DHE) fluorescence
staining
The cryopreserved lung tissue was embedded in
optimal cutting temperature compound (Beijing Kangwei Century
Biological Technology Ltd., Beijing, China), followed by sectioning
at 20 µm at −20°C and DHE fluorescence staining (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) at 37°C for 1 h. A fluorescence
microscope (BX51; Olympus Corporation, Tokyo, Japan) was used for
the observation (magnification, ×400), and the images were analyzed
using Image Pro Plus software version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Observation of pulmonary vascular
structures
Each paraffin block of rat lung tissue was cut into
3-µm-thick sections, followed by xylene dewaxing and graded alcohol
rehydration for hematoxylin and eosin (HE) staining (25°C for 10
min). Each section was observed in three visual fields with a
fluorescence microscope, and 10 antero-capillary pulmonary arteries
with a diameter <50 µm (determined according to the results of
the α-SMA immunofluorescence staining). were selected for analysis
of pulmonary vascular structure, medial thickness and degree of
muscularization, using Image Pro Plus software version 6.0 (Media
Cybernetics, Inc.).
Expression and localization of
Nox1
The 3-µm slice preparation was the same as for the
HE staining. The slices were rinsed with PBS, goat blocking serum
(AR1009; Boster Biological Technology) was applied and the slices
were incubated at room temperature for 30 min. Excess serum was
absorbed and primary anti-Nox1 antibody (BA3720; 1:100; Boster
Biological Technology) added for overnight incubation at 4°C.
Subsequently, the sections were rinsed three times in PBS and the
secondary antibody, Cy3 conjugated anti-mouse IgG (BA1031; 1:100;
Boster Biological Technology), was applied and incubated for 2 h at
37°C. The primary anti-α-SMA antibody and secondary antibody, HRP
conjugated anti-mouse IgG (LK2003; 1:100; Tianjin Sungene Biotech
Co., Ltd., Tianjin, China), were added following the same protocol
and the excess dye was rinsed out using PBS, followed by mounting
the section in neutral gum. A fluorescence microscope was used for
observation, and the results were analyzed using Image Pro Plus
software.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RNA of Nox1 and Nox4 were extracted (TRIzol,
Beijing Kaiao Technology Development Co., Ltd., Beijing, China)
from the lung tissue homogenates of the control, MCT and NAC
groups, and from the PASMCs of the N and C groups; RNA was
additionally extracted from the PASMCs of the C2 and M2 groups. The
RNA concentration and degree of degradation was determined using
colorimetry and electrophoresis, respectively. RT was performed
using an RT kit (EzOmics SYBR qPCR kit; Promega Corporation,
Madison, WI, USA) and the resulting cDNA was purified using the PCR
Purification kit (Qiagen GmbH, Hilden, Germany). The primers were
designed and synthesized by Beijing Kaiao Technology Development
Co., Ltd. The cDNA was amplified for 40 cycles, and the cycles in
each well were 20 sec at 90°C; 20 sec at 55°C and 20 sec at 72°C).
The mRNA expressions of different samples were quantified using the
2−ΔΔCq method (12).
The primer sequences were as follows: Hypoxanthine
phosphoribosyltransferase forward, 5′-CTCAGTCCCAGCGTCGTGAT-3′,
reverse, 5′-AGCACACAGAGGGCCACAAT-3′; Nox1 forward,
5′-ATGGTCCCTTTGGCACAGTC-3′, reverse, 5′-ATCCCAGCCAGTGAGGAAGA-3′;
and Nox4 forward, 5′-CCAGTGGTTTGCAGACTTGC-3′, reverse,
5′-CGAGGACGCCCAATAAAAAG-3′.
Western blot analysis
Total protein was obtained using Pierce protein
extraction buffer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) from the lung tissue homogenates of the control, MCT and NAC
groups, and from the PASMCs of groups C1, M1, and N, via
centrifugation (21,952 × g for 10 min at 23°C). Total protein was
quantified using the Bradford method and 15 µg protein/lane was
separated via SDS-PAGE on an 8% gel. The separated proteins were
transferred onto a nitrocellulose membrane and Ponceau staining
(0.2% for 10 min at 4°C) was performed, followed by ≥1 h blocking
in skimmed milk at 4°C. Membranes were incubated with anti-Nox1
polyclonal antibody for 12 h at 4°C and washed in TBS with Tween-20
(TBST). The labeled secondary antibody, Cy3 conjugated anti-mouse
IgG (BA1031; 1:100; Boster Biological Technology), was then added
and incubated at room temperature for 2 h, followed by washing with
TBST. The specific bands were detected using chemiluminescence
(Beijing Kaiao Technology Development Co., Ltd.) and developed onto
X-ray films. Each sample was repeated ≥3 times. TotalLab TL 100
program (Nonlinear Dynamics, Newcastle, UK) was used to analyze the
specific bands, with β-actin (BM0670; 1:100; Boster Biological
Technology) as the internal control.
Detection of cell proliferation and
apoptosis
The proliferation assay was performed on groups C1,
M1, N1, C2 and M2, according to the manufacturer's protocol of the
BrdU Cell Proliferation Assay kit (Roche Diagnostics, Basel,
Switzerland). Each sample was repeatedly tested ≥3 times, and the
count/minute values were obtained using a liquid scintillation
counter (Beckman Coulter, Inc., Brea, CA, USA). The apoptosis assay
was performed on groups C1, M1, N1, C2 and M2. The total protein
was extracted using the western blotting method and subsequently
quantified. The caspase-3 activity was detected using a
colorimetric kit (CaspACE colorimetric assay kit; Promega
Corporation), according to the manufacturer's protocol. Each sample
was assayed in three repeated wells. The protein-free control group
was additionally assayed, and the optical density was measured at
405 nm with a microplate reader. The differences between the mean
of each group and that of the control group were used to evaluate
the caspase-3 activity in each group.
Detection of total SOD activity
The total SOD activity in groups C1, M1, N1, C2 and
M2 was detected. The cells were collected, lysed and centrifuged at
4°C to obtain the supernatant as the test sample. The working
solutions were prepared according to the manufacturer's protocol of
the SOD activity detection kit (Beyotime Institute of
Biotechnology, Haimen, China). 96-well plates were used to set up
sample wells and blank control wells. The absorbance at 450 nm was
measured (VU2450 visible spectrophotometer; Shimadzu Corporation,
Kyoto, Japan) and the inhibition percentage was calculated.
Statistical analysis
The results are expressed as the mean ± standard
deviation. For the normally distributed data, the intergroup
comparisons were performed using analysis of variance followed by
Fisher's least significant difference test. The correlation was
analyzed using Pearson's correlation analysis, with the test level
set as α=0.05. P<0.05 was considered to indicate a statistically
significant difference. All of the results were analyzed using SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA).
Results
Alterations in hemodynamic parameters,
pulmonary vascular structures and RVHI
Following a single intraperitoneal injection of MCT,
mPAP was increased from 20.13±1.75 to 49.10±1.24 mmHg (P<0.001),
in addition to a significant increase in RVSP, from 19.78±1.84 to
51.56±1.44 mmHg (P<0.001), and right ventricular hypertrophy.
The measuring index was the increased RV/LV+S ratio, which was
increased from 0.22±0.11 to 0.85±0.08 (P<0.001), indicating that
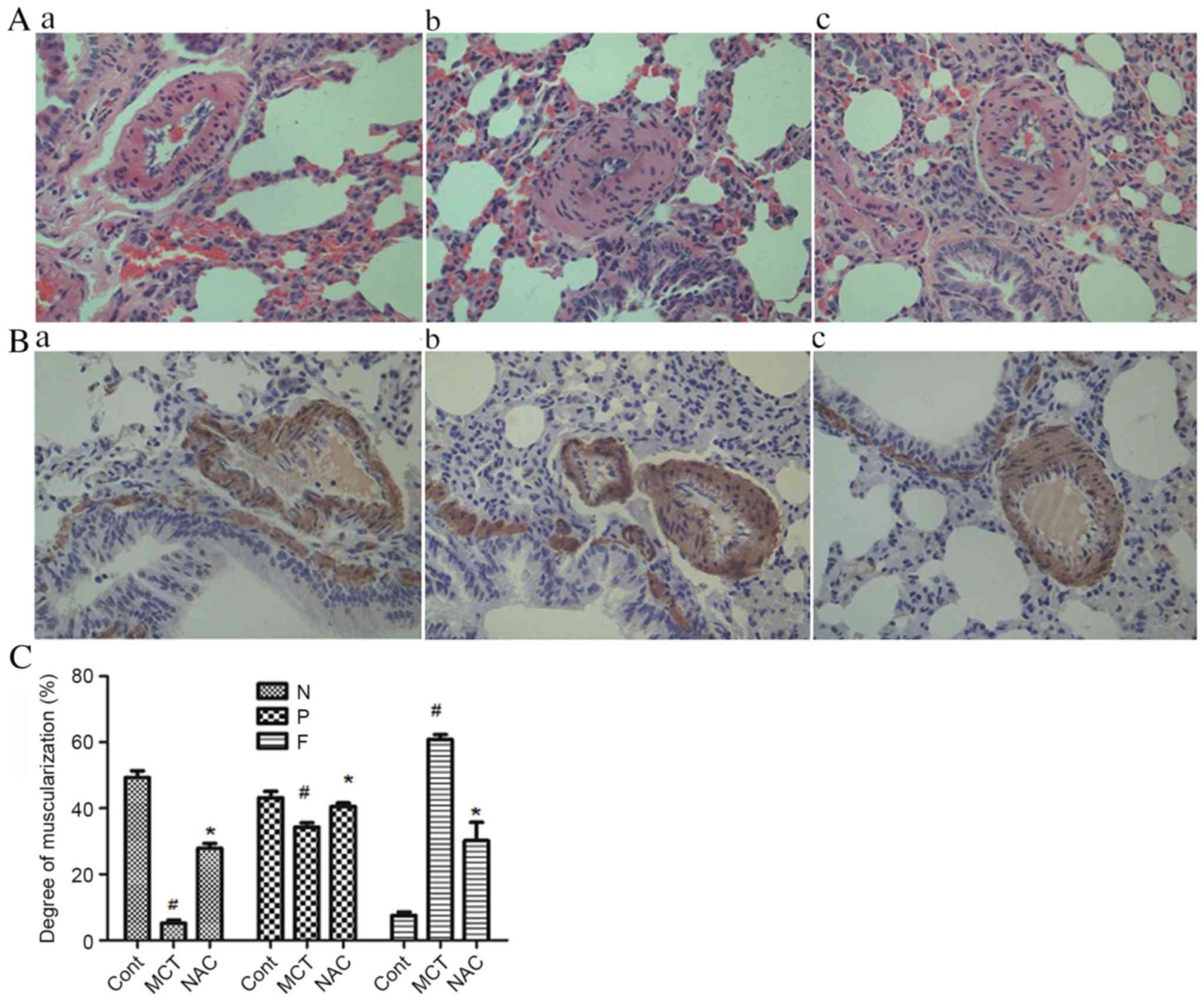
the rat PAH model was successfully generated (Table I). Compared with group M, group N
exhibited significantly decreased mPAP and RVSP, but the right
ventricular functions were improved (Table I), and the degree of medial
thickening and PVR were decreased (Fig. 1).
 | Table I.Hemodynamics and right heart
hypertrophy index. |
Table I.
Hemodynamics and right heart
hypertrophy index.
|
| Group C | Group M | Group N |
|---|
| RVSP, mmHg |
19.78±1.84 |
51.56±1.44a |
28.72±1.93b |
| mPAP, mmHg |
20.13±1.75 |
49.10±1.24a |
26.87±2.58b |
| RV/LV+S |
0.22±0.11 |
0.85±0.08a |
0.30±0.36b |
| WT, % |
53.66±0.54 |
57.28±0.62a |
54.20±0.44b |
Expression of Nox1 and Nox4 in PASMCs,
and positioning of Nox1
As presented in Fig.
2, PASMC was the primary location of vascular remodeling in
MCT-PAH. In order to clarify the roles of Nox1 and Nox4 in PAH, the
mRNA expression of Nox1 and Nox4 was measured in healthy and
MCT-treated rat lung tissue homogenates and PASMCs by RT-qPCR. Nox1
and Nox4 were detected in PASMCs of the MCT and control groups, and
in the MCT-treated lung tissue homogenates, Nox1 and Nox4 were
significantly upregulated (Fig.
2A; P<0.05); however, in PASMCs, the expression of Nox4 in
the MCT group and control groups exhibited no significant
difference (Fig. 2B). The
significant upregulation of Nox1 was additionally confirmed at the
protein level (Fig. 2D and E;
P<0.05). Following the NAC treatment, compared with the MCT
group, the expression of Nox1 and Nox4 in lung tissues was
significantly decreased (Fig. 2A;
P<0.05), whereas in PASMCs, only Nox1 was decreased
significantly (Fig. 2B;
P<0.05). The results of α-SMA and Nox1 dual immunofluorescence
staining (Fig. 2C) demonstrated
that in MCT-PAH, Nox1 was primarily expressed in PASMCs, and the
alterations in DHE fluorescence intensity were positively
correlated with the protein expression changes of Nox1 (r=0.850;
P<0.001; Fig. 2F and H).
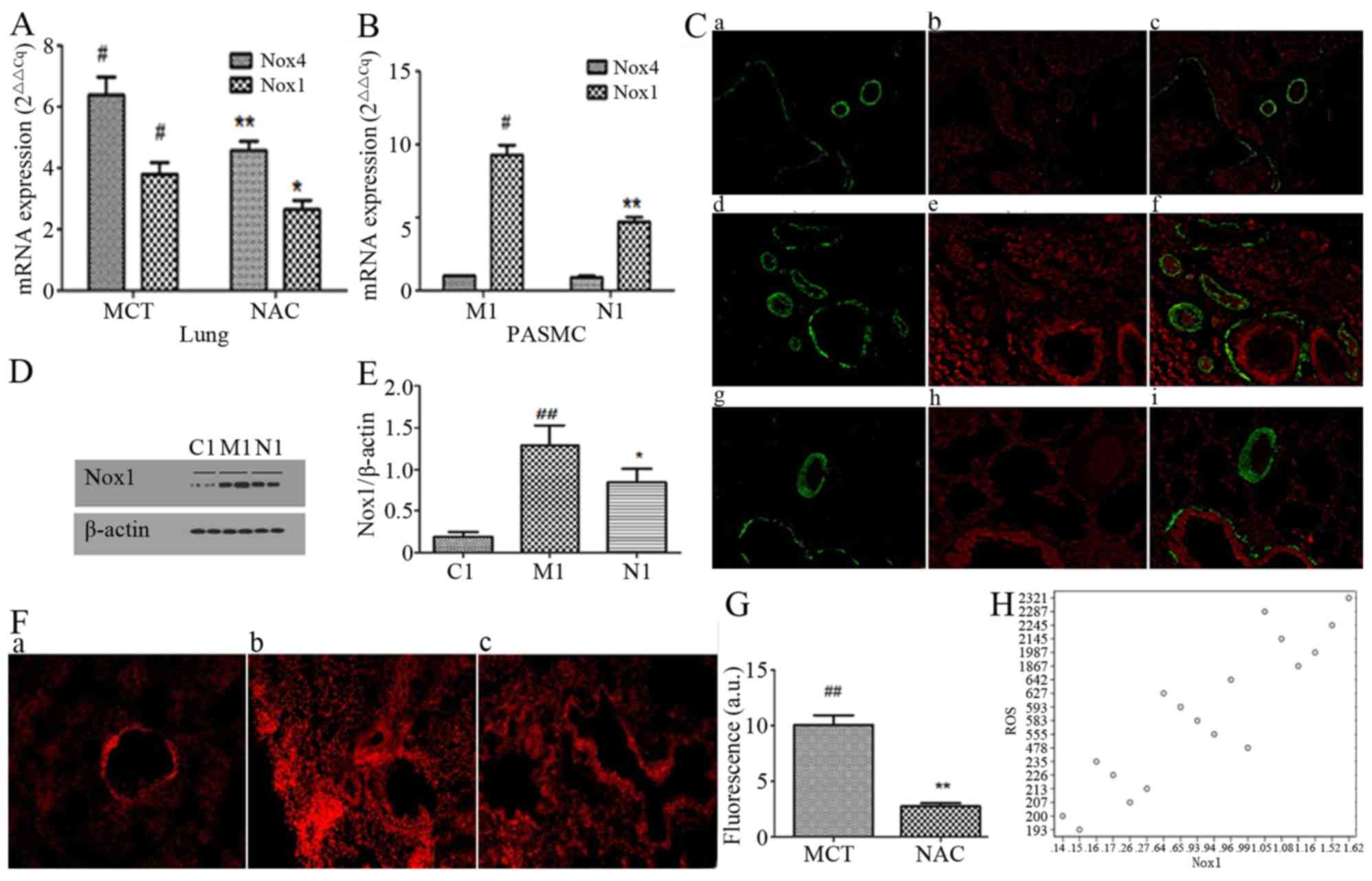 | Figure 2.Expression of Nox 1 and Nox4. (A) The
mRNA level of Nox1 and Nox4 in lung tissue. The standardization of
group Cont was 1. #P<0.05 vs. Cont; *P<0.05 vs.
MCT; **P<0.001 vs. MCT. (B) The mRNA level of Nox1 and Nox4 in
PASMCs. The standardization of group Cont was 1.
$P<0.05 vs. C1; +P<0.001 vs. M1. (C)
Double immunofluorescence staining of Nox1 and α-SMA in lung
tissue. Green, α-SMA; red, Nox1. (D) Western blotting and (E)
densitometric analysis of the expression of Nox1 protein in PASMCs
(n=6). β-actin was used as the internal control.
$$P<0.001 vs. C1; ++P=0.011 vs. M1. (F)
DHE fluorescence intensity of the lung tissue. (G) The
standardization of group Cont was 1. The data demonstrate the fold
change of group MCT and group NAC relative to group Cont.
##P<0.001 vs. Cont; **P<0.001 vs. MCT. (H)
Correlation analysis of Nox1 and DHE fluorescence intensity.
r=0.850; P<0.001. PASMC, pulmonary artery smooth muscle cell;
Nox, NADPH oxidase; Cont, control; α-SMA, smooth muscle actin; MCT,
monocrotaline; NAC, N-acetylcysteine; M, MCT-treated; N,
NAC-treated group; C, control subgroup; DHE, dihydroethidium. |
Impact of Nox1 on proliferation and
apoptosis
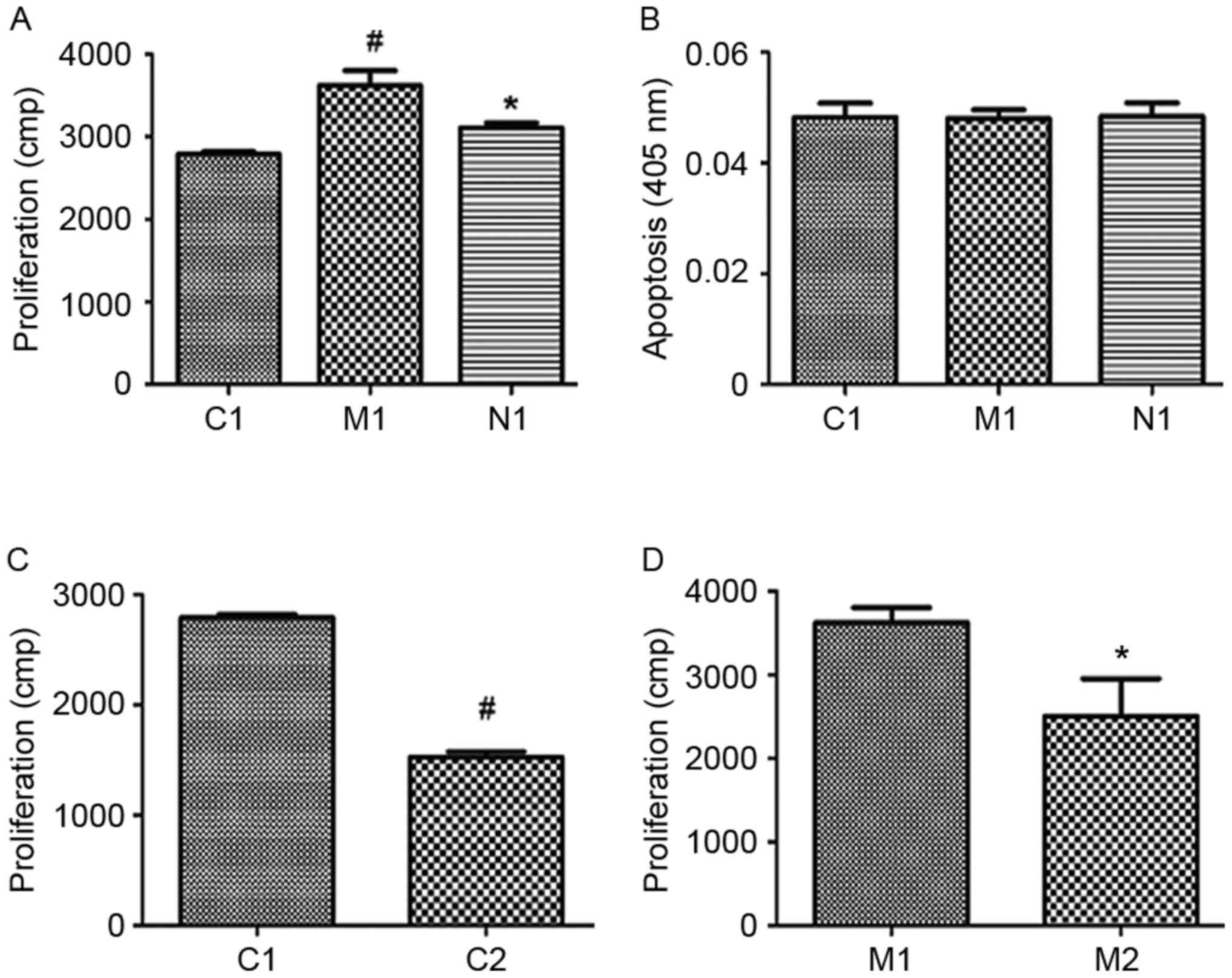
The MCT-treated PASMCs exhibited significantly
increased cell proliferation, but NAC was able to significantly
decrease cell proliferation (Fig.
3A; P<0.001). The apoptotic rates of PASMC among the three
groups exhibited no significant difference (Fig. 3B; P>0.05). Following treatment
with the Nox1 inhibitor ML171, the proliferation of PASMCs in
groups C1 and M1 were significantly decreased compared with C2 and
M2 (Fig. 3C and D;
P<0.001).
Total SOD activity
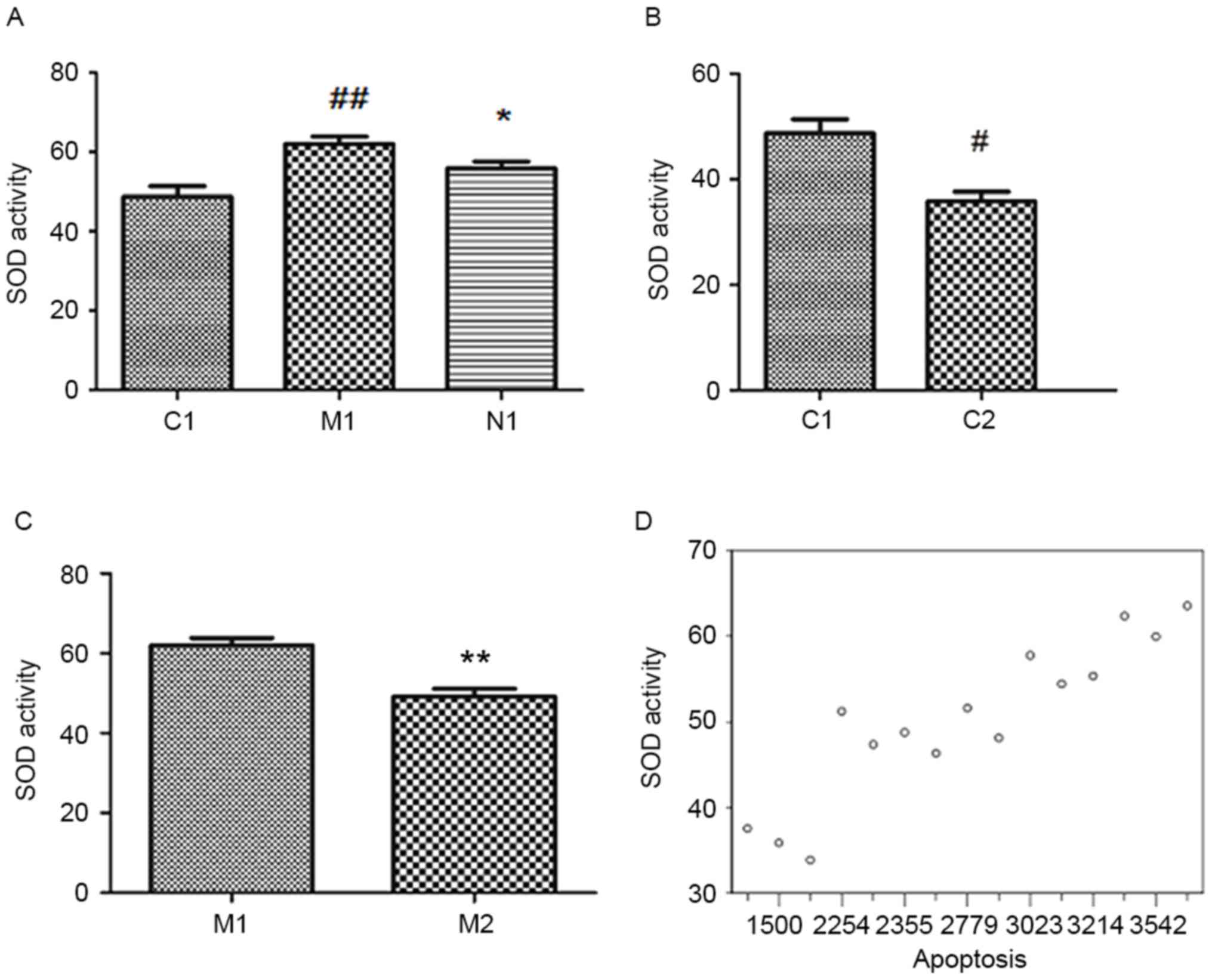
In response to oxidative stress, it was hypothesized
that the alterations in cellular antioxidant capacities may be
observed. The SOD activity was significantly increased in
MCT-PASMCs and, compared with group M1, group N1 exhibited
decreased SOD activity rather than the expected increase (Fig. 4A; P<0.05). The total SOD
activity was decreased by the inhibition of Nox1 (Fig. 4B and C; P<0.05). Therefore, the
total SOD activity was dependent on Nox1. The correlation between
SOD activity and cell proliferation demonstrated that SOD was
significantly correlated with the proliferation of PASMCs (Fig. 4D; r=0.949; P<0.001).
Discussion
Due to the unknown etiology, there remains a lack of
effective treatments for PAH and the prognosis is poor. The primary
mechanisms are the increasing of pulmonary vascular resistance and
pulmonary arterial pressure caused by the changes of pulmonary
vascular structures, thickening of the tunica media, stenosis and
non-muscle vascular muscularization, which eventually lead to right
heart failure. During this process, the proliferative and apoptotic
alterations of PASMCs are determined, and ROS serve important roles
in this process (3). However,
whether ROS are upregulated or downregulated in the process of PVR,
and whether the effects are beneficial or harmful, in addition to
the specific source, remains to be elucidated (13). In addition to hypoxia-induced PH,
the same questions in non-hypoxia-induced PH remain unresolved.
Experiments prior to the present study, and the
study by Ahmad et al (14)
demonstrated that an increase in Nox4-derived ROS may lead to PVR
in hypoxia-induced PAH. Seta et al (15) reported that Nox4 was increased in
MCT-PH rats. An additional study demonstrated that the excessive
proliferation and migration of PASMCs in MCT-PH was caused by the
Nox1-derived oxidation imbalance (4). In the present study, the mRNA and
corresponding protein expression of Nox1 and Nox4 in the lung
tissues of the MCT-treated group were increased compared with the
control group, and NAC was able to downregulate the expression of
Nox1 and Nox4. However, the mRNA samples extracted from PASMCs
exhibited increased expression of Nox1 only, and the α-SMA
fluorescence intensity used to measure the extent of vascular
muscularization was significantly positively correlated with the
expression level of Nox1, consistent with Veit et al
(5). These observations may be
associated with the fact that experiments performed prior to the
present study used the hypoxia-induced PH model, and the samples in
the study by Seta et al (15) originated from lung tissue
homogenates, indicating that different modeling methods and
different tissues may exhibit have different Nox isoforms. In
PASMCs, Nox4 and Nox1 were dominant, and the endothelial cells
appeared to be the primary location for the functioning of Nox4;
however, the expression of Nox1 appeared to be limited in PASMCs
and maintained at low concentrations in other vascular cells
(16,17), and this was the reason why Nox1 was
less well-studied in cardiovascular research compared with Nox4
(18,19).
In hypoxic PASMCs isolated from chronically hypoxic
mice, the expression of Nox4 was demonstrated to be increased
(20,21). Following treatment with MCT, the
expression level of Nox1 mRNA was significantly increased in
PASMCs, although the expression of Nox4 did not change, which was
associated with the different activation methods and distributions
of Nox1 and Nox4 in addition to different pathogeneses of PH. In
contrast to hypoxia-induced PH, the injection of MCT largely caused
the secretion of inflammatory factors, including platelet-derived
growth factors, thereby leading to strong inflammatory responses.
These inflammatory cytokines were able to stimulate an increased in
the expression and activity of Nox1 (22). NAC produces its MCT-PH protective
effects through anti-inflammatory and immunomodulatory mechanisms
(9–11). The present study compared pulmonary
arterial pressure and PVR in the MCT and NAC groups, and further
validated the conclusion that NAC acts via anti-inflammatory and
immunomodulatory mechanisms; in addition, it was demonstrated from
one lateral side that, different from hypoxia-induced PH,
MCT-PASMCs exhibited Nox1-mediated regulation of cell proliferation
rather than Nox4, and this was caused by different upstream
stimulatory factors mediating different signaling pathways. Nox4
appeared to be primarily associated with hypoxia-induced PH, and
Nox1 with inflammation and immune stimulation-induced PH.
It has been hypothesized that oxidative stress
induces inflammation, and that the inhibition of ROS enzymes (such
as Nox) and SOD may effectively decrease in vivo and in
vitro inflammation (23,24).
It has been suggested that inflammation may induce oxidative stress
and that Nox complexes serve key roles in this process (25). Therefore, the association between
Nox and inflammation-induced oxidative stress may be defined as a
vicious cycle, leading to vascular disease. In MCT-PH, in addition
to the increase in ROS-represented oxidative levels, the
antioxidant capacity may additionally be increased. In the present
study, it was specifically demonstrated that, compared with group
C1, the SOD activity in the PASMCs of group M1 was significantly
increased; following treatment with the Nox1 inhibitor ML171, the
generation of SOD returned to normal levels, and the proliferation
of MCT-PASMCs was significantly decreased. The correlation analysis
demonstrated that these two factors were strongly correlated,
indicating that SOD served important roles via the Nox1-SOD
signaling pathway.
Analysis of the correlation between Nox1 and DHE
demonstrated that when the Nox1-derived O2−
was increased, the SOD activity was additionally increased; it was
therefore hypothesized that the downstream signaling molecule of
this process was H2O2. Analysis of the
correlation between SOD activity and the proliferation of smooth
muscle cells, in addition to the alterations in SOD activity, led
to the hypothesis that the Nox-derived O2−
may produce more H2O2 following dismutation
by SOD, and that the latter served roles as an intra- and
extracellular messenger (26).
H2O2 could react with cysteine in a
reversible manner. H2O2 may lead to the
decreased production of NO, and may additionally release
intracellular Ca2+ and contract pulmonary vessels
(16). The results of the present
study, demonstrating that the total SOD activity in group N1 was
significantly decreased compared with group M1, additionally
demonstrated that NAC may exert functions on MCT-PASMCs by
affecting the Nox1-SOD signaling pathway, rather than by increasing
the antioxidant capacities so as to decrease ROS formation. Nox1
exhibited different intervention targets in MCT-PH models, and in
other animal and human PH models, and this requires further
study.
In conclusion, in lung tissues, Nox1 and Nox4 were
associated with the formation of PAH, and NAC was able to serve
therapeutic roles by decreasing the expression of Nox1 and Nox4. In
PASMCs, NAC was able to improve PVR by reducing Nox1-, rather than
Nox4-, derived ROS to reduce the proliferation of smooth muscle
cells, thereby reducing pulmonary arterial pressure and RVH. The
promotive roles of Nox1-derived ROS on the proliferation of smooth
muscle cells may be achieved by increasing SOD and the downstream
signaling molecule H2O2. The generation site,
type and downstream-involved oxidation and antioxidation imbalances
of Nox have not been demonstrated to be consistent in different
studies; thus, further studies are required in order to elucidate
the accurate mechanisms. In the present study, ML171 was less of a
focus; therefore, the results of the present study require
verification by gene knockout or inhibition studies prior to this
research being translated into clinical practice.
References
|
1
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Noordegraaf A Vonk,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension: The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS): Endorsed by: Association for European Paediatric and
Congenital Cardiology (AEPC), International Society for Heart and
Lung Transplantation (ISHLT). Eur Heart J. 37:67–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiao S, Fan K, Iwashita T, Ichihara M,
Yoshino M and Takahashi M: The involvement of reactive oxygen
species derived from NADPH oxidase-1 activation on the constitutive
tyrosine auto-phosphorylation of RET proteins. Free Radic Res.
48:427–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schermuly RT, Ghofrani HA, Wilkins MR and
Grimminger F: Mechanisms of disease: Pulmonary arterial
hypertension. Nat Rev Cardiol. 8:443–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freund-Michel V, Guibert C, Dubois M,
Courtois A, Marthan R, Savineau JP and Muller B: Reactive oxygen
species as therapeutic targets in pulmonary hypertension. Ther Adv
Respir Dis. 7:175–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veit F, Pak O, Egemnazarov B, Roth M,
Kosanovic D, Seimetz M, Sommer N, Ghofrani HA, Seeger W, Grimminger
F, et al: Function of NADPH oxidase 1 in pulmonary arterial smooth
muscle cells after monocrotaline-induced pulmonary vascular
remodeling. Antioxid Redox Signal. 19:2213–2231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ismail S, Sturrock A, Wu P, Cahill B,
Norman K, Huecksteadt T, Sanders K, Kennedy T and Hoidal J: NOX4
mediates hypoxia-induced proliferation of human pulmonary artery
smooth muscle cells: The role of autocrine production of
transforming growth factor-{beta}1 and insulin-like growth factor
binding protein-3. Am J Physiol Lung Cell Mol Physiol.
296:L489–L499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wassmann S, Wassmann K and Nickenig G:
Modulation of oxidant and antioxidant enzyme expression and
function in vascular cells. Hypertension. 44:381–386. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Millea PJ: N-acetylcysteine: Multiple
clinical applications. Am Fam Physician. 80:265–269.
2009.PubMed/NCBI
|
|
9
|
Kelly GS: Clinical applications of
N-acetylcysteine. Altern Med Rev. 3:114–127. 1998.PubMed/NCBI
|
|
10
|
Santus P, Corsico A, Solidoro P, Braido F,
Di Marco F and Scichilone N: Oxidative stress and respiratory
system: Pharmacological and clinical reappraisal of
N-acetylcysteine. COPD. 11:705–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaumais MC, Ranchoux B, Montani D,
Dorfmüller P, Tu L, Lecerf F, Raymond N, Guignabert C, Price L,
Simonneau G, et al: N-acetylcysteine improves established
monocrotaline-induced pulmonary hypertension in rats. Respir Res.
15:652014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Veit F, Pak O, Brandes RP and Weissmann N:
Hypoxia-dependent reactive oxygen species signaling in the
pulmonary circulation: Focus on ion channels. Antioxid Redox
Signal. 22:537–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmad M, Kelly MR, Zhao X, Kandhi S and
Wolin MS: Roles for Nox4 in the contractile response of bovine
pulmonary arteries to hypoxia. Am J Physiol Heart Circ Physiol.
298:H1879–H1888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seta F, Rahmani M, Turner PV and Funk CD:
Pulmonary oxidative stress is increased in cyclooxygenase-2
knockdown mice with mild pulmonary hypertension induced by
monocrotaline. PLoS One. 6:e234392011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Briones AM and Touyz RM: Oxidative stress
and hypertension: Current concepts. Curr Hypertens Rep. 12:135–142.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guzik TJ, Sadowski J, Guzik B, Jopek A,
Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG and
Channon KM: Coronary artery superoxide production and nox isoform
expression in human coronary artery disease. Arterioscler Thromb
Vasc Biol. 26:333–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng G and Lambeth JD: NOXO1, regulation
of lipid binding, localization, and activation of Nox1 by the Phox
homology (PX) domain. J Biol Chem. 279:4737–4742. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Helmcke I, Heumüller S, Tikkanen R,
Schröder K and Brandes RP: Identification of structural elements in
Nox1 and Nox4 controlling localization and activity. Antioxid Redox
Signal. 11:1279–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diebold I, Petry A, Hess J and Görlach A:
The NADPH oxidase subunit NOX4 is a new target gene of the
hypoxia-inducible factor-1. Mol Biol Cell. 21:2087–2096. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Judkins CP, Diep H, Broughton BR, Mast AE,
Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG and
Drummond GR: Direct evidence of a role for Nox2 in superoxide
production, reduced nitric oxide bioavailability, and early
atherosclerotic plaque formation in ApoE-/-mice. Am J Physiol Heart
Circ Physiol. 298:H24–H32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin W and Voit EO: Function and design of
the Nox1 system in vascular smooth muscle cells. BMC Syst Biol.
7:202013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valdivia A, Pérez-Alvarez S, Aroca-Aguilar
JD, Ikuta I and Jordán J: Superoxide dismutases: A
physiopharmacological update. J Physiol Biochem. 65:195–208. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaulmes A, Sansilvestri-Morel P,
Rolland-Valognes G, Bernhardt F, Gaertner R, Lockhart BP, Cordi A,
Wierzbicki M, Rupin A and Verbeuren TJ: Nox4 mediates the
expression of plasminogen activator inhibitor-1 via p38 MAPK
pathway in cultured human endothelial cells. Thromb Res.
124:439–446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anrather J, Racchumi G and Iadecola C:
NF-kappaB regulates phagocytic NADPH oxidase by inducing the
expression of gp91phox. J Biol Chem. 281:5657–5667. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schröder E and Eaton P: Hydrogen peroxide
as an endogenous mediator and exogenous tool in cardiovascular
research: Issues and considerations. Curr Opin Pharmacol.
8:153–159. 2008. View Article : Google Scholar : PubMed/NCBI
|