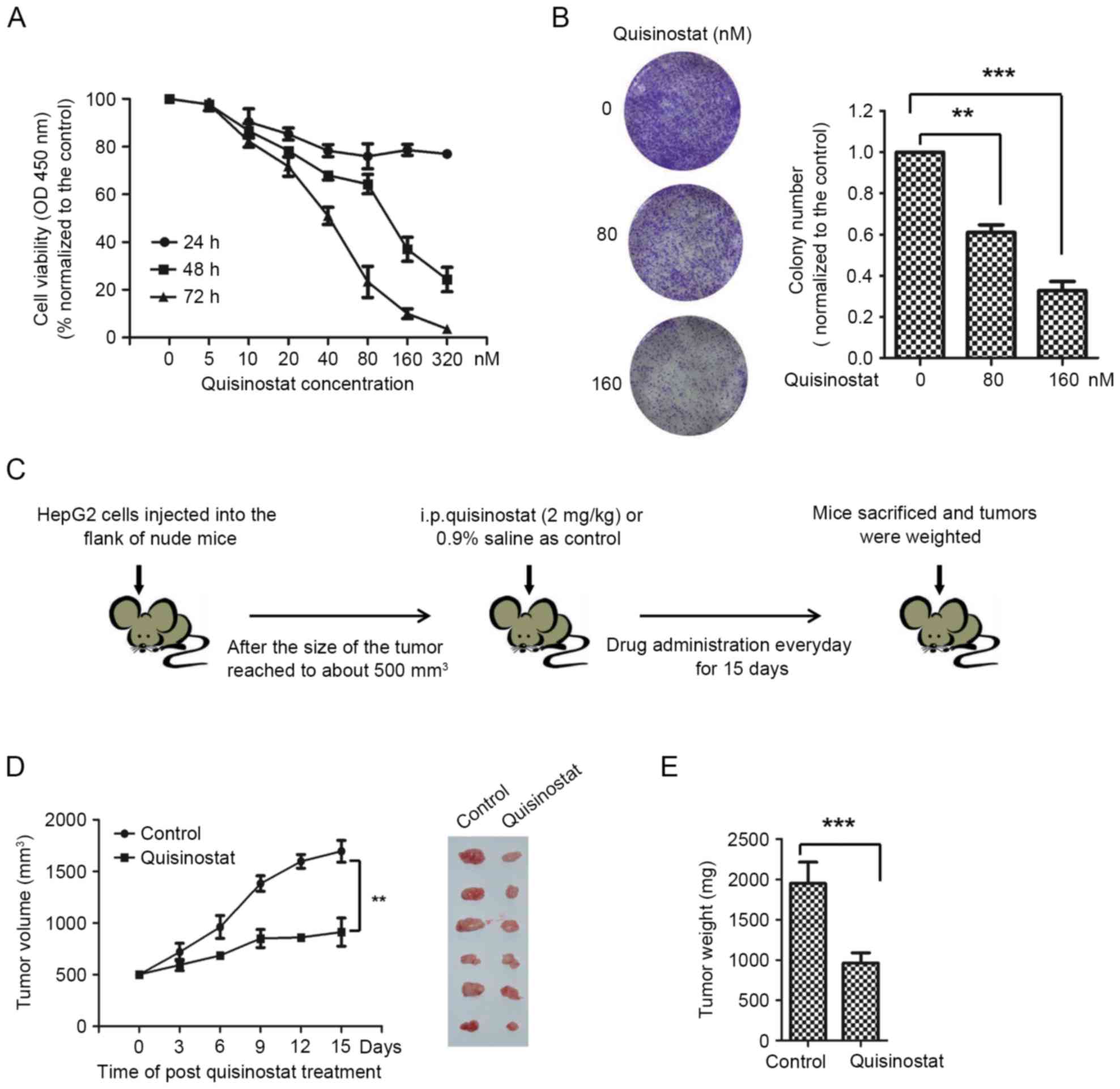
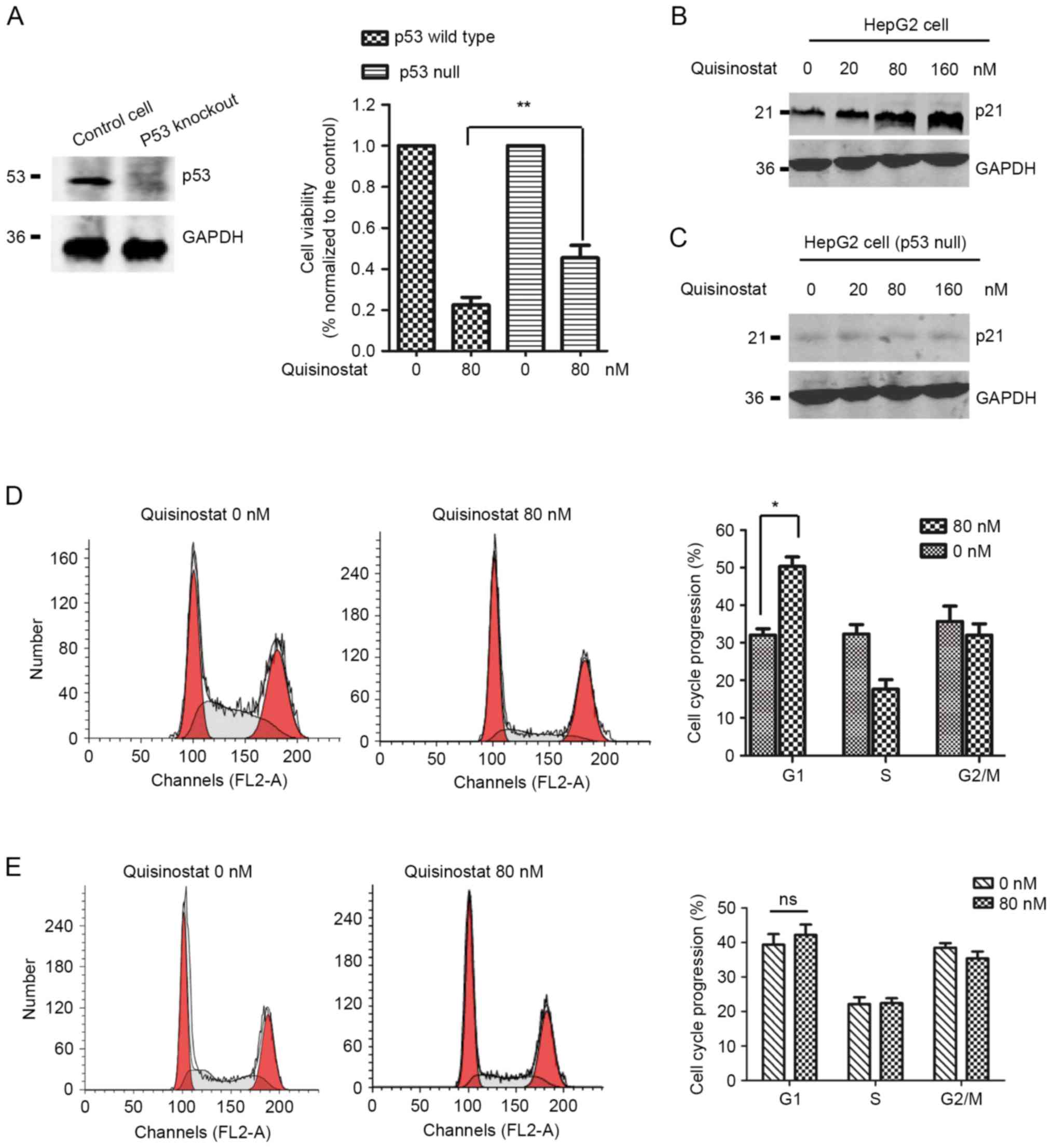
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erichsen R, Jepsen P, Jacobsen J, Norgaard
M, Vilstrup H and Sørensen HT: Time trends in incidence and
prognosis of primary liver cancer and liver metastases of unknown
origin in a Danish region, 1985–2004. Eur J Gastroenterol Hepatol.
20:104–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127 5 Suppl 1:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ray K: Gut microbiota: Obesity-induced
microbial metabolite promotes HCC. Nat Rev Gastroenterol Hepatol.
10:4422013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Starzl TE, Marchioro TL, Vonkaulla KN,
Hermann G, Brittain RS and Waddell WR: Homotransplantation of the
liver in humans. Surg Gynecol Obstet. 117:659–676. 1963.PubMed/NCBI
|
|
6
|
Liao PH, Hsu HH, Chen TS, Chen MC, Day CH,
Tu CC, Lin YM, Tsai FJ, Kuo WW and Huang CY: Phosphorylation of
cofilin-1 by ERK confers HDAC inhibitor resistance in
hepatocellular carcinoma cells via decreased ROS-mediated
mitochondria injury. Oncogene. 36:1978–1990. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ande SR, Nguyen KH, Grégoire Nyomba BL and
Mishra S: Prohibitin-induced, obesity-associated insulin resistance
and accompanying low-grade inflammation causes NASH and HCC. Sci
Rep. 6:236082016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baylin SB: Resistance, epigenetics and the
cancer ecosystem. Nat Med. 17:288–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lund AH and van Lohuizen M: Epigenetics
and cancer. Genes Dev. 18:2315–2335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao L, Diao H, Dong N, Su X, Wang B, Mo Q,
Yu H, Wang X and Chen C: Histone deacetylase inhibitor induces cell
apoptosis and cycle arrest in lung cancer cells via mitochondrial
injury and p53 up-acetylation. Cell Biol Toxicol. 32:469–482. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui Y, Gao D, Linghu E, Zhan Q, Chen R,
Brock MV, Herman JG and Guo M: Epigenetic changes and functional
study of HOXA11 in human gastric cancer. Epigenomics. 7:201–213.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Downs B and Wang SM: Epigenetic changes in
BRCA1-mutated familial breast cancer. Cancer Genet. 208:237–240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allfrey VG, Faulkner R and Mirsky AE:
Acetylation and methylation of histones and their possible role in
the regulation of Rna synthesis. Proc Natl Acad Sci USA.
51:786–794. 1964; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurdistani SK and Grunstein M: Histone
acetylation and deacetylation in yeast. Nat Rev Mol Cell Biol.
4:276–284. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon S and Eom GH: HDAC and HDAC
inhibitor: From cancer to cardiovascular diseases. Chonnam Med J.
52:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y and Seto E: HDACs and HDAC inhibitors
in cancer development and therapy. Cold Spring Harb Perspect Med.
6(pii): a0268312016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang XJ and Seto E: HATs and HDACs: From
structure, function and regulation to novel strategies for therapy
and prevention. Oncogene. 26:5310–5318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osada H, Tatematsu Y, Saito H, Yatabe Y,
Mitsudomi T and Takahashi T: Reduced expression of class II histone
deacetylase genes is associated with poor prognosis in lung cancer
patients. Int J Cancer. 112:26–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
West AC and Johnstone RW: New and emerging
HDAC inhibitors for cancer treatment. J Clin Invest. 124:30–39.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MS, Son MW, Kim WB, In Park Y and Moon
A: Apicidin, an inhibitor of histone deacetylase, prevents
H-ras-induced invasive phenotype. Cancer Lett. 157:23–30. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heinicke U, Kupka J, Fichter I and Fulda
S: Critical role of mitochondria-mediated apoptosis for
JNJ-26481585-induced antitumor activity in rhabdomyosarcoma.
Oncogene. 35:3729–3741. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arts J, King P, Mariën A, Floren W, Beliën
A, Janssen L, Pilatte I, Roux B, Decrane L, Gilissen R, et al:
JNJ-26481585, a novel ‘second-generation’ oral histone deacetylase
inhibitor, shows broad-spectrum preclinical antitumoral activity.
Clin Cancer Res. 15:6841–6851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheung ST, Wong SY, Lee YT and Fan ST: GEP
associates with wild-type p53 in hepatocellular carcinoma. Oncol
Rep. 15:1507–1511. 2006.PubMed/NCBI
|
|
24
|
Dai Q, Yin Y, Liu W, Wei L, Zhou Y, Li Z,
You Q, Lu N and Guo Q: Two p53-related metabolic regulators, TIGAR
and SCO2, contribute to oroxylin A-mediated glucose metabolism in
human hepatoma HepG2 cells. Int J Biochem Cell Biol. 45:1468–1478.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang CZ, Chen GG, Merchant JL and Lai PB:
Interaction between ZBP-89 and p53 mutants and its contribution to
effects of HDACi on hepatocellular carcinoma. Cell Cycle.
11:322–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laporte AN, Barrott JJ, Yao RJ, Poulin NM,
Brodin BA, Jones KB, Underhill TM and Nielsen TO: HDAC and
proteasome inhibitors synergize to activate pro-apoptotic factors
in synovial sarcoma. PLoS One. 12:e01694072017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faustino-Rocha A, Oliveira PA,
Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG,
Colaço B, Pires MJ, Colaço J, Ferreira R and Ginja M: Estimation of
rat mammary tumor volume using caliper and ultrasonography
measurements. Lab Anim (NY). 42:217–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Green DR: Apoptotic pathways: Ten minutes
to dead. Cell. 121:671–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang Y, Zhao W, Chen Y, Zhao Y and Gu W:
Acetylation is indispensable for p53 activation. Cell. 133:612–626.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ryu HW, Shin DH, Lee DH, Choi J, Han G,
Lee KY and Kwon SH: HDAC6 deacetylates p53 at lysines 381/382 and
differentially coordinates p53-induced apoptosis. Cancer Lett.
391:162–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding G, Liu HD, Huang Q, Liang HX, Ding
ZH, Liao ZJ and Huang G: HDAC6 promotes hepatocellular carcinoma
progression by inhibiting P53 transcriptional activity. FEBS Lett.
587:880–886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mariadason JM: HDACs and HDAC inhibitors
in colon cancer. Epigenetics. 3:28–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pelicci PG: A new class of anti-cancer
drugs: HDAC-inhibitors. Suppl Tumori. 1:S662002.PubMed/NCBI
|
|
34
|
Secrist JP, Zhou X and Richon VM: HDAC
inhibitors for the treatment of cancer. Curr Opin Investig Drugs.
4:1422–1427. 2003.PubMed/NCBI
|
|
35
|
Duvic M and Vu J: Vorinostat in cutaneous
T-cell lymphoma. Drugs Today (Barc). 43:585–599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duvic M and Vu J: Vorinostat: A new oral
histone deacetylase inhibitor approved for cutaneous T-cell
lymphoma. Expert Opin Investig Drugs. 16:1111–1120. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murata M, Towatari M, Kosugi H, Tanimoto
M, Ueda R, Saito H and Naoe T: Apoptotic cytotoxic effects of a
histone deacetylase inhibitor, FK228, on malignant lymphoid cells.
Jpn J Cancer Res. 91:1154–1160. 2000. View Article : Google Scholar : PubMed/NCBI
|