Introduction
Hepatic ischemia/reperfusion (I/R) injury, which
often occurs in response to shock or liver surgery, is caused by a
reduction in liver pedicle blood flow, and is an important factor
affecting liver function following liver transplantation and liver
lobe resection. The pathophysiological process associated with I/R
injury is complex, and there are currently no effective
preventative methods available; therefore, research regarding its
mechanism has recently garnered attention. Previous studies have
indicated that reactive oxygen species (ROS) are closely associated
with hepatic I/R injury (1–3). In
the early stage of hepatic I/R injury, ROS initiate lipid
peroxidation damage in liver cells, protein oxidation,
mitochondrial dysfunction and DNA damage (4). Kupffer cell activation and neutrophil
chemotaxis further induces liver cell death, resulting in acute
liver injury (5). Therefore,
studying the association between hepatic I/R injury and oxidative
damage may further aid the understanding of the mechanisms
underlying hepatic I/R injury, and provide a novel therapeutic
target and clinical treatment for hepatic I/R injury.
Nuclear factor, erythroid 2 like 2 (Nrf2) serves an
important role in cellular oxidative stress and exerts antioxidant
effects on numerous organs (6–9).
Under physiological conditions, Nrf2 and Kelch-like ECH-associated
protein 1 (Keap1) form a complex in the cytoplasm to inhibit the
activity of Nrf2. However, in response to various endogenous or
exogenous stimuli, Nrf2 and Keap1 dissociate, allowing Nrf2 to
translocate into the nucleus and combine with antioxidant response
elements (ARE) to initiate ARE-regulated phase II detoxification
enzyme and antioxidative enzyme gene expression. Heme oxygenase
(HO)-1 is a major phase II detoxifying enzyme regulated by Nrf2
(10–12) that protects the body from ROS
damage and is the main mechanism that protects cells against
oxidative damage (7,13,14).
Previous studies have reported that Nrf2 has an important role in
I/R injury of the small intestine and brain (15–17);
however, its role in hepatic I/R injury has yet to be
elucidated.
The δ-opioid receptor (DOR) belongs to a category of
seven-transmembrane G protein-coupled receptors, and
[D-Ala2, D-Leu5]-Enkephalin (DADLE) is a
synthetic DOR-specific agonist. Previous studies have indicated
that DADLE, as a DOR-specific agonist, exerts protective effects in
the damage repair of the brain, heart, liver and other organs
(18–20). However, whether DADLE exerts
protective effects on hepatic I/R injury, and its possible
mechanism, remain unclear.
The present study aimed to investigate the effects
of DADLE on acute liver injury induced by hepatic I/R injury in
mice and its possible mechanism. The results indicated that DADLE
was able to significantly improve liver I/R injury, possibly via
the Nrf2/HO-1 pathway, which may provide a novel theoretical basis
for the treatment of hepatic I/R injury.
Materials and methods
Animals
Male C57BL/6 mice (age, 35–42 days; weight, 18–22 g;
6 mice in each group) were provided by the Animal Experimental
Center of Guilin Medical University (animal quality certificate
number: 45000800000013). The mice were housed in cages, under
controlled temperature (24±2°C) and humidity (50±10%) conditions
with 12 h light/dark cycles and were given free access to food and
water All experiments were approved by the Animal Ethics Committee
of the Affiliated Hospital of Guilin Medical University (Guilin,
China).
Antibodies and reagents
DADLE, the DOR antagonist naloxone and pentobarbital
sodium were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Superoxide dismutase (SOD), glutathione (GSH), catalase
(CAT) and malondialdehyde (MDA) kits (catalog nos. A001-3, A006-2,
A007-1 and A003-1, respectively) were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Nucleic acid
protein and cytoplasmic protein extraction kit was purchased from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Nrf2 antibody
(catalog no. sc-722) was obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA); β-actin antibody (catalog no. TA890010) was
purchased from OriGene Technologies, Inc. (Beijing, China); HO-1
antibody (catalog no. ab13248) was purchased from Abcam (Cambridge,
UK); and proliferating cell nuclear antigen (PCNA) antibody
(catalog no. 13110) was obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA).
Animal models and groups
Forty-eight mice underwent 70% liver ischemia for 45
min. Six mice were sacrificed at each reperfusion time points (0,
1, 2, 4, 6, 8, 12 and 24 h). The alterations in serum alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) were
measured at different time points after reperfusion in mice. Thirty
mice were randomly divided into five groups (n=6 mice/group) and
intraperitoneally injected with different concentrations of DADLE
(1, 3, 5, 7 and 10 mg/kg) for 15 min prior to ischemia. After 45
min ischemia, mice were sacrificed at the same time point according
to the previous experiment. The effects of different concentrations
of DADLE on serum ALT and AST were observed. Twenty-four mice were
randomly divided into four groups (n=6 mice/group) as follows: Sham
group, Model group (I/R), DADLE group and DOR antagonist naloxone +
DADLE group (N/D). The mice in the Sham group underwent anesthesia,
laparotomy, separation of the perihepatic ligament and abdomen
closure. The mice in the I/R group were anesthetized with 2%
diethyl ether inhalation and were intraperitoneally injected with
50 mg/kg sodium pentobarbital; subsequently, their abdomens were
opened, and the blood vessels and bile ducts in the left and middle
lobes of the liver were occluded with vascular clamps to result in
70% liver ischemia. Blood supply was restored after 45 min and the
abdomen was immediate sutured after release of vascular clamps. In
the DADLE group, 5 mg/kg DADLE solution was injected
intraperitoneally 15 min prior to I/R, whereas in the N/D Group,
2.5 mg/kg naloxone was administered intraperitoneally at a fixed
time each day for a week prior to surgery, and 5 mg/kg DADLE was
injected intraperitoneally for 15 min prior to I/R as control. The
mice were sacrificed 6 h after the blood supply was restored, and
biological samples were taken for use in subsequent
experiments.
Determination of serum transaminase
levels
A total of 6 h postreperfusion, 1 ml whole blood was
collected from the mice via the inferior vena cava. The blood
samples were maintained at room temperature for 1 h and were then
centrifuged at 1006.2 × g, 4°C for 15 min. The serum levels of
alanine transaminase (ALT) and aspartate transaminase (AST) were
measured using an automated chemical analyzer (Roche Cobas 8000;
Roche Diagnostics, Basel, Switzerland). Values were expressed as
units per liter (U/l).
Liver histopathological observation
and assessment of liver injury
Liver sections (0.3–0.5 cm) were fixed in 10%
formalin, embedded in paraffin, dewaxed with xylene and passed
through a graded series of alcohol. Subsequently, the sections were
stained with hematoxylin at 25°C for 5 min and rinsed under running
water for 10 min; the color was differentiated using 1%
hydrochloric acid. The sections were further rinsed under running
water for 10 min, stained with eosin for 30 sec at 25°C, dehydrated
under an alcohol gradient, cleared with xylene and sealed with
neutral gum. The histopathological alterations of the liver tissue
were observed under an optical microscope. The degree of liver
injury was graded according to the Suzuki criteria (21): 0 to 4 according to occlusion of the
liver sinus, and necrosis, swelling and degeneration of liver
cells.
Measurement of SOD, GSH, CAT and MDA
in liver homogenates
The levels of SOD, GSH, CAT and MDA in the liver
tissue homogenates from each group were detected, according to the
manufacturer's protocols.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from the liver tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The absorbance (A) 260/A280 ratio was confirmed
to be between 1.8 and 2.0, and the purity was >90%.
Subsequently, RNA was reverse transcribed into cDNA (Tiangen
Biotech Co., Ltd., Beijing, China) and used as a template for PCR
amplification. The following primers were used: HO-1, upstream
5′-CAGAAGAGGCTAAGACCGCC-3′, downstream 5′-CTCTGACGAAGTGACGCCAT-3′;
and GAPDH (internal reference), upstream 5′-ACCACAGTCCATGCCATCAC-3′
and downstream 5′-TCCACCACCCTGTTGCTGTA-3′. PCR was conducted using
SYBR Premix Ex Taq II (Takara Biotechnology Co., Ltd., Tokyo,
Japan) according to the manufacturer's protocol in a 20-µl reaction
system under the following reaction conditions: 95°C for 2 min;
followed by 35 cycles of 94°C for 45 sec, 60°C for 30 sec and 72°C
for 2 min, and a final extension step at 72°C for 10 min. The
amplified products were analyzed by 1% agarose gel electrophoresis
and GelRed (Biotium, Inc., Freemont, CA, USA), and the target bands
were scanned using a gel imaging system; G:Box chemi-XR5 GENESys
version 1.2.5.0 (Syngene, Frederick, MD, USA).
Western blotting
Whole cell, nuclear and cytoplasmic proteins were
separately extracted from the samples using a Nuclear and
cytoplasmic protein extraction kit (cat. no. KGP1100) purchased
from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China) and the
bicinchoninic acid method was used to quantify protein levels. The
protein samples were mixed with loading buffer and boiled for 5 min
for protein denaturation. Subsequently, 5–10 µg samples were
separated by 10% polyacrylamide gel electrophoresis and were
transferred to polyvinylidene fluoride membranes under constant
pressure for 1 h. The membranes were washed three times with TBS
containing 0.05% Tween 20 (TBST; 10 min/wash), and were then
incubated with β-actin (1:1,000), Nrf2 (1:200), PCNA (1:1,000) and
HO-1 (1:500) antibodies at 4°C overnight. Subsequently, the
membranes were washed three times with TBST (15 mi/wash) and were
incubated with the corresponding secondary antibody (1:8,000) for 1
h at 37°C. The secondary antibodies were obtained from Santa Cruz
Biotechnology, Inc. (cat. no. ab6789 and ab150077). The membranes
were washed three times with TBST (10 min/wash), and the antibodies
were detected using an enhanced chemiluminescence system (Beyotime
Institute of Biotechnology, Haimen, China). Gray value analysis was
performed using Quantity One software version 4.62 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and were repeated 3 times, and were analyzed using SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). Initially, a
test of the homogeneity of variance was conducted for the data from
each group using a single-factor analysis of variance for multiple
group comparisons and a least significant difference test for the
homogeneity of variance. When variance was not homogeneous, the
data were converted to achieve homogeneity prior to analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of DADLE on hepatic
dysfunction caused by hepatic I/R injury
To determine the time course of hepatic I/R, the
serum levels of AST and ALT were detected in the I/R group at
various time points (0, 1, 2, 4, 6, 8, 12 and 24 h). The results
indicated that the ALT and AST levels were highest at 6 h following
I/R, confirming that the model was successful (Fig. 1A). To determine the appropriate
concentration of the DOR agonist for use in subsequent experiments,
the serum levels of ALT and AST were detected in response to
various concentrations of DADLE (1, 3, 5, 7 and 10 mg/kg). The
results demonstrated that the serum levels of ALT and AST were
markedly lower following treatment with 5 mg/kg DADLE. Therefore,
subsequent experiments were conducted 6 h following I/R, and DADLE
was used at a concentration of 5 mg/kg (Fig. 1B).
 | Figure 1.(A) Analysis of the serum levels of
ALT and AST in mice at various time points (0, 1, 2, 4, 6, 8, 12
and 24 h) after I/R. (B) Effects of various concentrations of DADLE
(1, 3, 5, 7 and 10 mg/kg) on hepatic I/R injury. Each experiment
was repeated three times. ALT, alanine aminotransferase; AST,
aspartate aminotransferase; DADLE, (D-Ala2,
D-Leu5)-Enkephalin; I/R, ischemia/reperfusion. |
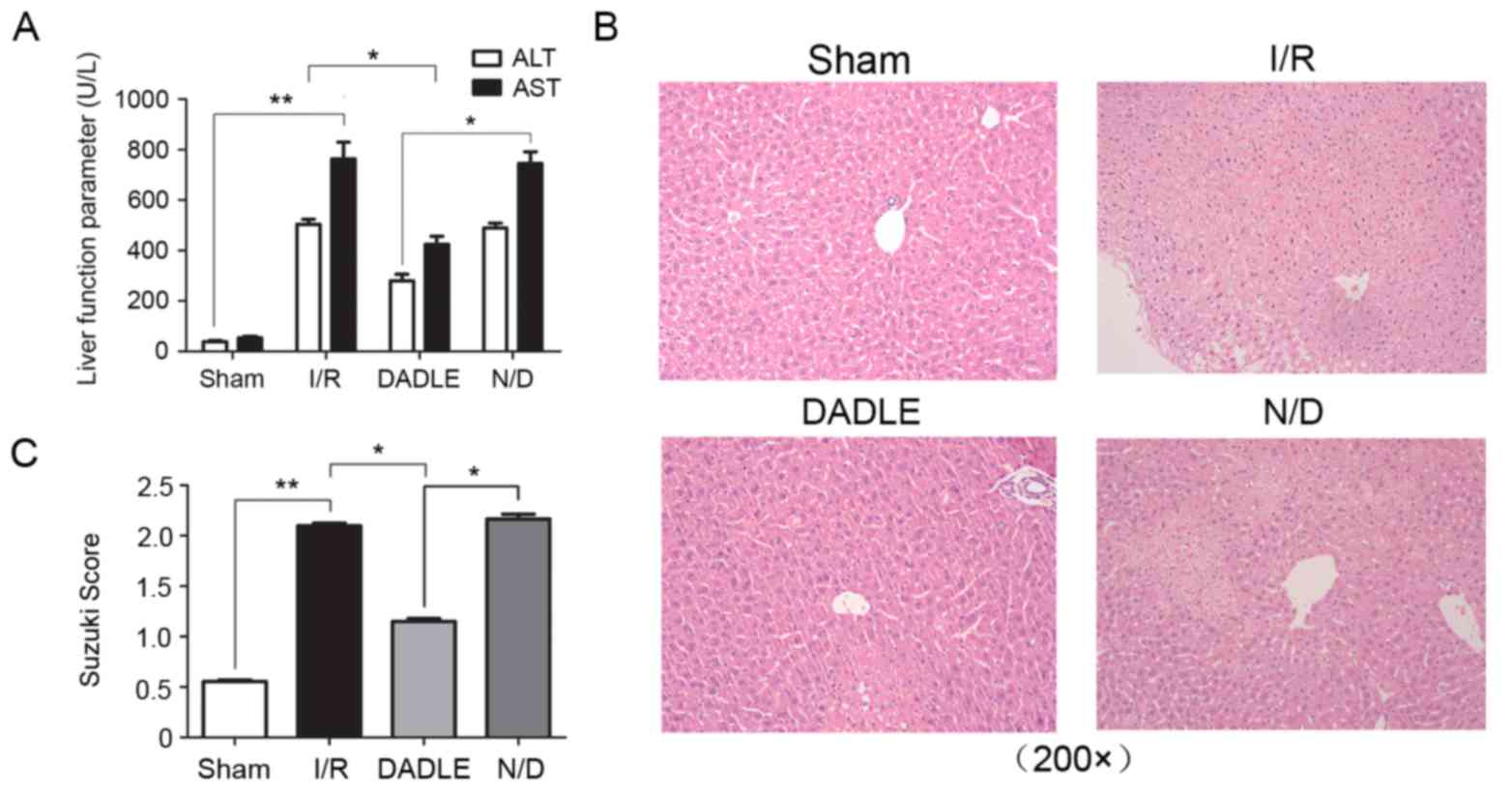
DADLE alleviates hepatic I/R
injury
Compared with in the Sham group, the AST and ALT
levels were significantly higher in the I/R group (P<0.01), and
hematoxylin and eosin (HE) staining detected obvious alterations in
lobe tissue structure, including hepatic sinus block, hepatocyte
swelling, degeneration and necrosis, and inflammatory cell
infiltration. Conversely, the serum levels of AST and ALT in the
serum DADLE group were significantly lower compared with the I/R
and N/D group (Fig. 2A;
P<0.05). HE staining detected fewer alterations in lobe
structure, reduced hepatocellular swelling and degeneration, and
decreased inflammatory cell infiltration in the DADLE group when
compared with the I/R and N/D group (Fig. 2B; however, there was no significant
alteration in the serum levels of AST and ALT, nor were there any
alterations in the histological structure of the lobe tissue in the
N/D group when compared with the I/R group (Fig. 2A; P>0.05). The Suzuki score
demonstrated that liver injury was increased in the I/R group
compared with in the Sham group (P<0.01; Fig. 2C and Table I), and the degree of injury was
significantly lower in the DADLE group compared with in the I/R and
N/D group (P<0.05; Fig.
2C).
 | Table I.Suzuki scores. |
Table I.
Suzuki scores.
| Group | Score |
|---|
| Sham |
0.55±0.0339a |
| I/R |
2.10±0.0663b |
| DADLE |
1.15±0.0731c |
| N/D |
2.17±0.1164 |
DADLE increases the ability of the
liver to resist oxidative damage
Compared with in the Sham group, the levels of SOD,
GSH and CAT in the I/R group were significantly decreased, whereas
the levels of MDA were significantly increased (P<0.05).
Compared with in the I/R group, the levels of SOD, GSH and CAT in
the DADLE group were significantly increased, whereas the MDA
levels were significantly decreased (Fig. 3; P<0.05). There were no
significant alterations in the levels of SOD, GSH, CAT and MDA
between the I/R and N/D groups (P>0.05; Fig. 3).
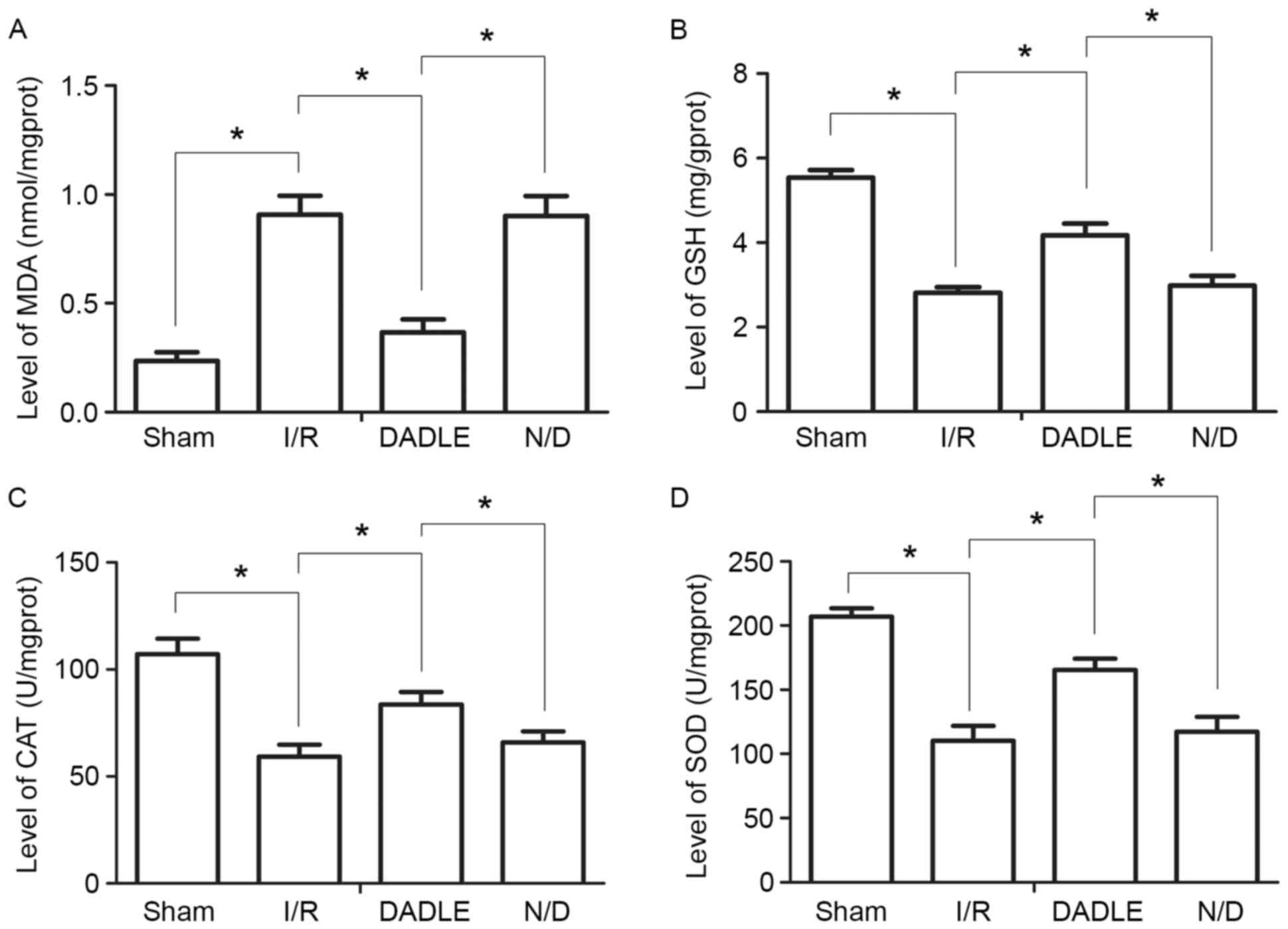 | Figure 3.Alterations in liver oxidative damage
in each group of mice. Levels of (A) MDA, (B) GSH, (C) CAT and (D)
SOD in liver tissue homogenates. Each experiment was repeated three
times, and the data are expressed as the mean ± standard deviation.
*P<0.05. CAT, catalase; DADLE, (D-Ala2,
D-Leu5)-Enkephalin; GSH, glutathione; I/R,
ischemia/reperfusion; MDA, malondialdehyde; N/D, naxolone + DADLE;
SOD, superoxide dismutase. |
DADLE promotes nuclear translocation
of Nrf2 and upregulates HO-1 expression
The expression levels of HO-1 and the nuclear
translocation of Nrf2 were analyzed using RT-PCR and western
blotting (Fig. 4). Compared with
in the Sham group, the mRNA expression levels of HO-1 were
significantly increased in the I/R group (P<0.05). In addition,
the mRNA expression levels of HO-1 were significantly increased in
the DADLE group compared with in the I/R group (P<0.01).
However, compared with in the I/R group, HO-1 mRNA expression was
not significantly altered in the N/D group (P>0.05; Fig. 4A and D). The western blotting
results indicated that the nuclear expression of Nrf2 protein was
significantly higher in the DADLE, I/R and N/D groups compared with
in the Sham group (P<0.01), and the nuclear expression of Nrf2
was significantly higher in the DADLE group compared with in the
I/R and N/D group (P<0.01; Fig. 4C
and F). The protein expression levels of cytoplasmic Nrf2 were
significantly different in all groups (Sham group vs. I/R group;
I/R group vs. DADLE group; DADLE group vs. N/D group), and the
trend in its expression was opposite to its trend in the nucleus
(P<0.05, P<0.01 and P<0.01, respectively); however, the
protein expression levels of total Nrf2 in all groups (Sham group
vs. I/R group; I/R group vs. DADLE group; DADLE group vs. N/D
group) were not statistically significant (P>0.05; Fig. 4B and E). In addition, the present
study detected the expression levels of HO-1, which is an
antioxidant protein downstream of Nrf2. The results indicated that
the protein expression levels of HO-1 were significantly increased
in the I/R, DADLE and N/D groups compared with in the Sham group,
and HO-1 expression was most obviously increased in the DADLE group
(P<0.01; Fig. 4B and E).
 | Figure 4.(A-F) Effects of DADLE on HO-1 and
Nrf2 expression in the liver of mice with I/R. (A and D) mRNA
expression levels of HO-1, as determined by polymerase chain
reaction. (B and E) Protein expression levels of HO-1, Nrf2-C and
Nrf2-T, as determined by western blotting. (C and F) Protein
expression levels of Nrf2-N, as determined by western blotting.
Each experiment was repeated three times, and the data are
presented as the mean ± standard deviation. DADLE,
(D-Ala2, D-Leu5)-Enkephalin; HO-1, heme
oxygenase-1; I/R, ischemia/reperfusion; N/D, naloxone + DADLE;
Nrf2, nuclear factor, erythroid 2 like 2; Nrf2-C, cytoplasmic Nrf2;
Nrf2-N, nuclear Nrf2; Nrf2-T, total Nrf2; PCNA, proliferating cell
nuclear antigen. *P<0.05, **P<0.01. |
Discussion
I/R injury is an important factor affecting liver
function following liver transplantation and liver resection. The
generation of ROS, and the activation of Kupffer cells, lymphocytes
and neutrophils, induces a series of damaging cellular responses,
leading to inflammation and cell death. DADLE is a synthetic
DOR-specific agonist, the molecular formula of which is
C29H39N5O7. DADLE has a
high affinity for the G protein-coupled DOR and is currently the
most studied DOR agonist. In recent years, numerous studies
(18–20) have focused on the protective
effects of DADLE on organs affected by I/R injury, and DADLE has
been reported to improve tissue oxygen utilization and maintain the
tissue oxygen metabolism balance, thus reducing disordered tissue
energy metabolism induced by hypoxia, and cell and tissue damage
(22). DADLE has also been
demonstrated to serve a protective role against cellular hypoxic
injury via various mechanisms. DADLE can activate protein kinase C
(PKC), and PKC in the cytoplasm can activate adenosine triphosphate
(ATP)-sensitive potassium channels (KATP channels),
resulting in the opening of mitochondrial KATP channels
to promote potassium efflux (23),
inhibit Ca2+ channel flow, shorten the action potential
duration and reduce the consumption of ATP, thereby serving a role
in organ protection. Activated PKC can also be translocated to the
membrane by phosphorylation. Nrf2 in the cytoplasm is a substrate
protein that can be activated by PKC and transferred to the
nucleus, where it may regulate the expression of various phase II
detoxification enzymes and antioxidant enzyme genes, including HO-1
(24). The subsequent increase in
tissue HO-1 expression enhances its antioxidative capacity and
reduces I/R injury (25). Finally,
the antioxidative activity may be attributed to the increased
production of carbon monoxide (CO), which is an end product of the
enzymatic activity of HO-1. Wei et al indicated that
exogenous CO released by CO-releasing molecule (CORM)-2 can be
applied to reduce hepatic I/R injury (26). Numerous studies have also
demonstrated that the immunomodulatory influence of CORM may occur
in some immunological diseases, including experimental autoimmune
uveoretinitis, type 1 diabetes, multiple sclerosis and allergic
encephalomyelitis (27–30). These studies demonstrated that CORM
as a potential therapeutic molecule for the treatment of
immunological diseases owing to its anti-inflammatory and
anti-apoptotic properties. Hepatic I/R injury induces formation of
reactive oxygen species, hepatocyte apoptosis and release of
pro-inflammatory cytokines, which together causes liver damage and
organ dysfunction. DADLE alleviates hepatic I/R injury and may be
used to exploit the potent antioxidant, anti-inflammatory and
cytoprotective effects of CORM.
In the present study, AST and ALT levels reached a
relative peak at 6 h after reperfusion, which is consistent with
the findings of previous studies (31,32);
therefore, these time points were used for subsequent experiments.
In the present study, the hepatoprotective effects of DADLE were
not dose-dependent; at lower concentrations, the levels of
transaminases were higher and protection was not significant. In
addition, at higher concentrations, the levels of transaminases
were also higher, which may be due to activation of the κ-opioid
receptor by the high concentration of DADLE, resulting in a
‘reverse preconditioning effect’ (33). Therefore, the present study
selected 5 mg/kg DADLE for subsequent experiments.
The results of the present study demonstrated that
hepatic I/R injury significantly increased the serum levels of ALT
and AST in mice; however, intraperitoneal injection with DADLE
prior to I/R resulted in a significant decrease in the serum levels
of ALT and AST. Histopathological analysis indicated that liver
cell degeneration, swelling and necrosis, and the degree of
inflammatory cell infiltration, were significantly reduced in
response to DADLE, and the Suzuki scores also further supported
these pathological observations. Mice in the N/D group were
intraperitoneally injected with naloxone every day for 1 week prior
to I/R, in order to block the opioid receptors, and were then
pretreated with DADLE. In the N/D group, neither ALT and AST levels
were decreased compared with in the I/R group, and
histopathological analysis detected hepatocellular degeneration,
swelling and necrosis, and inflammatory cell infiltration, thus
indicating that DADLE pretreatment had a protective effect on
hepatic function during hepatic I/R injury, whereas antagonizing
the DOR reversed the protective effects of DADLE on hepatic
function.
To evaluate liver oxidation, the levels of SOD, GSH,
CAT and MDA were detected in the liver tissue homogenate. The
results demonstrated that the levels of MDA were significantly
reduced in the DADLE pretreatment group, whereas the levels of SOD,
GSH and CAT were increased. The use of naloxone to antagonize the
opioid receptors offset these effects. These experimental results
confirmed that DADLE activation of the DOR may effectively reduce
hepatic I/R-induced oxidative damage.
It has previously been reported that DADLE activates
the DOR, thus resulting in DOR-associated neuronal protection, via
PKC (34). In addition, Cao et
al (35) demonstrated that
signal transduction via the DOR-PKC-Nrf2 pathway may exert a
protective effect against cell hypoxia-reoxygenation injury. The
present study indicated that the protein expression levels of total
Nrf2 were higher in the I/R, DADLE and N/D groups compared with in
the Sham group; however, this finding was not significant. In
addition, the expression levels of nuclear Nrf2 were significantly
higher in the I/R group compared with in the Sham group, whereas
the expression of nuclear Nrf2 in the DADLE group was more
obviously increased compared with in the I/R and N/D groups. These
findings suggested that DADLE exerts protective effects against
hepatic I/R injury. Activation of Nrf2 may therefore be considered
a potential target for the prevention of I/R injury, partly due to
the possibility that DADLE activates the DOR-PKC-Nrf2 axis,
resulting in Nrf2 nuclear translocation and activation of
downstream phase II detoxification enzymes and antioxidant genes.
HO-1 is a Nrf2-regulated phase II enzyme, and its antioxidative
effects have been confirmed (36).
Red blood cells damaged by I/R injury increase resistance to blood
flow, and heme released from red blood cells enhances the oxidation
process. HO-1 is a scavenger, which degrades heme and consumes
oxygen molecules in the degradation process, thus reducing free
oxygen radicals. The results of the present study demonstrated that
the mRNA and protein expression levels of HO-1 were significantly
higher in the I/R, DADLE and N/D groups compared with in the Sham
group, and the expression in the DADLE group was significantly
higher. These findings suggested that DADLE pretreatment may
activate the DOR-PKC-Nrf2 pathway to upregulate the expression of
HO-1, and possibly CO, which serves an antioxidative role during
I/R. However, DADLE may participate in protection against hepatic
I/R injury through various mechanisms and pathways; this hypothesis
requires further study.
CORM is an end product of the enzymatic activity of
HO-1, which serves an important role in protecting against several
immunoinflammatory and autoimmune diseases. Therefore, DADLE may
represent a promising therapeutic approach for the treatment of
I/R, and immunoinflammatory and autoimmune diseases, by activating
HO-1 and possibly CO (37–39).
Prevention of I/R injury in organs has garnered much
attention in recent years. Numerous studies have reported that some
compounds can induce upregulation of the Nrf2/HO-1 pathway to exert
protective effects on various I/R models (40–43).
The present study demonstrated that targeting the Nrf2/HO-1 pathway
may provide a novel strategy for preventing hepatic I/R injury.
In conclusion, DADLE pretreatment is able to improve
liver histopathology and liver function, significantly enhances
liver antioxidant capacity and reduces oxidative damage. The
underlying mechanism by which DADLE exerts its effects may be as
follows: DADLE activates Nrf2, resulting in its nuclear
translocation and the subsequent upregulation of HO-1, which is a
downstream antioxidative factor that may serve an antioxidative
role in hepatic I/R injury in mice. The present study may provide a
novel therapeutic strategy for the treatment of hepatic I/R injury
caused by hepatic resection, shock and liver transplantation.
Acknowledgements
The present study was partially supported by the
National Natural Science Foundation of China (grant no. 81360367),
the Natural Science Foundation of Guangxi (grant nos. 2015jjDA40010
and 2015GXNSFAA139175), the Scientific Research and Technology
Development Project for Guilin (grant no. 20140310-2-2), the
Guangxi Regional High-risk Tumors Early Prevention and Control of
Key Laboratory Open Research Project (grant no. GK2014-TKF01), the
Guangxi Science Fund for Distinguished Young Scholars Program
(grant no. 2016GXNSFFA380003) and the Prize Fund of Beijing Medical
Science (grant no. 346).
References
|
1
|
Jaeschke H and Woolbright BL: Current
strategies to minimize hepatic ischemia-reperfusion injury by
targeting reactive oxygen species. Transplant Rev (Orlando).
26:103–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y, Pu LY, Lu L, Wang XH, Zhang F and
Rao JH: N-acetylcysteine attenuates
reactive-oxygen-species-mediated endoplasmic reticulum stress
during liver ischemia-reperfusion injury. World J Gastroenterol.
20:15289–15298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reiniers MJ, van Golen RF, van Gulik TM
and Heger M: Reactive oxygen and nitrogen species in steatotic
hepatocytes: A molecular perspective on the pathophysiology of
ischemia-reperfusion injury in the fatty liver. Antioxid Redox
Signal. 21:1119–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemasters JJ, Qian T, He L, Kim JS, Elmore
SP, Cascio WE and Brenner DA: Role of mitochondrial inner membrane
permeabilization in necrotic cell death, apoptosis, and autophagy.
Antioxid Redox Signal. 4:769–781. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abu-Amara M, Yang SY, Tapuria N, Fuller B,
Davidson B and Seifalian A: Liver ischemia/reperfusion injury:
Processes in inflammatory networks-a review. Liver Transpl.
16:1016–1032. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niture SK, Kaspar JW, Shen J and Jaiswal
AK: Nrf2 signaling and cell survival. Toxicol Appl Pharmacol.
244:37–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Y, Gong J, Yoshida T, Eberhart CG, Xu
Z, Kombairaju P, Sporn MB, Handa JT and Duh EJ: Nrf2 has a
protective role against neuronal and capillary degeneration in
retinal ischemia-reperfusion injury. Free Radic Biol Med.
51:216–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka N, Ikeda Y, Ohta Y, Deguchi K, Tian
F, Shang J, Matsuura T and Abe K: Expression of Keap1-Nrf2 system
and antioxidative proteins in mouse brain after transient middle
cerebral artery occlusion. Brain Res. 1370:246–253. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang DD: Mechanistic studies of the
Nrf2-Keap1 signaling pathway. Drug Metab Rev. 38:769–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Pung D, Leong V, Hebbar V, Shen G,
Nair S, Li W and Kong AN: Induction of detoxifying enzymes by
garlic organosulfur compounds through transcription factor Nrf2:
Effect of chemical structure and stress signals. Free Radic Biol
Med. 37:1578–1590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong WS, Jun M and Kong AN: Nrf2: A
potential molecular target for cancer chemoprevention by natural
compounds. Antioxid Redox Signal. 8:99–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das BN, Kim YW and Keum YS: Mechanisms of
Nrf2/Keap1-dependent phase II cytoprotective and detoxifying gene
expression and potential cellular targets of chemopreventive
isothiocyanates. Oxid Med Cell Longev. 2013:8394092013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang AL, Niu Q, Shi N, Wang J, Jia XF,
Lian HF, Liu Z and Liu CX: Glutamine ameliorates intestinal
ischemia-reperfusion Injury in rats by activating the Nrf2/Are
signaling pathway. Int J Clin Exp Pathol. 8:7896–7904.
2015.PubMed/NCBI
|
|
16
|
Hu Y, Duan M, Liang S, Wang Y and Feng Y:
Senkyunolide I protects rat brain against focal cerebral
ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1
and inhibiting caspase 3. Brain Res. 1605:39–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo H, Li MJ, Liu QQ, Guo LL, Ma MM, Wang
SX, Yu B and Hu LM: Danhong injection attenuates
ischemia/reperfusion-induced brain damage which is associating with
Nrf2 levels in vivo and in vitro. Neurochem Res. 39:1817–1824.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Tang B, Tang F, Li Y, Zhang G,
Zhong L, Dong C and He S: Protection of rat intestinal epithelial
cells from ischemia/reperfusion injury by (D-Ala2,
D-Leu5)-enkephalin through inhibition of the MKK7-JNK signaling
pathway. Mol Med Rep. 12:4079–4088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SY, Duan YL, Zhao B, Wang XR, Zhao Z
and Zhang GM: Effect of delta opioid receptor activation on spatial
cognition and neurogenesis in cerebral ischemic rats. Neurosci
Lett. 620:20–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng YJ, Wang XR, Chen HZ, Wu XJ, Zhao YH
and Su DS: Protective effects of the delta opioid peptide [D-Ala2,
D-Leu5]enkephalin in an ex vivo model of ischemia/reperfusion in
brain slices. CNS Neurosci Ther. 18:762–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ
and Cejalvo D: Neutrophil infiltration as an important factor in
liver ischemia and reperfusion injury. Modulating effects of FK506
and cyclosporine. Transplantation. 55:1265–1272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung SP, Song FQ, Yu T, Weng Y, Sun S,
Weil MH and Tang W: Effect of therapeutic hypothermia vs δ-opioid
receptor agonist on post resuscitation myocardial function in a rat
model of CPR. Resuscitation. 82:350–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohnuma Y, Miura T, Miki T, Tanno M, Kuno
A, Tsuchida A and Shimamoto K: Opening of mitochondrial K(ATP)
channel occurs downstream of PKC-epsilon activation in the
mechanism of preconditioning. Am J Physiol Heart Circ Physiol.
283:H440–H447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keum YS: Regulation of Nrf2-mediated phase
II detoxification and anti-oxidant genes. Biomol Ther (Seoul).
20:144–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Volti G, Sorrenti V, Murabito P,
Galvano F, Veroux M, Gullo A, Acquaviva R, Stacchiotti A, Bonomini
F, Vanella L and Di Giacomo C: Pharmacological induction of heme
oxygenase-1 inhibits iNOS and oxidative stress in renal
ischemia-reperfusion injury. Transplant Proc. 39:2986–2991. 2007;
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei Y, Chen P, de Bruyn M, Zhang W, Bremer
E and Helfrich W: Carbon monoxide-releasing molecule-2 (CORM-2)
attenuates acute hepatic ischemia reperfusion injury in rats. BMC
Gastroenterol. 10:422010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nikolic I, Saksida T, Mangano K, Vujicic
M, Stojanovic I, Nicoletti F and Stosic-Grujicic S: Pharmacological
application of carbon monoxide ameliorates islet-directed
autoimmunity in mice via anti-inflammatory and anti-apoptotic
effects. Diabetologia. 57:980–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fagone P, Mangano K, Mammana S, Cavalli E,
Di Marco R, Barcellona ML, Salvatorelli L, Magro G and Nicoletti F:
Carbon monoxide-releasing molecule-A1 (CORM-A1) improves clinical
signs of experimental autoimmune uveoretinitis (EAU) in rats. Clin
Immunol. 157:198–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fagone P, Mangano K, Coco M, Perciavalle
V, Garotta G, Romao CC and Nicoletti F: Therapeutic potential of
carbon monoxide in multiple sclerosis. Clin Exp Immunol.
167:179–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fagone P, Mangano K, Quattrocchi C,
Motterlini R, Di Marco R, Magro G, Penacho N, Romao CC and
Nicoletti F: Prevention of clinical and histological signs of
proteolipid protein (PLP)-induced experimental allergic
encephalomyelitis (EAE) in mice by the water-soluble carbon
monoxide-releasing molecule (CORM)-A1. Clin Exp Immunol.
163:368–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stewart RK, Dangi A, Huang C, Murase N,
Kimura S, Stolz DB, Wilson GC, Lentsch AB and Gandhi CR: A novel
mouse model of depletion of stellate cells clarifies their role in
ischemia/reperfusion- and endotoxin-induced acute liver injury. J
Hepatol. 60:298–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou J, Xia Y, Jiang R, Chen D, Xu J, Deng
L, Huang X, Wang X and Sun B: PTPRO plays a dual role in hepatic
ischemia reperfusion injury through feedback activation of NF-κB. J
Hepatol. 60:306–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aitchison KA, Baxter GF, Awan MM, Smith
RM, Yellon DM and Opie LH: Opposing effects on infarction of delta
and kappa opioid receptor activation in the isolated rat heart:
Implications for ischemic preconditioning. Basic Res Cardiol.
95:1–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma MC, Qian H, Ghassemi F, Zhao P and Xia
Y: Oxygen-sensitive {delta}-opioid receptor-regulated survival and
death signals: Novel insights into neuronal preconditioning and
protection. J Biol Chem. 280:16208–16218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao S, Chao D, Zhou H, Balboni G and Xia
Y: A novel mechanism for cytoprotection against hypoxic injury:
δ-opioid receptor-mediated increase in Nrf2 translocation. Br J
Pharmacol. 172:1869–1881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martin D, Rojo AI, Salinas M, Diaz R,
Gallardo G, Alam J, De Galarreta CM and Cuadrado A: Regulation of
heme oxygenase-1 expression through the phosphatidylinositol
3-kinase/Akt pathway and the Nrf2 transcription factor in response
to the antioxidant phytochemical carnosol. J Biol Chem.
279:8919–8929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Motterlini R and Foresti R: Heme
oxygenase-1 as a target for drug discovery. Antioxid Redox Signal.
20:1810–1826. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ryter SW and Choi AM: Targeting heme
oxygenase-1 and carbon monoxide for therapeutic modulation of
inflammation. Transl Res. 167:7–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fagone P, Patti F, Mangano K, Mammana S,
Coco M, Touil-Boukoffa C, Chikovani T, Di Marco R and Nicoletti F:
Heme oxygenase-1 expression in peripheral blood mononuclear cells
correlates with disease activity in multiple sclerosis. J
Neuroimmunol. 261:82–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu T, Wei G, Xi M, Yan J, Wu X, Wang Y,
Zhu Y, Wang C and Wen A: Synergistic cardioprotective effects of
Danshensu and hydroxysafflor yellow A against myocardial
ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1
pathway. Int J Mol Med. 38:83–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee MH, Han MH, Lee DS, Park C, Hong SH,
Kim GY, Hong SH, Song KS, Choi IW, Cha HJ and Choi YH: Morin exerts
cytoprotective effects against oxidative stress in C2C12 myoblasts
via the upregulation of Nrf2-dependent HO-1 expression and the
activation of the ERK pathway. Int J Mol Med. 39:399–406. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen ZY, Sun Q, Xia ZY, Meng QT, Lei SQ,
Zhao B, Tang LH, Xue R and Chen R: Overexpression of DJ-1 reduces
oxidative stress and attenuates hypoxia/reoxygenation injury in
NRK-52E cells exposed to high glucose. Int J Mol Med. 38:729–736.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan YF, Yang WJ, Xu Q, Chen HP, Huang XS,
Qiu LY, Liao ZP and Huang QR: DJ-1 upregulates anti-oxidant enzymes
and attenuates hypoxia/re-oxygenation-induced oxidative stress by
activation of the nuclear factor erythroid 2-like 2 signaling
pathway. Mol Med Rep. 12:4734–4742. 2015. View Article : Google Scholar : PubMed/NCBI
|