Introduction
Glutamic acid (Glu) is an important source of energy
in the mucosa and the conditionally essential amino acid in early
weaned piglets (1). Glu has an
important function in the growth and repair of intestinal mucosa.
The absorption of Glu in the intestine requires specific
transporters. Excitatory amino acid transporters (EAATs) are
Na+-dependent high-affinity Glu transporters (2), that maintain the Glu balance of the
nervous system. Excitatory amino acid carrier 1 (EAAC1) belongs to
EAAT family and its transport rate is ~10 times of that of other
transporters in the nervous system (3). EAAC1 is also expressed in other
non-nervous tissues such as the small intestine. EAAC1 expression
is negatively regulated by glutamate transporter associate protein
3–18 (GTRAP3-18). In various types of human cancer and epilepsy,
GTRAP3-18 inhibits Glu transport via EAAC1 by binding to the
C-terminal of EAAC1, leading to Glu poisoning in the nervous system
(4). It is of note that, GTRAP3-18
may bind specifically to EAAC1 although not to other EAATs
(5). EAAC1 expression is
associated with the expression of GTRAP3-18. Previous studies have
focused on EAAC1 in the nervous system as opposed to the intestine
(6,7). A previous study determined that
deletion of the EAAC1 gene may lead to the loss of
age-dependent dopaminergic neurons and an increase in oxidative
stress in mice (8). In patients
with Alzheimer's disease, abnormal EAAC1 accumulation was located
in the hippocampal neurons (9). Fu
et al (10) cloned the
jejunal EAAC1 protein in suckling piglets and investigated the
dynamic changes of EAAC1 protein expression, which was low in the
ileum of piglets with low birth weight (10). These previous findings imply that
EAAC1 expression may be associated with abnormal development or the
stress and disease status of the piglets. Low EAAC1 expression may
reduce the transport rate of Glu and impair development and
absorption function of the mucosa in piglets (10). Weaning induces stress piglet stress
and the uptake of maternal Glu is interrupted. The present study
aimed to investigate the changes of the expression levels of EAAC1
and its regulatory protein GTRAP3-18 in the jejunum and ileum of
weaned piglets. The effect of weaning on EAAC1 expression was
analyzed; therefore, providing insight into the underlying
molecular mechanism of intestinal mucosa repair and potential
measures that may be implemented in order to improve the growth
performance of weaned piglets.
Materials and methods
Grouping and feeding management
The experiment was performed at Pig Breeding
Demonstration Base (Qiqihar, China) in May 2010. A total of 40
hybrid piglets (Landrace × Large White × Duroc) born to 40
different sows, with similar body weight were selected. A
single-factor experiment design was adopted. The piglets were
randomly divided into two groups (n=20 per group): Control group
(suckling piglets) and experimental group (weaned piglets, reared
in isolation). The experiment continued for 10 days once the
piglets were 10 days old.
The suckling piglets were reared by sows in the
piglet house for the first 10 days of life. Following this, the
weaned piglets were then reared in a nursery piglet house for a
further 10 days. Weaned piglets were fed with corn-soybean
meal-based starter diet for 10 days, 3 times daily (morning, noon
and evening), and allowed free access to water. Each weaned piglet
was fed in a separate cage and the quantity of food consumed by
each weaned piglet (the feed-to-gain ratio) was calculated by
weighing the amount of food originally provided and the amount of
food leftover each morning. The weaned piglets were weighed on the
first day they were fed separately (10 days old) and on the last
day when the study was complete (20 days old). The composition and
the nutrition of the basal diet are presented in Table I.
 | Table I.Weaning diet of piglets used in the
present study (dry basis). |
Table I.
Weaning diet of piglets used in the
present study (dry basis).
| A, Contents |
|---|
|
|---|
| Ingredient | Quantity (%) |
|---|
| Lactose | 15 |
| Glucose | 8.52 |
| Corn | 15.95 |
| Soybean | 56.00 |
| L-threonine | 0.00 |
| Corn oil | 0.8 |
| Limestone | 1.05 |
|
CaHPO4 | 1.58 |
| Iodized salt | 0.5 |
| Vitamin-mineral
premix | 0.5 |
| Lincomycin
antibiotic | 0.1 |
|
| B, Nutrition |
|
| Ingredient | Quantity |
|
| CP (%) | 26.0 |
| DE (MJ/kg) | 14.27 |
| P (%) | 0.92 |
| Ca (%) | 0.68 |
| Ca:P | 0.74 |
| Arginine (mg/kg) | 1.87 |
| Histidine
(mg/kg) | 0.69 |
| Isoleucine
(mg/kg) | 1.16 |
| Leucine (mg/kg) | 2.07 |
| Lysine (mg/kg) | 1.63 |
| Methionine
(mg/kg) | 0.37 |
| Cysteine (mg/kg) | 0.42 |
| Phenylalanine
(mg/kg) | 1.28 |
| Tyrosine (mg/kg) | 0.99 |
| Threonine
(mg/kg) | 1.02 |
| Tryptophan
(mg/kg) | 0.35 |
| Valine (mg/kg) | 1.22 |
The nursery piglet house provided a closed
environment with good ventilation and cement flooring. Segregated
early weaning (SEW) was adopted with 1 piglet per pen. An infrared
heat lamp was hung 0.8 m above the ground at the corner of the pen.
The ground directly under the lamp was paved with a 0.5×1 m
heat-absorbing pad to ensure the warmth of the piglets. The present
study was approved by the Ethics Committee of Northeast
Agricultural University (Harbin, China) (approval no. SYXK (Hei)
2012–2067).
Sample collection and processing
On the morning of the 11th day, 12 piglets were
randomly selected from each group (n=24). Piglets were sedated and
maintained under anesthesia by inhalation of 8% anesthetic
isoflurane via a facial mask. The abdominal cavity was opened to
harvest the jejunum and ileum, which were washed with ice-cold
normal saline. Samples were collected from the middle of the
intestine and preserved in liquid nitrogen, as previously described
(5). The samples were ground with
liquid nitrogen to powder using a mortar and subsequently preserved
at −80°C. At the end of tissue sampling, piglets were euthanized
via an intra-cardiac injection of 50 mg/kg sodium pentobarbital
(Schering-Plough Canada, Inc., Pointe-Claire, QC, Canada) (11).
Sample preparation
Cryopreserved homogenate small intestine sample
weighing ~1.3 g was used and mixed with homogenate buffer (50 mM
D-mannitol, 10 mM HEPES and 2.0 µg·ml-1 each of aprotinin,
leupetin, pepstatin A, N-tosyl-L-phenylalanine chloromethyl-ketone,
N-a-p-tosyl-L-lysine ketone and 0.20 mM PMSF, pH 7.4; all Sigma;
Merck KGaA, Darmstadt, Germany) at the proportion of 1 g/20 ml.
Subsequently, the sample was thawed in the homogenate buffer
containing the protease inhibitor. The sample was homogenized using
a multi-layer homogenizer at 1,523 × g for 3 min at 4°C (with a ~20
sec pause every min). The homogenate was weighed and the total
volume was recorded with 2 ml cryopreserved at −80°C. The endoplasm
sample was prepared by Mg2+ precipitation and differential
centrifugation at 4°C as previously described (5). The remaining endoplasm sample was
used for further differential centrifugation to obtain the apical
membrane of the cells. Briefly, the remaining supernatant was mixed
with 1 M MgCl2 to produce 10 mM MgCl2, stirred on ice for 15 min
and then centrifuged at 4°C and 2,400 × g for 15 min. After
discarding the top foam layer, the resultant supernatant was
centrifuged at 4°C and 19,000 × g for 30 min using the Ti 55.2
rotor on a Beckman L8-55 Ultracentrifuge to generate crude apical
membrane pellets. The supernatant obtained was regarded as the
cytosolic fraction and sampled for protein content analyses as well
as western blotting analyses of the abundance of target proteins.
The crude apical membrane pellets were then suspended in 15 ml of
membrane suspension buffer (300 mM D-mannitol, 25 mM HEPES and 2.0
µg·ml-1 each of aprotinin, leupetin, pepstatin A,
N-tosyl-L-phenylalanine chloromethyl-ketone, N-α-p-tosyl-L-lysine
ketone and 0.20 mM PMSF, pH 7.4) and centrifuged at 4°C and 39,000
× g for 30 min to generate the final apical membrane vesicle
pellets. The final pellets were re-suspended with a 25-gauge needle
in 5 ml of the same membrane suspension buffer to yield the apical
membrane fraction samples for protein content and western blotting
analyses.
Detection of EAATs expression
levels
The protein concentration assay was performed with
PMSF buffer (Sigma; Merck KGaA) as previously described (6). Protein in the homogenate, endoplasm
and apical membrane of the cells was quantified using a Bradford
assay dye reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and proteins in fetal bovine serum (grade IV) as the standard. A
total of 20 µg protein/lane was loaded and separated on a 10%
SDS-PAGE gel.
Western blotting
Western blotting was performed using 1 µg/µl protein
sample and β-actin as the internal control. Proteins were
transferred to PVDF membranes, which were blocked at room
temperature for 1 h with 6% non-fat dry milk powder dissolved in 1X
TBS (25 mM Tris-HCl, 0.15 M NaCl, pH 7.4) and then incubated at 4°C
with primary antibodies overnight. The primary antibody for EAAC1
was goat anti-human EAAC1 polyclonal antibody (cat. no. sc-7761;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) diluted to
1:2,000, the primary antibody for GTRAP3-18 was mouse anti-human
GTRAP3-18 polygonal antibody (cat. no. H00010550-A01; Abnova,
Atlanta, GA, USA) diluted to 1:2,000; primary antibody to β-actin
was mouse anti-human monoclonal antibody (cat. no. VMA00014;
Bio-Rad Laboratories, Inc.) diluted to 1:10,000. The membranes were
then incubated at room temperature for 1 h with the purified
secondary antibody rabbit anti-human IgG (cat. no. STAR195; Bio-Rad
Laboratories, Inc.) diluted to 1:10,000.
Detection of gene expression
The primers for the target genes and the
housekeeping gene were designed using Primer Premier version 5.0
software (Premier Biosoft International, Palo Alto, CA, USA) based
on GenBank cDNA sequences (Table
II). The primers were synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). To avoid contamination of the
primers by non-specific genomic DNA, mRNA sequences from all
samples were aligned against the pig genome using Spidey v0.7
software (now Splign software; https://www.ncbi.nlm.nih.gov/sutils/splign/splign.cgi).
Each primer contained 2 exons. Total RNA was extracted from the
samples using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
as previously described (6).
Following digestion with DNA enzyme (Invitrogen; Thermo Fisher
Scientific, Inc.), cDNA synthesis was performed using iScript cDNA
synthesis kit (Bio-Rad, Laboratories, Inc.) according to the
manufacturer's protocol. Quantitative polymerase chain reaction
(qPCR) was performed using iQ SYBR-Green supermix (Qiagen, Inc.,
Valencia, CA, USA) with total reaction volume of 25 µl. The
procedures of reverse transcription (RT)-qPCR were as follows: RT
at 50°C for 30 min, denaturation at 95°C for 15 min, amplification
and quantification for 45 cycles, denaturation at 95°C for 15 sec,
annealing at 54°C for 15 sec, extension at 72°C for 15 sec;
plotting of dissociation curve, 60–99°C, temperature rise at
0.1°C/sec, with fluorescence detection. The relative expression
ratio of target gene to the housekeeping gene was calculated as
follows:
 | Table II.Primer sequences and product size. |
Table II.
Primer sequences and product size.
| Gene | Forward (5′→3′) | Reverse (5′→3′) | Size (bp) | GenBank no. |
|---|
| EAAC1 |
CAAACTGGGCCTTTACATGG |
TGTTGCTGAACTGGAGGAGA | 169 | AY195622 |
| GTRAP3-18 |
CTGGTATTCACGGGCTTTGT |
CCCCCAAACATGGATATGAG | 131 | NM_001048073 |
| β-actin |
GGATGCAGAAGGAGATCACG |
ATCTGCTGGAAGGTGGACAG | 150 | AY550069 |
R=2-Cq (target gene-housekeeping gene) (12)
where R is relative expression ratio of the target
gene; Cq is the quantification cycle value. Under this threshold
cycle value, the target gene and the β-actin housekeeping gene were
amplified by over 30 fluorescence units. The optimal RT-qPCR
efficiency was obtained from the formula of −1+10 (−1/slope) with
serial dilution of RNA. The efficiency was consistent for the
target gene and β-actin. Experiments were repeated three times with
three duplicates of each.
Quantification of free amino acids in
the small intestine of piglets
High-performance liquid chromatography (HPLC) was
performed as previously described (13). Briefly, HPLC was performed using
the LC-9A liquid chromatograph system (Shimadzu Corporation, Kyoto,
Japan). The analytical column Inertsil ODS-2 (150×4.6 mm ID 5lm; GL
Sciences, Inc., Tokyo, Japan) was fixed at 40°C and connected
through a corresponding guard column (10×4.0 mm ID 5 lm; GL
Sciences, Inc.) with a HPLC workstation; the flow rate of the
eluate was 1.0 ml/min. All samples were injected into the column
with an Auto Injector (Shimadzu Corporation). An RF-530
fluorescence spectromonitor (Shimadzu Corporation) was used with
excitation and emission set at 380 and 510 nm, respectively. The
signals from the detector were recorded on a Chromatopac C-R4A
(Shimadzu Corporation). O-phthalaldehyde was used as an internal
standard.
Statistical analysis
The protein expression levels were expressed
relative to the expression levels of the EAAC1 and GTRAP3-18 genes.
The protein blots were scanned and analyzed using Quantity One
software v19.0 (Bio-Rad Laboratories, Inc.). The optical density
was calculated. The relative expression of the target protein was
characterized as the optical density ratio of the target gene to
β-actin. One-way analysis of variance was performed using SAS
version 9.2 (SAS Institute, Inc., Cary, NC, USA). Data were
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference. The
findings of the present study were plotted as histograms and curves
using The Scientific Fig. Processor software (Fig. P Software
Incorporated, Hamilton, ON, Canada).
Results
Effect of weaning on growth
performance
Weaned piglets were fed 148.50±16.90 g/day (n=20)
with feed-to-gain ratio of 3.58±2.34 during the experiment. The
weaned piglets showed had significantly reduced body weight at the
end of the experiment, compared with the suckling piglets
(P<0.05) and the daily weight gain of the weaned piglets was
also significantly lower (P<0.001; Table III). Therefore, early weaning had
a negative impact on the growth performance of piglets.
 | Table III.Effect of weaning on growth
performance of piglets. |
Table III.
Effect of weaning on growth
performance of piglets.
| Characteristic | Suckling | Weaning | SEM |
|---|
| Initial body weight
(kg) | 4.18 | 4.77 | 0.16 |
| Terminal body
weight (kg) | 7.68 | 5.19a | 0.24 |
| Average daily gain
(g/day) | 350 | 41.50b | 24.49 |
| Average daily food
intake (g/day) | – | 148.50 | 16.90 |
| ADFI/ADG (g/g) | – | 3.58 | 2.34 |
Effect of weaning on EAAC1 protein
expression
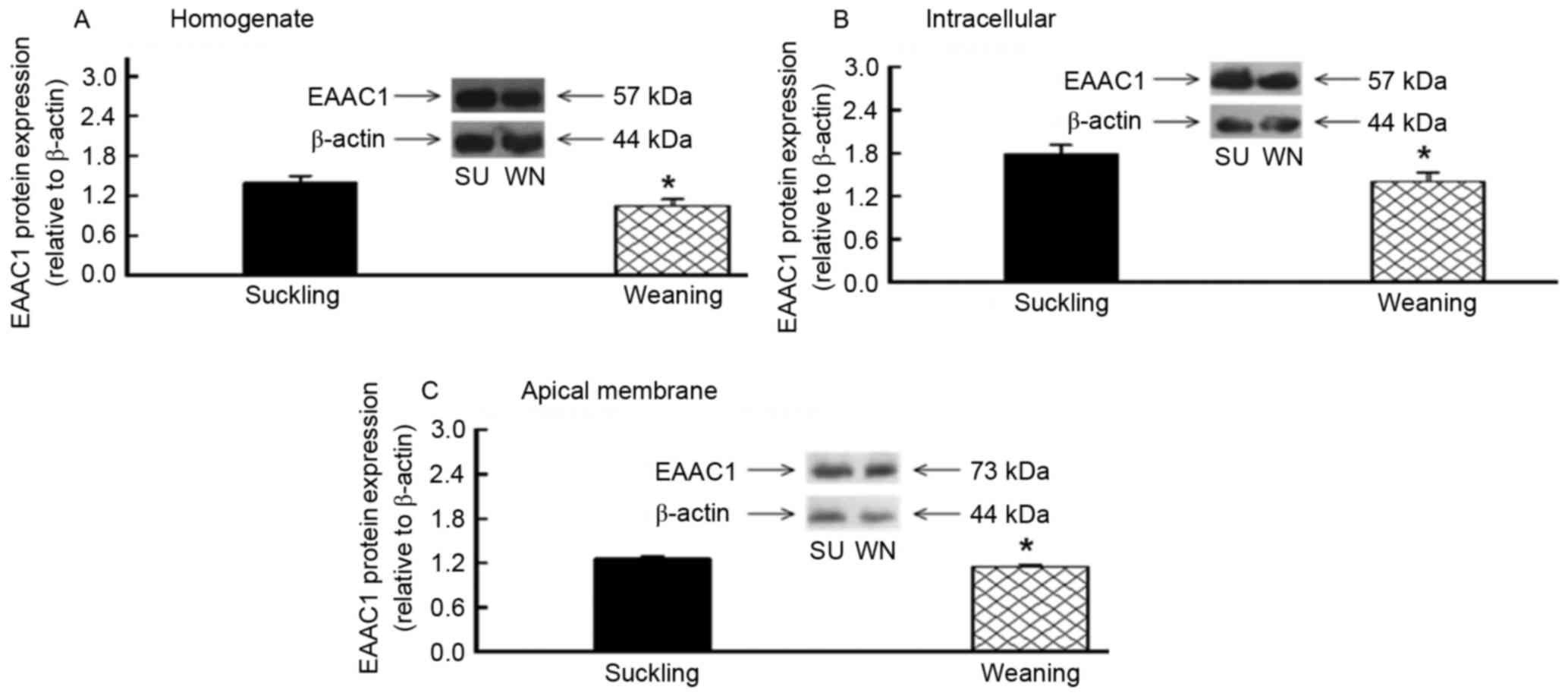
EAAC1 protein content was detected in the
homogenate, endoplasm and apical membrane of the small intestine
using western blotting. The molecular weight of the EAAC1 was 57
kDa in the homogenate and endoplasm and 73 kDa in the apical
membrane of the cells. With β-actin as the internal control, the
EAAC1 protein expression in the homogenate, endoplasm and apical
membrane of the jejunum was significantly reduced, by 25, 21 and
9%, respectively (P<0.05; Fig.
1). The EAAC1 protein expression in the homogenate, endoplasm
and apical membrane of the ileum was also significantly reduced, by
32, 22 and 14%, respectively (P<0.05; Fig. 2). Therefore, EAAC1 protein was
expressed in the small intestine of piglets, and its expression
levels were reduced following early weaning.
Effect of early weaning on EAAC1 mRNA
expression
EAAC1 mRNA expression was compared between the
suckling piglets and the weaned piglets, as presented in Table IV. β-actin was used as an internal
reference, the EAAC1 mRNA expression in the jejunum of the weaned
piglets decreased by 88% as compared with the suckling piglets
(P<0.05); the EAAC1 mRNA expression in the ileum of the weaned
piglets decreased by 73% (P<0.05).
 | Table IV.Intestinal excitatory amino acid
carrier 1 mRNA levels (relative to β-actin) in piglets. |
Table IV.
Intestinal excitatory amino acid
carrier 1 mRNA levels (relative to β-actin) in piglets.
| Location | Suckling | Weaning | SEM |
|---|
| Jejunum | 0.0201 | 0.0024a | 0.0026 |
| Ileum | 0.0179 | 0.0048a | 0.0026 |
Correlations of EAAC1 protein and mRNA
expression levels in different parts of the small intestine
Pearson's correlation was used to determine that the
EAAC1 protein and mRNA expression levels in the homogenate (r=0.52;
P=0.042; n=24), endoplasm (r=0.56; P=0.021; n=24) and apical
membrane of cells (r=0.49; P=0.008; n=24) in the jejunum were
positively correlated for weaned and suckling piglets.
Additionally, the EAAC1 protein expression in the homogenate of the
jejunum was positively correlated with the endoplasm of the
jejunum. The EAAC1 protein expression in the endoplasm of the
jejunum was positively correlated with that in the apical membrane
of the cells for weaned and suckling piglets. The EAAC1 mRNA and
protein expression levels in the homogenate (r=0.51; P=0.021;
n=24), endoplasm (r=0.51; P=0.016; n=24) and apical membrane of
cells (r=0.41; P=0.016; n=24) in the ileum were positively
correlated for weaned and suckling piglets. EAAC1 protein
expression in the homogenate of ileum was positively correlated
with that in the endoplasm of the ileum. The EAAC1 protein
expression in the endoplasm of ileum was positively correlated with
that in the apical membrane of the cells (P<0.05) for weaned and
suckling piglets.
Effect of early weaning on GTRAP3-18
protein expression
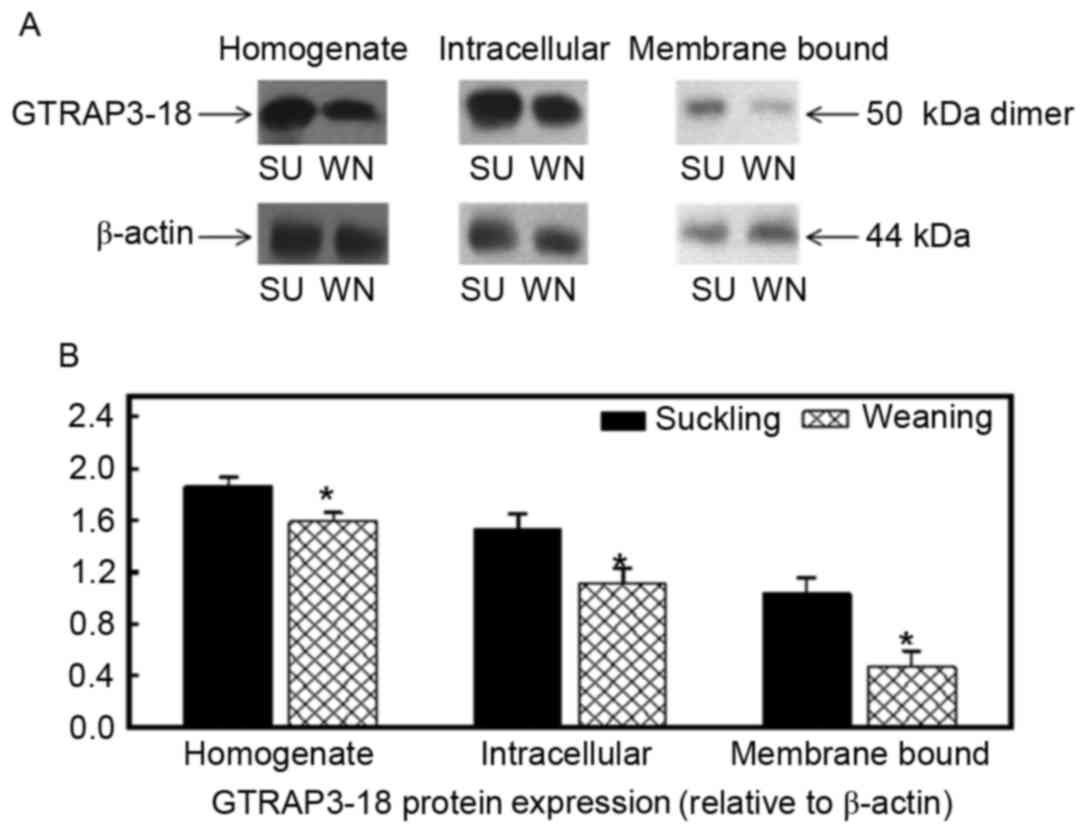
The molecular weight of GTRAP3-18 in the homogenate,
endoplasm and apical membrane of cells in the jejunum and ileum was
50 kDa, as presented in Figs. 3
and 4. β-actin was used as an
internal reference and the GTRAP3-18 protein expression in the
homogenate, endoplasm and apical membrane of the jejunum of the
weaned piglets was reduced by 15, 28 and 55% when compared with the
suckling piglets (P<0.05; Fig.
3). The GTRAP3-18 protein expression in the homogenate,
endoplasm and apical membrane of the ileum was also significantly
reduced by 16, 7 and 27%, respectively (P<0.05; Fig. 4).
Effect of early weaning on GTRAP3-18
mRNA expression
The GTRAP3-18 mRNA expression levels in the small
intestine of the weaned piglets and suckling piglets are presented
in Table V. With β-actin as
internal reference, the GTRAP3-18 mRNA expression in the jejunum of
the weaned piglets was reduced by 70% compared with the suckling
piglets (P<0.05) and expression in the ileum of the weaned
piglets was reduced by 52% (P<0.05).
 | Table V.Intestinal glutamate transporter
associate protein 3–18 mRNA levels (relative to β-actin) in
piglets. |
Table V.
Intestinal glutamate transporter
associate protein 3–18 mRNA levels (relative to β-actin) in
piglets.
| Location | Suckling | Weaning | SEM |
|---|
| Jejunum | 0.0067 | 0.0020a | 0.001 |
| Ileum | 0.0056 | 0.0027a | 0.0007 |
Correlations of GTRAP3-18 expression
levels in different parts of the small intestine
Pearson's correlation revealed that the GTRAP3-18
protein and mRNA expression levels in the homogenate (r=0.33;
P=0.027; n=24), endoplasm (r=0.54; P=0.019; n=24) and apical
membrane of cells (r=0.56; P=0.028; n=24) in the jejunum were
positively linearly correlated for weaned and suckling piglets.
Additionally, the GTRAP3-18 protein expression in the homogenate of
the jejunum was positively correlated with that in the endoplasm
and apical membrane of the jejunum. The GTRAP3-18 protein
expression in the endoplasm of the jejunum was positively
correlated with that in the apical membrane of the cells
(P<0.05) for weaned and suckling piglets. The GTRAP3-18 protein
and mRNA expression in the homogenate (r=0.42; P=0.014; n=24),
endoplasm (r=0.42; P=0.047; n=24) and apical membrane of cells
(r=0.15; P=0.029; n=24) in the ileum were positively linearly
correlated (P<0.05) for weaned and suckling piglets. GTRAP3-18
protein expression in the homogenate of ileum was positively
correlated with that in the endoplasm and apical membrane of the
ileum. The GTRAP3-18 protein expression in the endoplasm of ileum
was positively linearly correlated with that in the apical membrane
of the cells (P<0.05) for the weaned and suckling piglets.
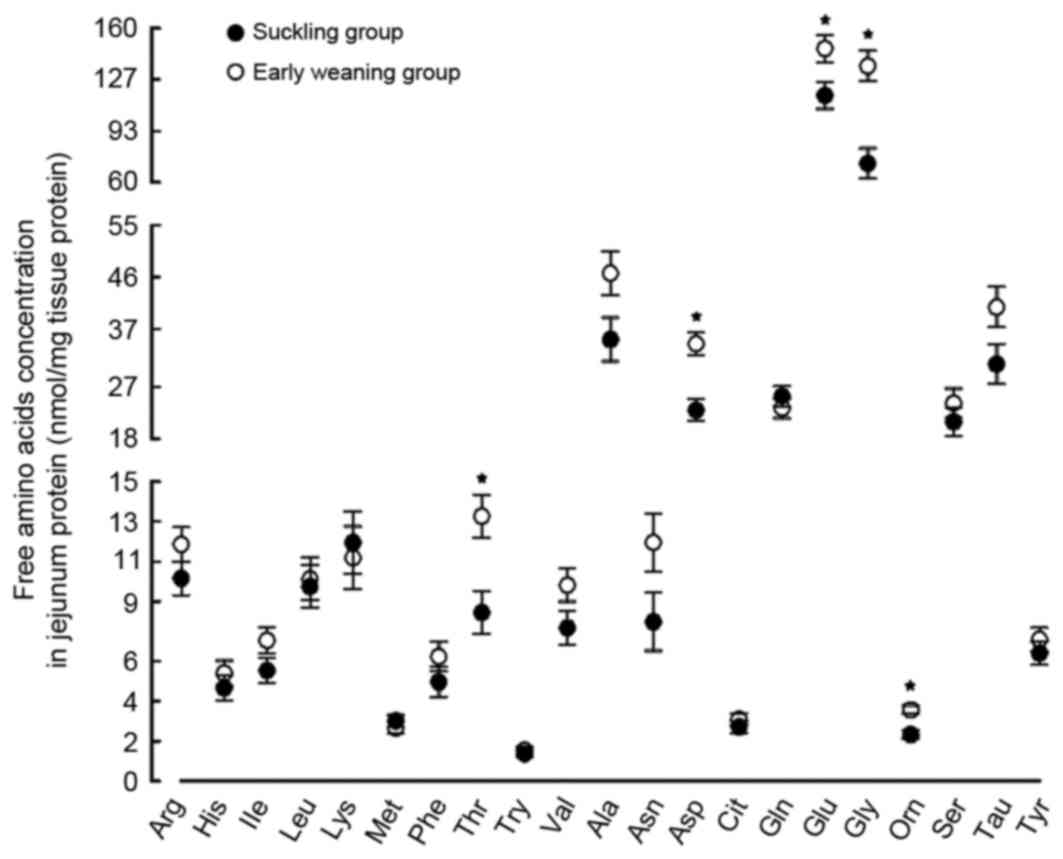
Effect of early weaning on free amino
acid content in the jejunum
Compared with the suckling piglets, The Glu content
in the jejunum was significantly greater in the weaned piglets
compared with the suckling piglets (by 26%; P<0.05; Fig. 5). However, no significant
difference was identified between the content of glutamine (Gln),
the substrate for the ASC amino-acid transporter 2 (ASCT2) protein
in the weaned and suckling piglets. The contents of other free
amino acids, including threonine, glycine and ornithine, increased
by 57, 88 and 53%, respectively (P<0.05; Fig. 5).
Effect of early weaning on free amino
acid content in the ileum
The changes of the free amino acid content in the
ileum of the weaned piglets and the suckling piglets were opposite
to those observed in the jejunum. The Glu content, the substrate
for the EAAC1 protein, were reduced by 43% in the weaned piglets
(P<0.05). However, the content of Gln, the substrate for the
ASCT2 protein, was reduced by 52% in the weaned piglets
(P<0.05). The contents of the remaining free amino acids,
including lysine, methionine, phenylalanine, trypophan, arginine,
taurine and tyrosine, were all reduced by >42% (P<0.05;
Fig. 6).
Discussion
Weaning may induce a series of changes in the
morphology of piglets, including nutritional level, digestion and
metabolism (14), stress,
neuroendocrine (15) and gene
functions. Daily weight gain following weaning is an important
apparent indicator of the piglet's growth. If the daily weight gain
does not change considerably after weaning as compared with the
suckling piglets, this means that the daily diet had sufficient
nutrients for the piglets and they have adapted to weaning with
normal intestinal development. However, in practice, ~10% of the
weaned piglets die from physiological stress of two major sources,
the change of the external environment and abnormal intestinal
mucosa development due to a novel feeding diet. Weaning may induce
changes of gastrointestinal functions and affects the regulation of
gut-brain axis (16). The quantity
of feed consumed by the piglets frequently decreases following
weaning due to changes in nutrition and environment. The
gastrointestinal tract is underdeveloped in piglets and weaning may
lead to a decline in their growth performance. In the present
study, the piglets in SEW treatment group exhibited signs of
weaning-associated stress. The daily feeding quantity was 148.5 g,
which was lower compared with the standard of 240 g/day in the
requirements of swine from the National Research Council (NRC).
Additionally, the daily weight gain of the weaned piglets was
significantly lower compared with the suckling piglets of the same
age (P<0.001). Gu et al (17) determined that weaning-associated
stress may lead to significant structural and functional changes of
the intestine, thus affecting the normal growth of piglets.
EAAC1 is a crucial Glu transporter with wide
distribution in the entire cell body. A previous study (6) focused on Glu transport by EAAC1 in
the central nervous system as opposed to the intestinal mucosa of
piglets. The present study detected the EAAC1 protein and mRNA
expression levels were significantly reduced in the intestine of
weaned piglets compared with the suckling piglets. Watabe et
al (18) revealed that
GTRAP3-18 was a negative regulator of EAAC1 and intracellular
glutathione content using HEK293 cells (18). Additionally, GTRAP3-18 had a
sequential impact on the susceptibility to oxidative stress.
Differentiation, heat stress and oxidative stress may also lead to
the separation of GTRAP3-18 protein (6). This involves the upregulation of
GTRAP3-18 protein or mRNA expression in response to oxidative
stress. The present study determined that early weaning reduced the
protein and mRNA expression of GTRAP3-18 in piglets, which was
contrary to the response to heat and oxidative stress. In order to
manage the increased stress, the piglets require more Glu from
their daily diet for intestinal mucosa repair. The findings of a
previous study revealed that the increased efficiency of Glu uptake
may led to the downregulation of GTRAP3-18 protein expression
levels (18). Increased Glu
content in the jejunum led to reduced GTRAP3-18 protein expression
in the jejunum, which was in accordance with previous findings by
Lin et al (19). However,
the Glu content and GTRAP3-18 expression in the ileum was reduced
in weaned piglets. The function of GTRAP3-18 in stress and disease
of piglets remains to be fully elucidated (5).
Glu is the excitatory amino acid transported by
EAAC1. The present study determined that weaning led to a reduction
in the content of free amino acids in the ileum and an increase of
free amino acids in the jejunum of the weaned piglets. Previous
studies revealed that EAAC1 was primarily expressed in the jejunum
(20) and the efficiency of Glu
transmembrane transport in the intestine was regulated by EAAC1
(21). Therefore, it is possible
that the Glu transport efficiency was increased in the jejunum of
weaned piglets in order to compensate for the reduced Glu uptake.
In the meantime, EAAC1 was consumed and the EAAC1 expression was
reduced. Conversely, the EAAC1 expression in the ileum was reduced,
leading to lower Glu transport efficiency. The content of free Glu
in the ileum was reduced in the weaned piglets compared with the
suckling piglets. It is of note that the Glu content is not solely
determined by transport, but also by the Glu-Gln cycle.
In conclusion, the present study determined that
EAAC1 expression was reduced in the jejunum of the weaned piglets
and the consumed EAAC1 was associated with the increased transport
of Glu in the jejunum of the weaned piglets. Therefore, the content
of amino acids such as Glu was increased in the jejunum. This was
accompanied by the reduced expression of GTRAP3-18, the regulator
of EAAC1. As EAAC1 expression was downregulated in the ileum of the
weaned piglets, the Glu transport rate was reduced, leading to
reduction of amino acid content, such as Glu in the ileum.
Acknowledgements
The present study was supported by the China
Postdoctoral Science Foundation (grant no. 2013M531011) and the
Heilongjiang Province Natural Science Foundation of China (grant
nos. C201444 and C2015040).
References
|
1
|
Ruth MR and Field CJ: The immune modifying
effects of amino acids on gut-associated lymphoid tissue. J Anim
Sci Biotechnol. 4:272013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bianchi MG, Bardelli D, Chiu M and
Bussolati O: Changes in the expression of the glutamate transporter
EAAT3/EAAC1 in health and disease. Cell Mol Life Sci. 71:2001–2015.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aoyama K and Nakaki T: Neuroprotective
properties of the excitatory amino acid carrier 1 (EAAC1). Amino
Acids. 45:133–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butchbach ME, Lai L and Lin CL: Molecular
cloning, gene structure, expression profile and functional
characterization of the mouse glutamate transporter (EAAT3)
interacting protein GTRAP3-18. Gene. 292:81–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoyama K and Nakaki T: Inhibition of
GTRAP3-18 may increase neuroprotective glutathione (GSH) synthesis.
Int J Mol Sci. 13:12017–12035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang BG, Won SJ, Kim JH, Choi BY, Lee MW,
Sohn M, Song HK and Suh SW: EAAC1 gene deletion alters zinc
homeostasis and enhances cortical neuronal injury after transient
cerebral ischemia in mice. J Trace Elem Med Biol. 26:85–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoyama K, Watabe M and Nakaki T:
Modulation of neuronal glutathione synthesis by EAAC1 and its
interacting protein GTRAP3-18. Amino Acids. 42:163–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berman AE, Chan WY, Brennan AM, Reyes RC,
Adler BL, Suh SW, Kauppinen TM, Edling Y and Swanson RA:
N-acetylcysteine prevents loss of dopaminergic neurons in the
EAAC1-/-mouse. Ann Neurol. 69:509–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duerson K, Woltjer RL, Mookherjee P,
Leverenz JB, Montine TJ, Bird TD, Pow DV, Rauen T and Cook DG:
Detergent-insoluble EAAC1/EAAT3 aberrantly accumulates in
hippocampal neurons of Alzheimer's disease patients. Brain Pathol.
19:267–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu D, Yang H, Kong X, Blachier F, Wang W
and Yin Y: Molecular cloning and expression profiling of excitatory
amino acid carrier 1 in suckling Huanjiang mini-piglets with large
or small body weight at birth. Mol Biol Rep. 40:3341–3350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bregendahl K, Yang X, Liu L, Yen JT,
Rideout TC, Shen Y, Werchola G and Fan MZ: Fractional 485 protein
synthesis rates are similar when measured by intraperitoneal or
intravenous flooding 486 doses of L-[ring-2H5]phenylalanine in
combination with a rapid regimen of sampling in piglets. J Nutr.
138:1976–1981. 2008.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiele B, Stein N, Oldiges M and Hofmann
D: Direct analysis of underivatized amino acids in plant extracts
by LC-MS-MS. Methods Mol Biol. 828:317–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takita M and Kikusui T: Early weaning
influences short-term synaptic plasticity in the medial
prefrontal-anterior basolateral amygdala pathway. Neurosci Res.
103:48–53. 2006. View Article : Google Scholar
|
|
15
|
Xiong X, Yang H, Tan B, Yang C, Wu M, Liu
G, Kim SW, Li T, Li L, Wang J, et al: Differential expression of
proteins involved in energy production along the crypt-villus axis
in early-weaning pig small intestine. Am J Physiol Gastrointest
Liver Physiol. 309:G229–G237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Candeias EM, Sebastião IC, Cardoso SM,
Correia SC, Carvalho CI, Plácido AI, Santos MS, Oliveira CR,
Moreira PI and Duarte AI: Gut-brain connection: The neuroprotective
effects of the anti-diabetic drug liraglutide. World J Diabetes.
6:807–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu X, Li D and She R: Effect of weaning on
small intestinal structure and function in the piglet. Arch
Tierernahr. 56:275–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watabe M, Aoyama K and Nakaki T: A
dominant role of GTRAP3-18 in neuronal glutathione synthesis. J
Neurosci. 28:9404–9413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CI, Orlov I, Ruggiero AM, Dykes-Hoberg
M, Lee A, Jackson M and Rothstein JD: Modulation of the neuronal
glutamate transporter EAAC1 by the interacting protein GTRAP3-18.
Nature. 410:84–88. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burrin DG and Stoll B: Metabolic fate and
function of dietary glutamate in the gut. Am J Clin Nutr.
90:850S–856S. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan MZ, Matthews JC, Etienne NM, Stoll B,
Lackeyram D and Burrin DG: Expression of apical membrane
L-glutamate transporters in neonatal porcine epithelial cells along
the small intestinal crypt-villus axis. Am J Physiol Gastrointest
Liver Physiol. 287:G385–G398. 2004. View Article : Google Scholar : PubMed/NCBI
|