Introduction
The temporomandibular joint (TMJ), a synovial joint,
similar to other articulating joints in the human body, provides
diarthrodial articulation between the mandibular condyle and
temporal bone. Its synovial membrane covers all of TMJ
intra-articular structures, except for the articular cartilage of
the eminence, fossa and mandibular condyle, and the articular disc
(1). Pathological conditions of
the TMJ, such as internal derangement and/or osteoarthritis, in
patients with temporomandibular joint disorder (TMD) have been
reported to be accompanied by inflammation of the synovial membrane
(2,3). Synovitis, is defined as inflammation
of the synovial membrane and characterized by chronic inflammatory
changes, such as hyperplasia of the synovial lining as well as
increases in new capillaries and small vessels along with immune
cell infiltration (1), while
various inflammatory mediators, such as cytokines have been
detected in synovial fluid and tissue samples obtained from
affected patients (4,5). Inflammatory mediators that modulate
the functions of cells that compose the synovial membrane, such as
synovial fibroblasts are considered to promote and shape the
pathological condition of the TMJ.
TNF-α is produced by monocytes and macrophages, and
known to be a pro-inflammatory cytokine with roles in inflammatory
mediation and immune response (6).
Furthermore, reported evidence has shown that this cytokine likely
mediates acute and chronic inflammation associated with connective
tissue degeneration in TMD. Indeed, TNF-α has been detected in the
synovial fluid of patients with TMD (4,5),
while transgenic mice with over-expression of TNF-α were found to
develop remarkable arthritic changes in the TMJ (7). In other studies, TNF-α was reported
to increase inflammatory chemokines, small peptides that induce
leucocyte activation and migration, such as IL-8 and CCL20, in
synovial fibroblasts obtained from patients with TMJ (8,9). On
the other hand, IFN-γ is also known as a Type II interferon
produced by T-lymphocytes and natural killer cells (10). Although IFN-γ as well as TNF-α have
been found in synovial fluid samples from TMD patients, in contrast
to those from healthy individuals (5), it is unknown whether either
participates in induction of inflammatory chemokines, such as IL-8
in synovial fibroblasts from the TMJ.
CXCR3-agonistic chemokines including CXCL10 bind to
the chemokine receptor CXCR3 expressed by activated T cells, and
play important roles in inflammation via their T cell chemotactic
and adhesion-promoting activities (11). In a previous study, CXCL10 was
detected in the majority of synovial fluid samples from patients
with internal derangement of the TMJ (12). Furthermore, TNF-α and IFN-γ have
been reported to be main inducers of CXCL10 in monocytes, skin
fibroblasts, and endothelial cells (13). Together, these findings suggest
regulation of CXCR3 chemokines such as CXCL10 in synovial
fibroblasts by TNF-α and IFN-γ, and also implicate their
involvement in the development of pathological processes in the
TMJ.
In the present study, we examined whether TNF-α and
IFN-γ participate in regulation of expression of various chemokines
in pathological processes in the TMJ. We first investigated their
effects on the expression of several different chemokines including
CXCL10 in synovial fibroblasts, then examined the effects of IFN-γ
on regulation of expression of those chemokines and transcription
factors affected by TNF-α.
Materials and methods
Cultures of synovial fibroblast from
TMJs
After obtaining informed consent for acquisition
according to a protocol approved by the Ethical Committee of
Hiroshima University (no. 930), human synovial tissue samples were
obtained from a patient with condyle hypertrophy. Synovial
fibroblasts were then isolated from the synovial membrane using an
outgrowth method, as previously reported (9,14).
Briefly, the samples were washed with PBS, then minced, placed in
tissue culture flasks, and grown in Dulbecco's modified Eagle's
medium (Sigma Chemical Co, St. Louis, MO, USA) containing 10% fetal
calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin in a
humidified atmosphere of 5% CO2 in air. Confluent cells
were detached with 0.025% trypsin (Gibco, Grand Island, NY, USA)
and 0.02% EDTA in PBS, then sub-cultured in medium. For the
experiments, we used synovial fibroblasts obtained from 4 to 8
passages.
RNA preparation
Cells were exposed to either recombinant human IFN-γ
(10 ng/ml) or TNF-α (10 ng/ml) (both from R & D Systems,
Minneapolis, MI, USA), or those in combination at various
concentrations for 12 h. RNA from each culture was extracted using
an RNAeasy Mini kit (Qiagen, Hilden, Germany).
RNA extraction, RT-PCR, and real-time
PCR
We used gene-specific oligonucleotide primers for
PCR analysis, as follows (Table
I). Total RNA was prepared from the cell using an RNeasy Total
RNA Isolation kit (Qiagen). One-step RT-PCR was performed using an
RT-PCR High Plus System (Toyobo Co., Ltd., Osaka, Japan), according
to the manufacturer's instructions. Single-stranded cDNA for RT-PCR
and a quantitative real-time PCR template were synthesized using a
First Strand cDNA Synthesis kit (Amersham Biosciences, Uppsala,
Sweden). The RT-PCR conditions for assays of the chemokines were 1×
(95°C, 15 min), 35× (95°C, 2 min; 60°C, 30 sec; 72°C, 1 min) and 1×
(72°C, 7 min), while those for β-actin were 1× (95°C, 15 min), 25×
(95°C, 2 min; 60°C, 30 sec; 72°C, 1 min), and 1× (72°C, 7 min).
Quantitative real-time PCR was performed using SYBR-Green Master
Mix (Applied Biosystems, Carlsbad, CA, USA) for 40 cycles at 95°C
for 15 sec and 60°C for 60 sec. Quantitative PCR analysis was
performed using a CFX Connect Real-Time PCR Detection System
(Bio-Rad Laboratories, Hercules, CA, USA). Values for
quantification of chemokine mRNA levels are presented as the fold
increases in chemokine mRNA expression in cells treated with TNF-α
and IFN-γ were calculated in comparison with mRNA expression in
non-treated cells after normalization to that of β-actin, and shown
as the mean ± standard deviation from 3 independent
experiments.
 | Table I.Effects of TNF-α and IFN-γ on
chemokine mRNA expression levels. |
Table I.
Effects of TNF-α and IFN-γ on
chemokine mRNA expression levels.
|
| mRNA expression
levelsa |
|
|---|
|
|
|
|
|---|
| Chemokines | TNF-α | IFN-γ | Primer sequences |
|---|
| CXCL9 | 29.9±3.4b |
5.6±1.5b |
5′-CATGCTGGTGAGCCAAGCAGTTTGAA-3′ |
|
|
|
|
5′-CACTTCTGTGGGGTGTTGGGGACAAG-3′ |
| CXCL10 |
212.9±27.6b |
10.3±0.82b |
5′-TGCAAGCCAATTTTGTCCACGTGTTG-3′ |
|
|
|
|
5′-GCAGCTGATTTGGTGACCATCATTGG-3′ |
| CXCL11 |
6.1±1.1b |
6.8±1.9b |
5′-AGAGGACGCTGTCTTTGCAT-3′ |
|
|
|
|
5′-GTCCTTTCACCCACCTTTCA-3 |
| CCL20 |
10,268.4±1,425.6b | 1.3±0.2 |
5′-TACTCCACCTCTGCGGCGAATCAGAA-3′ |
|
|
|
|
5′-GTGAAACCTCCAACCCCAGCAAGGTT-3′ |
| IL-8 |
592.1±78.7b | 0.9±0.2 |
5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ |
|
|
|
|
5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ |
| CXCL1 |
82.2±5.4b | 1.2±0.3 |
5′-TACTCCACCTCTGCGGCGAATCAGAA-3′ |
|
|
|
|
5′-AACTATGGGGGATGCAGGA-3′ |
Chemokine determination
Cells were pre-cultured for 1 h in the presence or
absence of Bay 11-7082, an NF-κB inhibitor (Invivogen, San Diego,
CA, USA) and AG490, a JAK/STAT inhibitor (Cayman Chemical Co., Ann
Arbor, MI, USA), then incubated with TNF-α or IFN-γ for 24 h.
Supernatants from the cultures were collected, and the
concentrations of IL-8 and CXCL10 were measured using a Duoset
ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA).
Western blot analysis
Cells were harvested using a Mammalian Cell Lysis
kit (Sigma-Aldrich, St. Louis, MO, USA). Protein concentrations
were determined using a protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and proteins from each sample were
separated on 10% SDS-polyacrylamide gels, then transferred to
polyvinylidene fluoride membranes (Amersham Biosciences). After
incubation with the specific antibody, immunoblots were labeled
with an HRP-conjugated secondary antibody and developed using an
ECL Advance Western Blotting Detection kit (GE Healthcare Life
Sciences, Tokyo, Japan). Image data were analyzed using an LAS 4000
mini imaging system (Fuji Film, Tokyo, Japan). Phosphorylation of
the proteins was evaluated by comparing the integrated density of
the phosphorylated-bands revealed by western blotting.
Densitometric scanning was performed using Kodak Digital Science 1D
Software (Eastman Kodak, Rochester, USA) and the levels of
phosphorylated proteins were compared with total protein
values.
NF-κB activity
Nuclear extracts were prepared using a nuclear
extraction kit (Cayman Chemical Co.), according to the
manufacturer's instructions. Nuclear extract concentrations were
determined using a protein assay kit (Bio-Rad Laboratories, Inc.).
The DNA-binding activity of NF-κB p65 was detected by use of an
NF-κB (p65) Transcription Factor Assay kit (Cayman Chemical Co.).
Nuclear extracts (each containing 10 µg of protein) were added to
96-well plates coated with a specific double stranded DNA sequence
containing the NF-κB response element. NF-κB was then detected by
addition of a specific primary antibody directed against NF-κB,
followed by an HRP-conjugated secondary antibody to provide a
colorimetric readout obtained using a microplate reader (Bio-Rad
Laboratories, Inc.). Relative NF-κB p65 DNA-binding activity in the
nuclear extracts was normalized to that of the control cells.
Statistical analysis
Data were analyzed using Student's t-test or one-way
analysis of variance (ANOVA), and the results are presented as the
mean ± standard deviation.
Results
Effects of TNF-α and IFN-γ on various
chemokine mRNA expressions in synovial fibroblasts
We initially examined the effects of TNF-α on the
mRNA expressions of various chemokines in synovial fibroblasts
obtained from the TMJ. The mRNA levels of CXCL9, 10, 11, 20, IL-8,
and CXCL1 were increased by TNF-α (Table I, Fig.
1A), whereas, addition of IFN-γ to the culture resulted in only
slight increase in CXCL9-11 expression (Table I, Fig.
1B), while no increase in expression of CCL20, IL-8, or CXCL1
expression observed (Table I,
Fig. 1B).
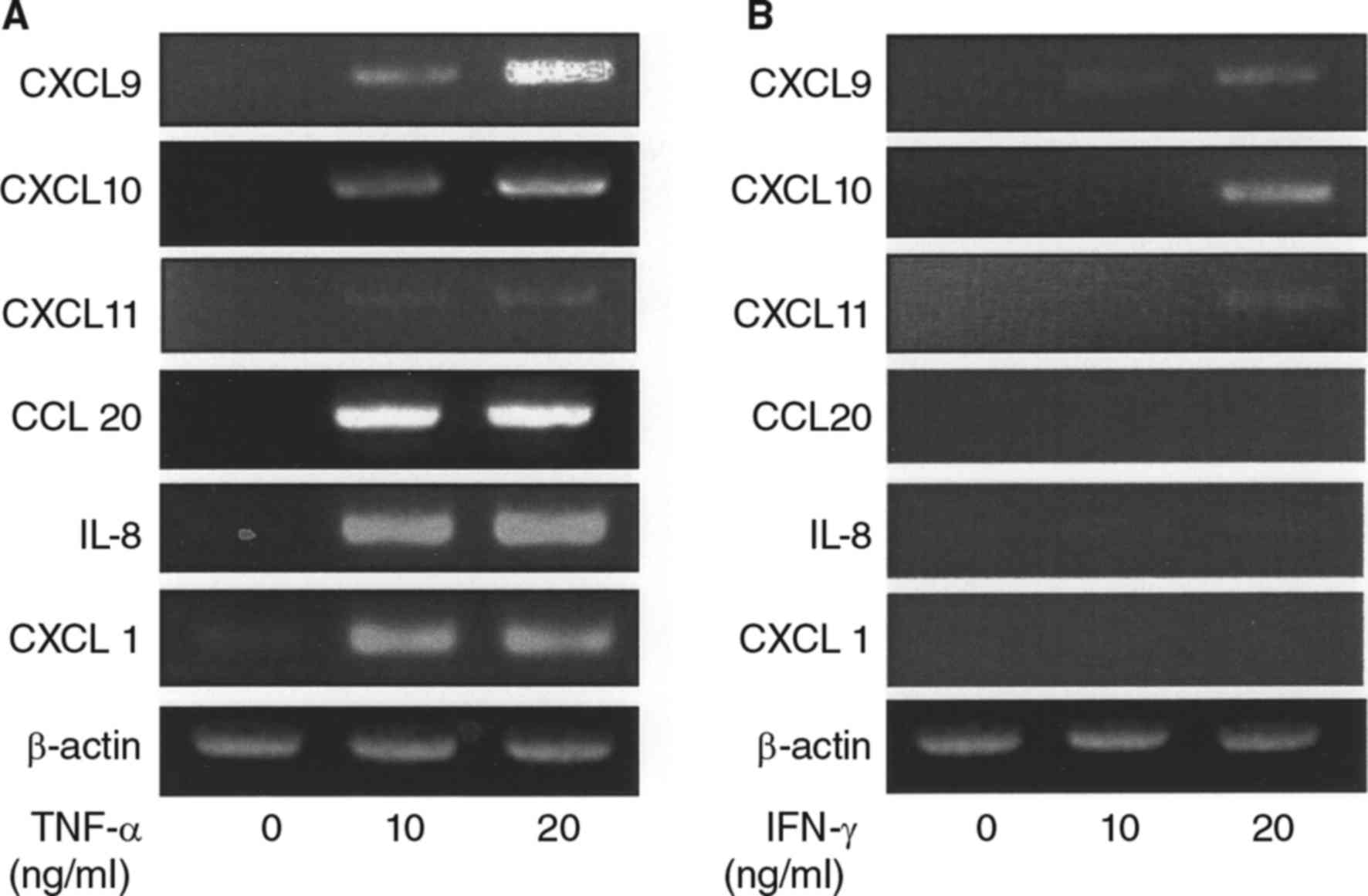 | Figure 1.Effects of TNF-α and IFN-γ on
induction of mRNA expression of several chemokines in synovial
fibroblasts from temporomandibular joint. (A) Cells were exposed to
TNF-α (10, 20 ng/ml) for 12 h, then total RNA was isolated from the
cells, and chemokine expression was examined using RT-PCR assays.
Results are shown as relative to β-actin, the internal control. (B)
Cells were exposed to IFN-γ (10, 20 ng/ml) for 12 h, then total RNA
was isolated from the cells, and expressions of chemokines were
examined using RT-PCR. Results are shown as relative to β-actin,
the internal control. TNF, tumor necrosis factor; IFN, interferon;
CXCL, C-X-C motif chemokine ligand; IL, interleukin; RT-PCR,
reverse transcription-polymerase chain reaction. |
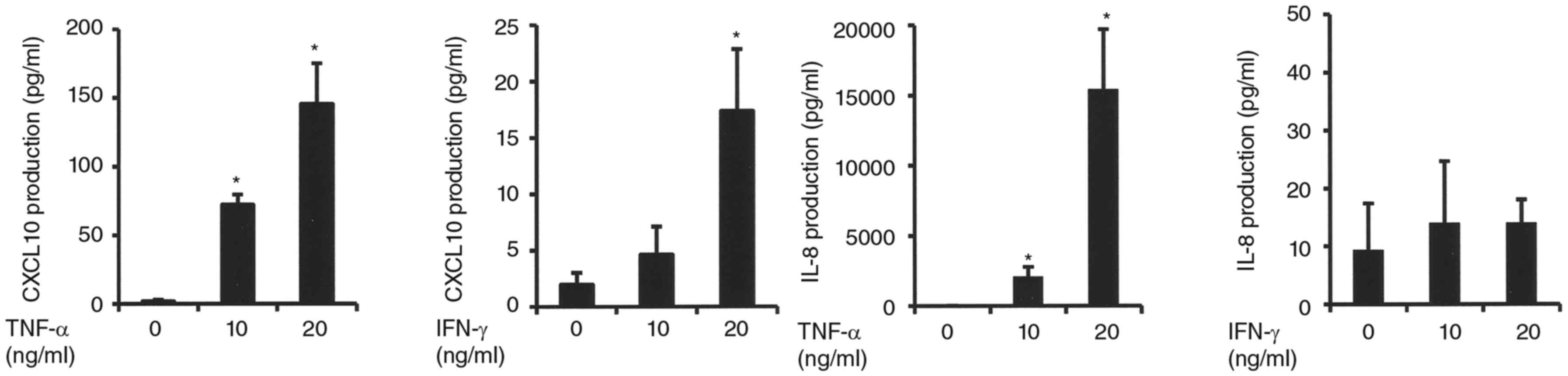
Effects of TNF-α and IFN-γ on CXCL10
and IL-8 protein expressions in synovial fibroblasts
To confirm the variations of mRNA expressions when
stimulated with TNF-α or IFN-γ, we examined CXCL10 and IL-8 protein
expressions that were especially affected by TNF-α in CXCR3 and
CXCR2 agonists, respectively. Those results showed that TNF-α
increased CXCL10 and IL-8 expressions in a manner, similar to the
increase in mRNA expression (Fig.
2). In contrast, addition of IFN-γ resulted in an increase in
CXCL10, but not IL-8 (Fig. 2).
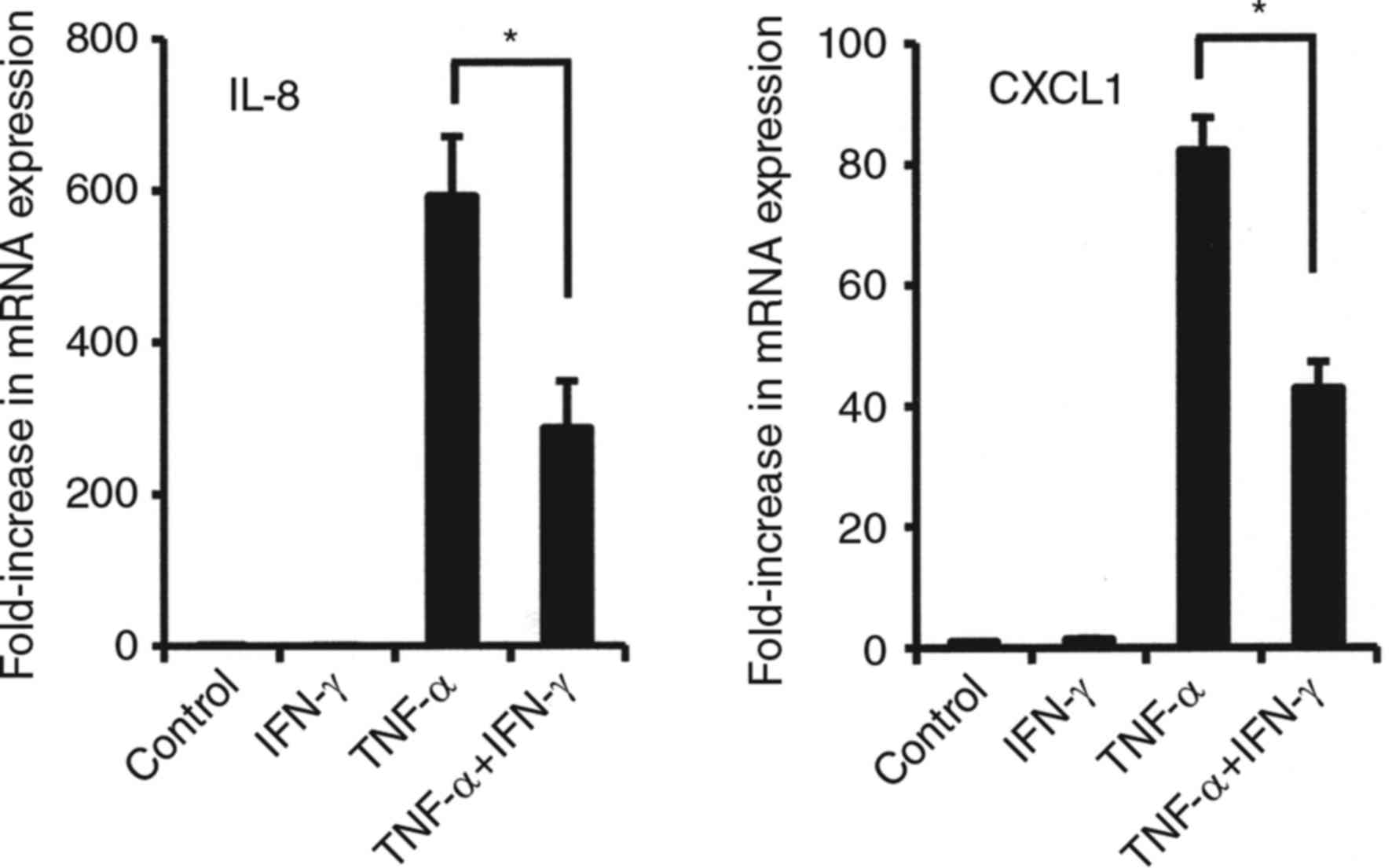
Effects of combined TNF-α and IFN-γ on
mRNA expressions of various cytokines in synovial fibroblasts
We also examined the effects of the combination of
IFN-γ and TNF-α on the mRNA expression of various cytokines in
synovial fibroblasts from TMJ. That combination enhanced CXCL9,
CXCL10, CXCL11, and CCL20 mRNA expressions in comparison to
stimulation with either alone (Fig.
3). Notably, the combinations of IFN-γ and TNF-α dramatically
increased the mRNA levels of CXCL9, CXCL10 and CXCL11 (CX3CR1
agonists) in comparison to TNF-α alone (Fig. 3). On the other hand, exposure to
that in combination resulted in decreased mRNA levels of CXCR2
agonists, IL-8 and CXCL1 as compared to TNF-α alone (Fig. 4).
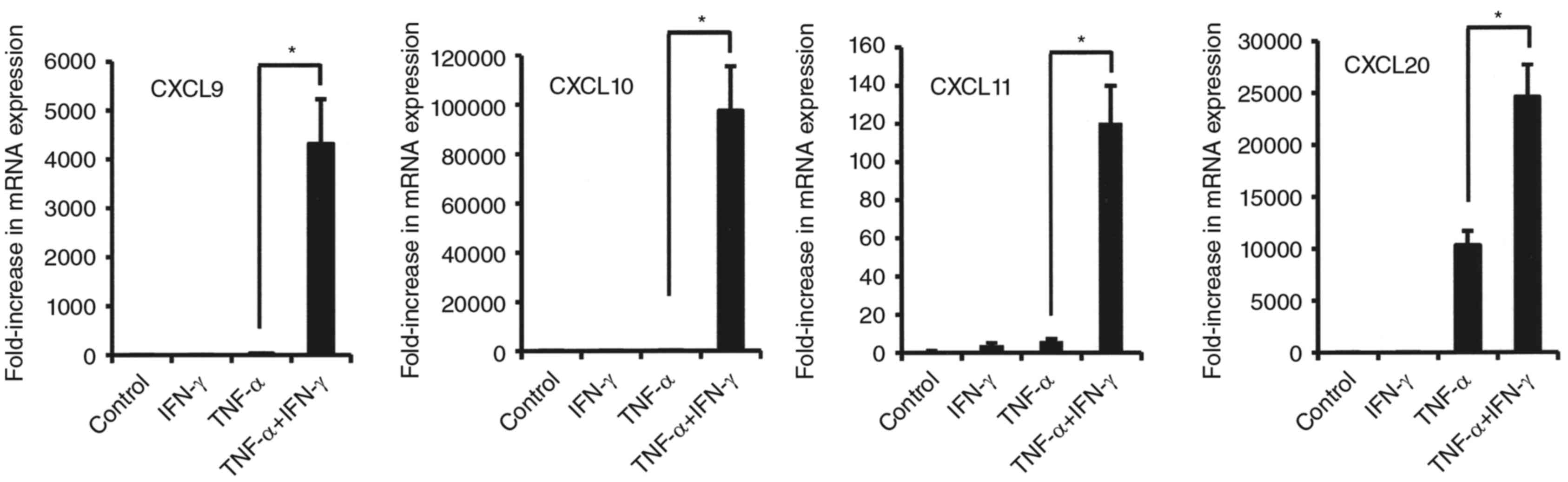 | Figure 3.Effects of combination of TNF-α and
IFN-γ on mRNA expression of CCL20, CXCL9, CXCL10, and CXCL11 in
synovial fibroblasts from temporomandibular joint. Cells were
exposed to 20 ng/ml of TNF-α, IFN-γ, or those in combination for 12
h, then mRNA expression of the indicated chemokines was examined.
Results are shown as relative to β-actin, the internal control.
Values are presented as the mean ± standard deviation of 3
independent experiments. *Significantly different as compared to
TNF-α (P<0.05). TNF, tumor necrosis factor; IFN, interferon;
CXCL, C-X-C motif chemokine ligand. |
Effects of TNF-α on NF-κB activation
in synovial fibroblasts from TMJ
NF-κB, an inducible transcription factor well known
for its involvement in inflammatory and immune responses, is
activated by phosphorylation of IκBα, then activated NF-κB is
translocated to the nucleus, and induces target gene expression
(15). We examined the effects of
TNF-α and IFN-γ on NF-κB activation in synovial fibroblasts from
the TMJ. TNF-α increased the phosphorylation of IκBα (Fig. 5A), as well as NF-κB p65 DNA-binding
activity in the nucleus (Fig. 5B),
indicating that TNF-α participates in NF-κB activation. However,
IFN-γ did not have an effect on NF-κB activation in the presence or
absence of TNF-α (Fig. 5B). To
examine CXCL10 and IL-8 expressions mediated by TNF-α via an NF-κB
dependent pathway, we investigated the effects of Bay 11-7082, an
NF-κB inhibitor, on expressions of these cytokines mediated by
TNF-α. Pre-treatment with Bay 11-7082 resulted in an increase of
TNF-α-induced IL-8 and CXCL10 protein levels in both the presence
and absence of IFN-γ (Fig.
5C).
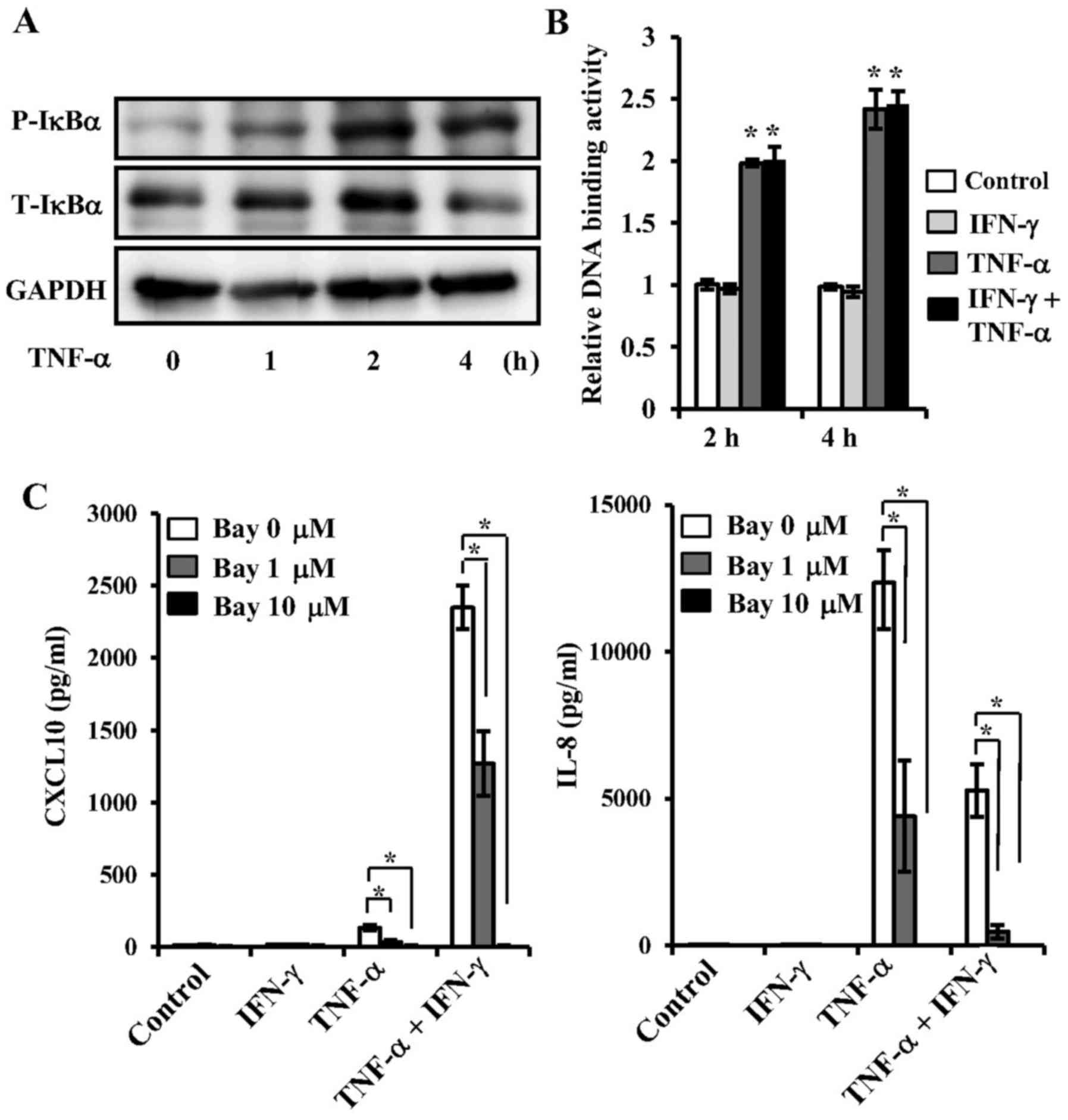 | Figure 5.Effects of TNF-α on NF-κB activation,
and effects of NF-κB inhibitor on TNF-α-mediated CXCL10 and IL-8
expressions in synovial fibroblasts from temporomandibular joint.
(A) Effect of TNF-α on phosphorylation of IKBα. Cells were exposed
to TNF-α (20 ng/ml) for various time periods, after which cell
extracts were subjected to SDS-PAGE. Phosphorylation of IKBα was
examined by western blotting analysis with antibodies against
phospho-specific IKBα (P-IKBα), total IKBα (T-IKBα), and GAPDH. (B)
Effect of TNF-α on NF-κB activation. Cells were exposed to 20 ng/ml
of TNF-α, IFN-γ, or those in combination for 2 or 4 h, after which
nuclear extracts were subjected to NF-κB (p65) transcription factor
assays. NF-κB p65 DNA-binding activity was examined and the results
are expressed as fold changes relative to the non-treated control.
*Significantly different from non-treated cells (P<0.05). (C)
Effect of NF-κB inhibitor on TNF-α-mediated CXCL10 and IL-8
expressions. Cells were pre-incubated with Bay-11-7082 (Bay; 1 or
10 µM) for 1 h, then exposed to 20 ng/ml of TNF-α, IFN-γ or those
in combination for 24 h, after which the levels of CXCL10 and IL-8
in culture supernatants were measured by ELISA. Data are shown as
the mean ± standard deviation of 3 independent experiments.
*Significant difference as compared to Bay at 0 µM (Student's
t-test, P<0.05). TNF, tumor necrosis factor; IFN, interferon;
CXCL, C-X-C motif chemokine ligand; IL, interleukin; NF, nuclear
factor; T, total; P, phosphorylated. |
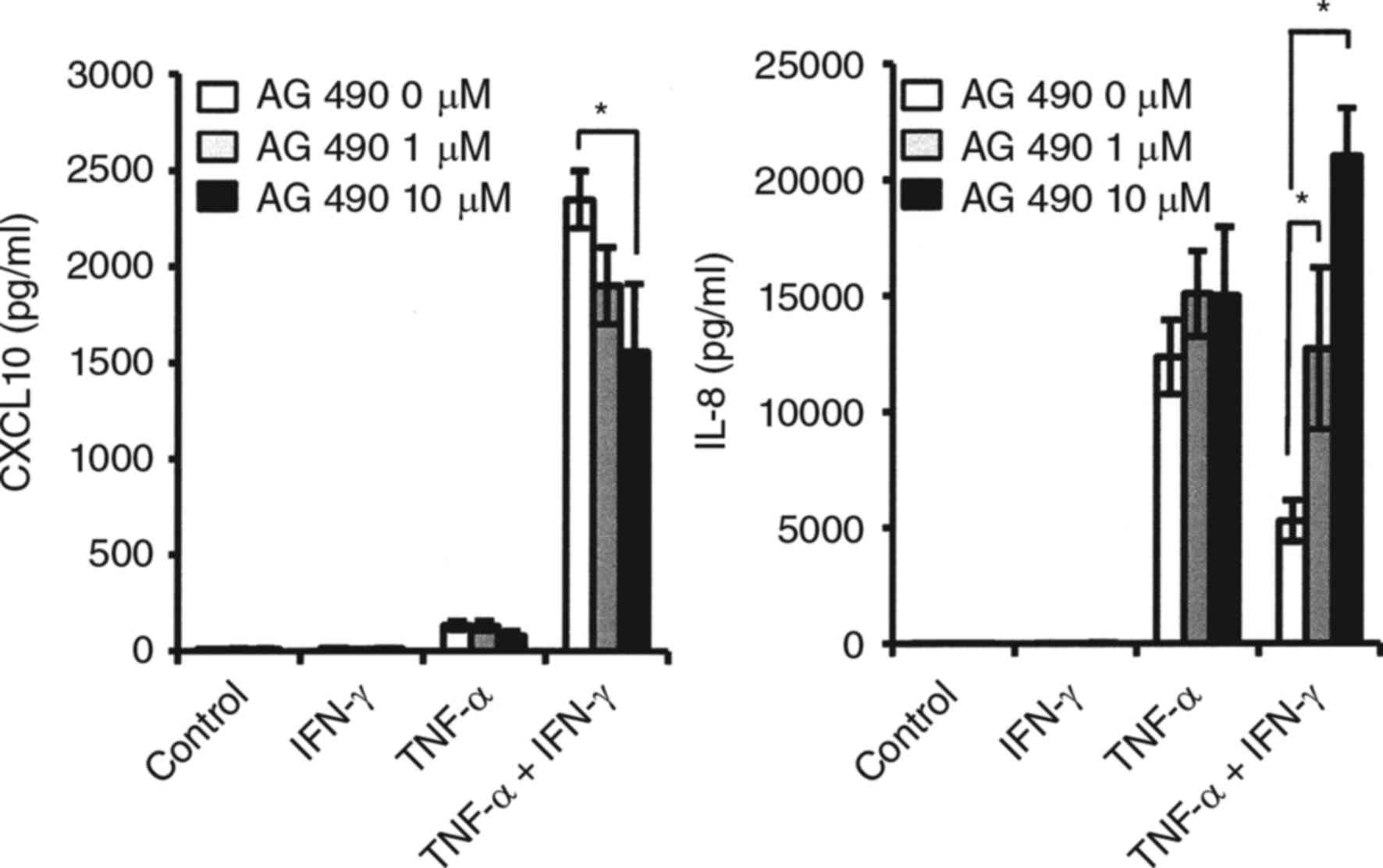
Effects of IFN-γ on STAT1 activation
in synovial fibroblasts from TMJ
STAT1 is a key mediator of gene expression induced
by type II interferons, such as IFN-γ, and activated STAT1 directly
regulates the expression of CXCL10 (16). We examined whether STAT1
phosphorylation in synovial fibroblasts from the TMJ activated by
IFN-γ, and IFN-γ was increased in a time-dependent manner (Fig. 6A). No effect of TNF-α on IFN-γ
induced-STAT-1 activation was observed (Fig. 6B). Exposure to the JAK/STAT
inhibitor AG490 resulted in partial inhibition of CXCL10 expression
when fibroblasts were simultaneously stimulated with IFN-γ and
TNF-α, while the decrease in TNF-α-induced IL-8 caused by exposure
to IFN-γ was recovered by addition of AG 490. (Fig. 7).
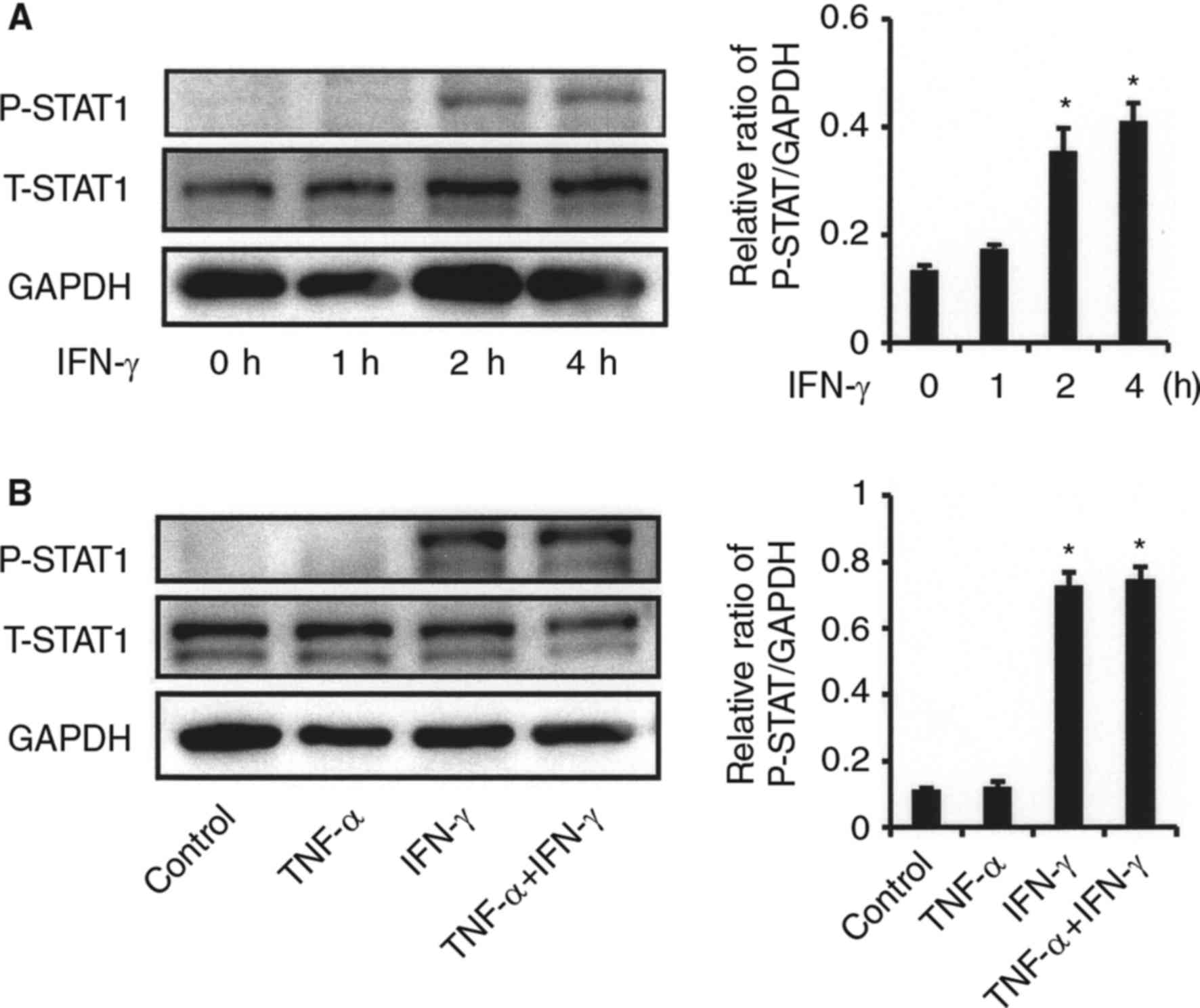 | Figure 6.Effects of IFN-γ on STAT1 activation
in synovial fibroblasts from TMJ. (A) Effect of IFN-γ on STAT1
phosphorylation. Cells were exposed to IFN-γ (20 ng/ml) for various
time periods, after which cell extracts were subjected to SDS-PAGE.
Phosphorylation of STAT1 was examined by western blot analysis with
antibodies against phospho-specific STAT1 (P-STAT1), total STAT1
(T-STAT1), and GAPDH. Phosphorylation of the proteins was evaluated
by comparing the integrated density of the phosphorylated-bands,
then the relative ratio of phosphorylated proteins in comparison
with GAPDH values was determined. *Significantly different from
non-treated cells (P<0.05). (B) Effect of TNF-α on IFN-γ
induced-STAT1 phosphorylation. Cells were exposed to 20 ng/ml of
TNF-α, IFN-γ, or those in combination for 2 h, after which cell
extracts were subjected to SDS-PAGE. Phosphorylation of STAT1 was
examined by western blotting analysis with antibodies against
P-STAT1, T-STAT1, and GAPDH. The relative ratio of phosphorylated
proteins in comparison with the GAPDH values was determined.
*Significantly different as compared to non-treated cells
(P<0.05). TNF, tumor necrosis factor; IFN, interferon; T, total;
P, phosphorylated; STAT, signal transducer and activator of
transcription. |
Discussion
TNF-α, a pro-inflammatory cytokine produced by
immune cells, such as Th1 cells, is thought to be involved in TMJ
destruction. Takahashi et al (4) reported detection of TNF-α in synovial
fluid samples from TMD patients affected by disc derangement with
locking or clicking as compared to those from healthy individuals.
In addition, Suzuki et al (17) demonstrated that TNF-α was
predominantly expressed in cells of the synovial lining and blood
vessels in synovial specimens obtained from patients with TMJ
internal derangement), while, others have shown that TNF-α
increased the production of several chemokines, such as IL-8,
CXCL1, and CCL20, in synovial fibroblasts from the TMJ (8,9). On
the other hand, TNF-α is also known as a potent inducer of NF-κB,
an inflammatory inducible transcription factor. Binding of TNF-α
with the cell surface receptors, TNF receptor 1 (TNFR1) and TNFR2
was found to lead to phosphorylation of IκBα, then activated NF-κB
translocated to the nucleus to induce the target inflammatory gene
(15). In another study, Ke et
al (18) demonstrated that
activation of NF-κB is responsible for TNF-α-induced cyclooxygenase
2 expression in synovial fibroblasts from the TMJ. In the present
study, similar to previous reports, TNF-α shown to increase the
expression of various chemokines and also active NF-κB. In
addition, we found that addition of an NF-κB inhibitor to the
cultures resulted in dramatic decreases in TNF-α-mediated IL-8 and
CXCL10 expressions in synovial fibroblasts from the TMJ. Therefore,
NF-κB plays an important role in regulation of TNF-α-mediated
expression of various inflammatory chemokines in synovial
fibroblasts in the TMJ.
IFN-γ produced by activated T cells is known to
induce CXCR3 chemokines (13),
such as CXCL10, CXCL9, and CXCL11, which share an approximately 40%
amino acid sequence identity and bind to the chemokine receptor
CXCR3, which is mainly expressed by activated T cells (11). IFN-γ-induced CXCR3 chemokines have
been found in synovial fluid from rheumatoid arthritis (RA)
patients, and are thought to contribute to development of Th1
immune responses in the joints (19,20).
Also, high levels of CXCR3 chemokines and CD4+ T cells expressing
the CXCR3 receptor were found in inflamed synovial tissues from RA
patients (21), and the serum
level of CXCL10 in RA patients was reported to be correlated with
disease activity (22). In the
present study, stimulation with IFN-γ alone slightly increased the
expression of CXCR3 chemokines in synovial fibroblasts obtained
from the TMJ, while that in combination with TNF-α led to dramatic
increases in expression of those chemokines. A few reports have
noted the contribution of T-cell-induced inflammation to the
pathogenesis of TMD, including a study that showed the presence of
CD45RO+ T cells and CD68+ macrophages in
samples from patients with generalized osteoarthritis and
rheumatoid arthritis of TMD (23).
We speculate that the synergistic effect of IFN-γ and TNF-α on
induction of CXCR3 chemokines, such as CXCL10, mobilizes a large
number of T cells toward the site of inflammation, which may
promote and shape the pathological condition of the TMJ.
IL-8 and CXCL1 are functional homologues, and have
been shown to be primarily associated with neutrophil recruitment
and inflammation (24). IL-8 binds
to both the CXCR1 and CXCR2 receptors, which are found on the
surface of neutrophils, while CXCL1 binds only to the CXCR2
receptor (25). In an in
vivo study that used rabbit models of TMJ arthritis, TNF-α and
IL-8 expressions were observed in immune cells and synovial
fibroblasts from the TMJ, while IL-8 was shown to be mainly
produced in infiltrating inflammatory cells and synovial cells
during the acute stage (26). In
the present study as well, IL-8 expression in synovial fibroblasts
from the TMJ was dramatically increased by TNF-α. In contrast, some
investigators have reported that IFN-γ inhibited TNF-α-mediated
inflammatory responses in various cell types. Kohara et al
(27) found that IFN-γ directly
inhibited induction of osteoclastogenesis in bone marrow
macrophages and another showed that IFN-γ inhibited TNF-α-induced
collagenase expression in chondrocytes (28). Our results revealed that IFN-γ
inhibited increases in IL-8 and CXCL1 caused by TNF-α. In addition,
Kristense et al (29)
reported that TNF-α was consistently detected in healthy young
individuals and high levels were associated with a high level of
IFN-γ, which was sporadically found in those subjects. Therefore,
IFN-γ has both pro-inflammatory and anti-inflammatory properties,
while IFN-γ and TNF-α may control the pro-/anti-inflammatory
balance of homeostatic levels of both cytokines under normal TMJ
conditions.
IFN-γ has been shown to trigger prolonged activation
of the transcription factor STAT1 via the IFN-γ receptor and
JAK1/2, which induces expression of various genes, such as CXL10
(30,31). Activation of STAT1 involves
phosphorylation of tyrosine and serine residues, which are required
for the protein to exert its function (31,32),
while the JAK2 inhibitor AG490 prevents site-specific
phosphorylation of STAT1 by JAK kinase. It was also reported that
most IFN-γ-inducible genes expressed in the synovium of RA patients
are likely targets of STAT1 (30).
Kasperkovis et al (33)
investigated STAT1 expression in synovial tissues of RA patients
using immunohistochemistry, and found elevated levels of total
STAT1 protein, with both its activated tyrosine and serine
phosphorylated forms seen in RA synovium specimens as compared with
the control group. Although it remains unknown whether activation
of STAT1 is associated with the pathogenesis of TMD, IFN-γ
increased STAT1 phosphorylation in synovial fibroblasts from the
TMJ in the present study. Furthermore, AG490 partially decreased
the combined effect of IFN-γ and TNF-α on induction of CXCL10
expression, and recovered the decrease in IL-8 induced by that
combination. It is possible that IFN-γ participates in differential
regulation of those TNF-α-induced chemokines via JAK/STAT signaling
in synovial fibroblasts, and also contributes to modulation of the
inflammatory process in the TMJ.
In the present study, we used human synovial cells
derived from a patient with condyle bone hypertrophy without TMJ
disease, because it was difficult to obtain human synovial cells
from the TMJ of a healthy donor. Although these synovial
fibroblasts are considered to have characteristics similar to those
of normal synovial fibroblasts from the TMJ, comparisons of
response to TNF-α and IFN-γ by synovial cells between those from
healthy controls and subjects with TMJ diseases in vivo
models may be needed in the future to more clearly elucidate
the factors involved.
In summary, our results demonstrated that the
expression of several chemokines including CXCL10 were increased by
TNF-α and IFN-γ in synovial fibroblasts obtained from the TMJ. In
addition, TNF-α-mediated IL-8 and CXCL10 production was associated
with NF-κB signaling. Also, IFN-γ was shown to differentially
regulate IL-8 and CXCL10 production induced by TNF-α via JAK/STAT
signaling. We concluded that TNF-α and IFN-γ cooperatively regulate
the expressions of several chemokines including CXCL10 in synovial
fibroblasts from the TMJ, and may contribute to its pathological
condition of the TMJ.
References
|
1
|
Dijkgraaf LC, de Bont LG, Boering G and
Liem RS: Structure of the normal synovial membrane of the
temporomandibular joint: A review of the literature. J Oral
Maxillofac Surg. 54:332–338. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Israel HA, Langevin CJ, Singer MD and
Behrman DA: The relationship between temporomandibular joint
synovitis and adhesions: pathogenic mechanisms and
rheumatoidarthritis:aclinical, arthroscopic, histologic and
immunohistochemical study. Int J Oral Maxillofac Surg. 26:10–16.
1997.PubMed/NCBI
|
|
3
|
Israel HA, Diamond B, Saed-Nejad F and
Ratcliffe A: Osteoarthritis and synovitis as major pa arthroscopic
morphology. J Oral Maxillofac Surg. 56:1023–1027. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi T, Kondoh T, Fukuda M, Yamazaki
Y, Toyosaki T and Suzuki R: Proinflammatory cytokines detectable in
synovial fluids from patients with temporomandibular disorders.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 85:135–141. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaneyama K, Segami N, Nishimura M, Suzuki
T and Sato J: Importance of proinflammatory cytokines in synovial
fluid from 121 joints with temporomandibular disorders. Br J Oral
Maxillofac Surg. 40:418–423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baud V and Karin M: Signal transduction by
tumor necrosis factor and its relativs. Trends Cell Biol.
11:372–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puzas JE, Landeau JM, Tallents R, Albright
J, Schwarz EM and Landesberg R: Degradative pathways in tissues of
the temporomandibular joint. Use of in vitro and in vivo models to
characterize matrix metalloproteinase and cytokine activity. Cells
Tissues Organs. 169:248–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogura N, Tobe M, Sakamaki H, Nagura H,
Abiko Y and Kondoh T: Tumor necrosis factor-alpha increases
chemokine gene expression and production in synovial fibroblasts
from human temporomandibular joint. J Oral Pathol Med. 34:357–363.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akutsu M, Ogura N, Ito K, Kawashima M,
Kishida T and Kondoh T: Effects of interleukin-1β and tumor
necrosis factor-α on macrophage inflammatory protein-3α production
in synovial fibroblast-like cells from human temporomandibular
joints. J Oral Pathol Med. 42:491–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodig S, Kaplan D, Shankaran V, Old L and
Schreiber RD: Signaling and signaling dysfunction through the
interferon gamma receptor. Eur Cytokine Netw. 9 3 Suppl:S49–S53.
1998.
|
|
11
|
Qin S, Rottman JB, Myers P, Kassam N,
Weinblatt M, Loetscher M, Koch AE, Moser B and Mackay CR: The
chemokine receptors CXCR3 and CCR5 mark subsets of T cells
associated with certain inflammatory reactions. J Clin Invest.
101:746–754. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto K, Honda K, Ohshima M, Yamaguchi
Y, Nakajima I, Micke P and Otsuka K: Cytokine profile in synovial
fluid from patients with internal derangement of the
temporomandibular joint: A preliminary study. Dentomaxillofac
Radiol. 35:432–441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee EY, Lee ZH and Song YW: CXCL10 and
autoimmune diseases. Autoimmun Rev. 8:379–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alaaeddine N, DiBattista JA, Pelletier JP,
Cloutier JM, Kiansa K, Dupuis M and Martel-Pelletier J:
Osteoarthritic synovial fibroblasts possess an increased level of
tumor necrosis factor-receptor 55 (TNF-R55) that mediates
biological activation by TNF-alpha. J Rheumatol. 24:1985–1994.
1997.PubMed/NCBI
|
|
15
|
Thanos D and Maniatis T: NF-kappa B: A
lesson in family values. Cell. 80:529–532. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gough DJ, Messina NL, Hii L, Gould JA,
Sabapathy K, Robertson AP, Trapani JA, Levy DE, Hertzog PJ, Clarke
CJ and Johnstone RW: Functional crosstalk between type I and II
interferon through the regulated expression of STAT1. PLoS Biol.
27:e10003612010. View Article : Google Scholar
|
|
17
|
Suzuki T, Segami N, Nishimura M and Nojima
T: Co-expression of interleukin-1beta and tumor necrosis factor
alpha in synovial tissues and synovial fluids of temporomandibular
joint with internal derangement: Comparison with histological
grading of synovial inflammation. J Oral Pathol Med. 31:549–557.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke J, Long X, Liu Y, Zhang YF, Li J, Fang
W and Meng QG: Role of NF-kappaB in TNF-alpha-induced COX-2
expression in synovial fibroblasts from human TMJ. J Dent Res.
86:363–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel DD, Zachariah JP and Whichard LP:
CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin
Immunol. 98:39–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohan K, Ding Z, Hanly J and Issekutz TB:
IFN-gamma-inducible T cell a chemoattractant is a potent stimulator
of normal human blood T lymphocyte transendothelial migration:
Differential regulation by IFN-gamma and TNF-alpha. J Immunol.
168:6420–6428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ueno A, Yamamura M, Iwahashi M, Okamoto A,
Aita T, Ogawa N and Makino H: The production of CXCR3-agonistic
chemokines by synovial fibroblasts from patients with rheumatoid
arthritis. Rheumatol Int. 25:361–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuan WP, Tam LS, Wong CK, Ko FW, Li T, Zhu
T and Li EK: CXCL 9 and CXCL 10 as sensitive markers of disease
activity in patients with rheumatoid arthritis. J Rheumatol.
37:257–264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gynther GW, Holmlund AB, Reinholt FP and
Lindblad S: Temporomandibular joint involvement in generalized
osteoarthritis and rheumatoid arthritis: A clinical, arthroscopic,
histologic, and immunohistochemical study. Int J Oral Maxillofac
Surg. 26:10–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godiska R, Chantry D, Dietsch GN and Gray
PW: Chemokine expression in murine experimental allergic
encephalomyelitis. J Neuroimmunol. 58:167–176. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murphy PM: Neutrophil receptors for
interleukin-8 and related CXC chemokines. Semin Hematol.
34:311–318. 1997.PubMed/NCBI
|
|
26
|
Sukedai M, Tominaga K, Habu M, Matsukawa
A, Nishihara T and Fukuda J: Involvement of tumor necrosis
factor-alpha and interleukin-8 in antigen-induced arthritis of the
rabbit temporomandibular joint. J Oral Pathol Med. 33:102–110.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kohara H, Kitaura H, Fujimura Y,
Yoshimatsu M, Morita Y, Eguchi T, Masuyama R and Yoshida N: IFN-γ
directly inhibits TNF-α-induced osteoclastogenesis in vitro and in
vivo and induces apoptosis mediated by Fas/Fas ligand interactions.
Immunol Lett. 30:53–61. 2011. View Article : Google Scholar
|
|
28
|
Meyer FA, Yaron I and Yaron M:
Synergistic, additive, and antagonistic effects of
interleukin-1beta, tumor necrosis factor-alpha and gamma-interferon
on prostaglandin E, hyaluronic acid, and collagenase production by
cultured synovial fibroblasts. Arthritis Rheum. 33:1518–1525. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kristensen KD, Alstergren P, Stoustrup P,
Küseler A, Herlin T and Pedersen TK: Cytokines in healthy
temporomandibular joint synovial fluid. J Oral Rehabil. 41:250–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: New surprises in the Jak/Stat pathway.
Cell. 109 Suppl:S121–S131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kasperkovitz PV, Verbeet NL, Smeets TJ,
van Rietschoten JG, Kraan MC, van der Pouw Kraan TC, Tak PP and
Verweij CL: Activation of the STAT1 pathway in rheumatoid
arthritis. Ann Rheum Dis. 63:233–239. 2004. View Article : Google Scholar : PubMed/NCBI
|