Introduction
Primary cilia are microtubule-based organelles that
protrude from most eukaryotic cells (1). Primary cilia consist of nine pairs of
peripheral microtubules organized in a ring without a central
microtubule. The outer peripheral microtubule structure is
collectively termed the 9+0 axoneme (2,3).
With the exception of cells undergoing division, most eukaryotic
cells have a single primary cilium (4,5).
Primary cilia were believed to have no important functions in
normal cells; however, previous studies have indicated that they
can act as sensory receptors for mechanical and chemical stimuli,
and they may transmit these signals intracellularly (6–8).
The intraflagellar transport (IFT) complex consists
of >20 different IFT proteins that assemble into macromolecular
complexes, which can be transported through the action of kinesin
or dynein motors along the flagellar axoneme (9,10).
IFTs are responsible for the assembly and maintenance of cilia and
flagella in eukaryotic cells (9,11).
Intraflagellar transport protein 88 (IFT88) is a central component
of the IFT-B complex, and is required for ciliogenesis in
vertebrates and for flagellar assembly in Chlamydomonas
(12,13). IFT88 has a critical role in cilia
formation; however, the regulation of IFT88 expression in
chondrocytes remains to be fully elucidated.
Basic fibroblast growth factor (bFGF) is a member of
the FGF family with mitogenic properties, which may exert numerous
functions and a wide range of effects in cells. bFGF may bind
heparin and heparin sulfate (14)
and regulate the migration, differentiation, proliferation and
survival of various types of cells (15–17).
bFGF is also involved in the regulation of articular cartilage
homeostasis; however, the exact mechanical and biochemical
processes involved in cartilage degeneration and the function of
bFGF in these processes remain to be fully elucidated. In addition,
the effects of bFGF on IFT88 expression and the formation and
maintenance of cilia in chondrocytes remain unclear. Therefore, the
present study aimed to identify the effects of bFGF on IFT88
expression and primary cilia formation in chondrocytes in
vitro.
Materials and methods
Cells and reagents
Murine ATDC5 chondrogenic cells were purchased from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were plated at a density of
1×104 cells/cm2 and cultured in Dulbecco's
modified Eagle's medium/nutrient mixture F12 (DMEM/F12; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 5%
fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin
(100 U/ml). Cells were maintained at 37°C in a 5% CO2
atmosphere. Media were replaced every other day.
Primary cultured chondrocytes were prepared from the
femoral cartilage of 6 male C57BL mice (age, 2 weeks; weight,
8.85±1.07 g). Mice were maintained in an environment with a 12 h
light/dark cycle and at 23±1°C and with free access to food and
water. All mice were supplied by the Experimental Animal Center of
Tongji Hospital (Wuhan, China) and the procedures were approved by
the Ethics Committee on Animal Experimentation of Tongji Medical
College, Huazhong University of Science and Technology (Wuhan,
China). The cartilage was dissected, enzymatically digested with
0.25% trypsin solution (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 37°C for 30 min and 0.1% type I collagenase solution
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C for 6 h. The single cell suspensions that were obtained were
resuspended in DMEM/F12 supplemented with 10% FBS following washing
in phosphate-buffered saline (PBS).
bFGF was purchased from Cyagen Biosciences Inc.
(Santa Clara, CA, USA), PD0325901 and BGJ398 were purchased from
Selleck Chemicals (Houston, TX, USA). ATDC5 cells were treated at
37°C with different concentrations of bFGF (0, 2.5, 5, 10, 20 or 50
ng/ml for 24 h) and for different durations (5 ng/ml for 0, 12, 24,
36, 48 or 72 h). The control group received a DMSO vehicle
treatment. The ERK and FGFR inhibitors PD0325901 (11 nM) and BGJ398
(1 nM), respectively, were used to treated cells at 37°C for 24
h.
Western blot analysis
ATDC5 cells were lysed in radioimmunoprecipitation
assay lysis buffer (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) containing a protease inhibitor cocktail. Extracted
protein concentrations were measured using a bicinchoninic acid
assay and equal quantities (20 µg) were separated by 10% SDS-PAGE
and transferred onto polyvinylidene difluoride membranes. The
proteins were then blocked with 5% BSA (Biosharp, Hefei, China) at
37°C for 1 h. Blots were incubated at 4°C overnight with the
following primary antibodies: IFT88 (AP11138b, 1:1,000 dilution;
Abgent, Inc., San Diego, CA, USA), β-actin (BM0627, 1:400 dilution;
Wuhan Boster Biological Technology, Ltd.), extracellular
signal-regulated protein kinase (ERK, #4695; 1:1,000 dilution), and
phosphorylated (p)-ERK (#4370, 1:1,000 dilution) (both from Cell
Signaling Technology, Inc., Danvers, MA, USA), followed by
incubation with horseradish peroxidase-conjugated goat anti-rabbit
or goat anti-mouse immunoglobulin (Ig)G (BA1054 and BA1050, 1:5,000
dilution; Wuhan Boster Biological Technology, Ltd.) secondary
antibodies at 37°C for 1 h. An enhanced chemiluminescence (ECL)
western blot detection kit (Thermo Fisher Scientific, Inc.) was
used to visualize the protein bands on an ECL system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Blots were semi-quantified
by densitometric analysis using Image-Lab software version 4.0.1
(Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells following
incubation using the RNeasy Mini kit (Qiagen GmbH, Hilden,
Germany), according to the manufacturer's protocol. cDNA was
synthesized from total RNA using the SuperScript First-Strand
Synthesis system for RT-PCR (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. qPCR was performed
on cDNA using a SYBR Green Real-Time PCR Master Mix kit (Toyobo
Life Science, Osaka, Japan). The thermocycling conditions were as
follows: Pre-denaturation at 95°C for 1 min, followed by 40 cycles
of denaturation at 95°C for 15 sec, annealing at 60°C for 15 sec
and extension at 72°C for 45 sec. Gene expression levels were
normalized to those of β-actin and the method of quantification was
2−ΔΔCq (18). The
primer sequences used in the present study were as follows: IFT88
forward, (F) 5′-TGGCCAACGACCTGGAGATTAACA-3′ and reverse, (R)
5′-ATAGCTGCTGGCTTGGGCAAATTC-3′; and β-actin F,
5′-CTTCTTGGGTATGGAATCCTGTGG-3′ and R,
5′-TGTGTTGGCATAGAGGTCTTTACG-3′.
Small interfering (si)RNA
transfection
ATDC5 cells (1×106) were transfected with
50 nM siRNA targeting IFT88 or with a scrambled sequence (negative
control siRNA) for 72 h using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) as the transfection reagent. The
knockdown efficiency of IFT88 siRNA was assessed using RT-qPCR and
western blot analysis, as aforementioned. IFT88 siRNA and negative
control siRNA (siG151225113820 and siN05815122147) were synthesized
by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Immunofluorescence
ATDC5 cells were seeded on coverslips at a density
of 5×103 cells/cm2 and stimulated with the
various reagents. The cells were fixed in 4% paraformaldehyde for
15 min following 24 h of treatment, blocked with 0.5% bovine serum
albumin (Biosharp) at room temperature for 1 h, and then incubated
with a primary acetylated α-tubulin (acTub) antibody (ab24610,
1:500 dilution; Abcam, Cambridge, UK) overnight at 4°C. The cells
were then incubated with cyanine 3-conjugated goat anti-mouse IgG
secondary antibody (BA1031, 1:200 dilution; Wuhan Boster Biological
Technology, Ltd.) at room temperature for 1 h, and the nuclei were
stained with 1 µg/µl DAPI at 37°C for 5 min. The cells were washed
with PBS 3 times for 10 min after each step. Stained cells were
visualized under a fluorescence microscope and photomicrographs
were captured using an EVOS FL Auto Cell Imaging System (Thermo
Fisher Scientific, Inc.).
Statistical analysis
Each experiment was performed at least 3 times. Data
are presented as the mean ± standard deviation. The statistical
significance of the differences between groups was assessed using
Student's t-test and two-way analysis of variance followed by a
Bonferroni post-hoc test were used. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS version 20.0 (IBM Corporation,
Armonk, NY, USA).
Results
bFGF treatment increasesIFT88
expression in a time- and dose-dependent manner in ATDC5 cells
To examine the effects of bFGF on IFT88 mRNA and
protein expression levels, ATDC5 cells were treated with 5 ng/ml
bFGF for up to 72 h. bFGF treatment was demonstrated to upregulate
the mRNA and protein expression of IFT88 within 12 h, a maximal
effect was evident following 24 h of treatment; the effects of bFGF
continued for 72 h (Fig. 1A-C). To
investigate whether the effects of bFGF on IFT88 expression were
dose-dependent, RT-qPCR and western blot analysis were performed
using ATDC5 cells treated with various doses of bFGF. ATDC5 cells
were treated with bFGF at concentrations of 0, 2.5, 5, 10, 20, and
50 ng/ml for 24 h. bFGF treatment was revealed to potentiate the
protein expression of IFT88 in a dose-dependent manner; this effect
was evident at 2.5 ng/ml and peaked at 5 ng/ml (Fig. 1D and E). The effect of bFGF on
IFT88 protein expression gradually decreased at concentrations
>5 ng/ml, but expression remained above the IFT88 levels in
control cells, with the exception of cells treated with 50 ng/ml
bFGF (Fig. 1D and E). In addition,
bFGF treatment increased the mRNA expression of IFT88 in a
dose-dependent manner, and the results were similar to those
regarding protein expression (Fig.
1F).
 | Figure 1.bFGF treatment enhanced the mRNA and
protein expression of IFT88 in a time- and dose-dependent manner in
murine ATDC5 chondrocytes in vitro. (A-C) ATDC5 cells were
cultured for the indicated times (0, 12, 24, 36, 48 and 72 h) in
the presence of bFGF (5 ng/ml) or (D-F) with the indicated
concentrations of bFGF (0, 2.5, 5, 10, 20 and 50 ng/ml) for 24 h.
(A, B, D, E) IFT88 protein expression levels were assessed using
western blot analysis with β-actin as a loading control. (C and F)
IFT88 mRNA expression was detected using reverse
transcription-quantitative polymerase chain reaction and normalized
to β-actin mRNA levels. Data are expressed as the mean ± standard
deviation of 3 independent experiments. *P<0.05, **P<0.01, as
indicated. bFGF, basic fibroblast growth factor; IFT88,
intraflagellar transport protein 88. |
ERK inhibition counteracts the effect
of bFGF on IFT88 protein expression
To investigate the putative signaling pathways
implicated in the regulatory effects of bFGF on IFT88 expression,
ATDC5 cells were treated with bFGF in the presence or absence of
the ERK inhibitor PD0325901. The present results demonstrated that
bFGF treatment enhanced the phosphorylation of ERK, as indicated by
western blot analysis of p-ERK expression (Fig. 2). Conversely, treatment with
PD0325901 significantly downregulated the protein expression levels
of p-ERK in ATDC5 cells, and suppressed IFT88 protein expression
(Fig. 2B), however, no differences
were evident across total ERK expression levels. bFGF treatment in
the presence of PD0325901 appeared to upregulate the expression of
IFT88; however, the effect was significantly reduced when compared
with bFGF treatment alone (Fig.
2B). A similar trend was revealed in p-ERK protein expression
levels, suggesting that PD0325901 may reduce the expression of
IFT88 by inhibiting ERK phosphorylation. These findings suggested
that the ERK signaling pathway may be involved in the regulation of
IFT88 expression by bFGF in chondrocytes in vitro.
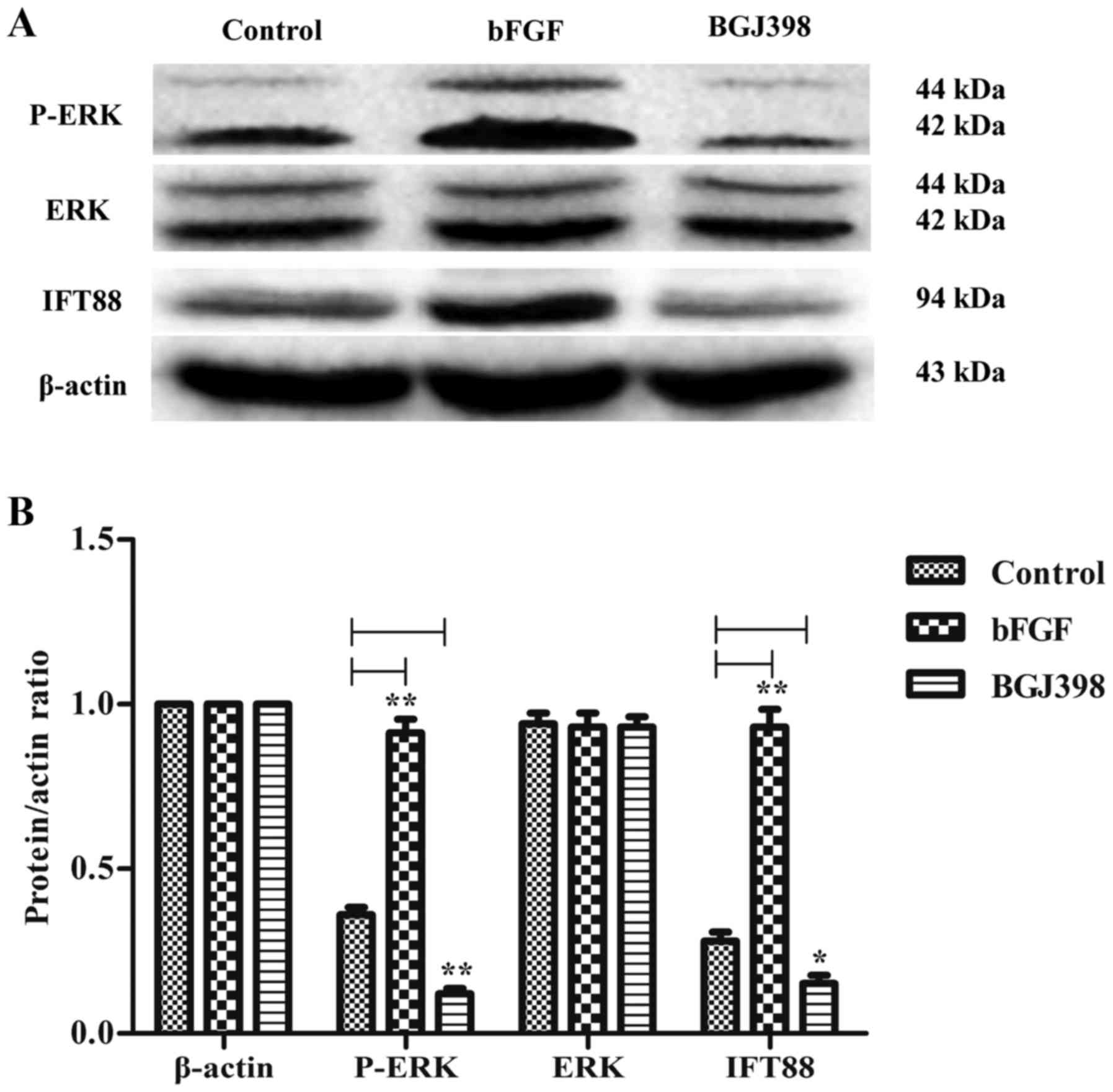
Inhibition of the FGF receptor (FGFR)
suppresses IFT88 protein expression in ATDC5 cells
To investigate the effects of FGFR inhibition on
IFT88 protein expression, ATDC5 cells were treated with the FGFR
inhibitor BGJ398. BGJ398 primarily inhibits FGFR1, FGFR2 and FGFR3.
The present findings revealed that BGJ398 downregulated the protein
expression levels of IFT88 in ATDC5 cells, which was accompanied by
a decrease in p-ERK expression levels (Fig. 3). These findings suggested that
FGFR may participate in the molecular mechanisms underlying the
regulation of IFT88 expression in chondrocytes.
Knockdown of IFT88 downregulates the
protein expression of p-ERK in ATDC5 cells
To investigate the effects of IFT88 downregulation
on the mitogen-activated protein kinase (MAPK)/ERK signaling
pathway, RNA interference was used to silence IFT88 expression in
ATDC5 cells. The efficacy of IFT88 siRNA transfection was confirmed
(Fig. 4A), and the protein
expression levels of IFT88, p-ERK and ERK were evaluated using
western blot analysis. IFT88 knockdown was revealed to suppress the
protein expression of IFT88 and p-ERK in ATDC5 cells, whereas it
had no effect on ERK protein expression (Fig. 4B and C). These findings suggested
that the MAPK/ERK signaling pathway may be involved in the
regulation of IFT88 expression in ATDC5 cells.
bFGF exposure increases the number of
ATDC5 cells exhibiting primary cilia
To investigate the effects of bFGF on the
development of primary cilia, the presence of primary cilia in
ATDC5 cells was detected using an acTub antibody. Following
staining with the anti-acTub antibody, primary cilia were
visualized as red acetylated α-tubulin-positive structures in ATDC5
cells under a fluorescence microscope. bFGF exposure increased the
percentage of ciliated cells compared with the control group
(Fig. 5). bFGF treatment increased
the number of ciliated cells by ~80%, suggesting that the
bFGF-mediated regulation of IFT88 expression may be involved in the
development of primary cilia in chondrocytes.
PD0325901 or BGJ398 treatment and
IFT88 knockdown decreases the number of ciliated ATDC5 cells
To further investigate the effects of IFT88
downregulation on primary cilia development, primary cilia were
visualized in ATDC5 cells using an anti-acTub antibody. Treatment
with PD0325901 or BGJ398, and transfection with IFT88 siRNA,
resulted in fewer ciliated cells compared with the control group
(Fig. 6). These findings suggested
that the regulation of IFT88 expression, and MAPK/ERK- and
FGFR-mediated pathways may be involved in primary cilium
maintenance.
 | Figure 6.Treatment with the ERK inhibitor
PD0325901 or the FGFR inhibitor BGJ398, and IFT88 knockdown
suppressed the formation of primary cilia in murine ATDC5
chondrocytes. ATDC5 cells were cultured in the presence or absence
of (A) PD0325901 or BGJ398 for 24 h, or (B) transfected with
negative control or IFT88-targeting siRNA for 72 h. The cells were
then stained with an anti-acTub (red) antibody to detect primary
cilia. DAPI was used to visualize the nuclei (blue). PD0325901 or
BGJ398 treatment and IFT88 knockdown decreased the number of
ciliated cells (white arrows) compared with the control group.
Magnification, ×200. ERK, extracellular signal-regulated protein
kinase; FGF, fibroblast growth factor; FGFR, FGF receptor; IFT88,
intraflagellar transport protein 88; si, small interfering; acTub,
acetylated α-tubulin. Treatment with the ERK inhibitor PD0325901 or
the FGFR inhibitor BGJ398, and IFT88 knockdown suppressed the
formation of primary cilia in murine ATDC5 chondrocytes. (C) The
percentage of ciliated cells was calculated in 10 randomly selected
fields of view and >100 cells were counted in each field. Data
are expressed as the mean ± standard deviation. *P<0.05,
**P<0.01, as indicated. ERK, extracellular signal-regulated
protein kinase; FGF, fibroblast growth factor; FGFR, FGF receptor;
IFT88, intraflagellar transport protein 88; si, small interfering;
acTub, acetylated α-tubulin. |
bFGF treatment upregulates IFT88
expression and promotes primary cilium development in primary
chondrocytes
To investigate whether primary chondrocytes may
exhibit a similar response to bFGF to ATDC5 cells in vitro,
the mRNA and protein expression of IFT88 was assessed in primary
chondrocytes. Primary chondrocytes were prepared from newborn mice
and treated with bFGF at a concentration of 5 ng/ml for 24 h. bFGF
treatment upregulated the IFT88 mRNA and protein expression in
primary chondrocytes (Fig. 7A-C).
An immunofluorescence assay was used to evaluate the effects
of exogenous bFGF on cilium development, and results revealed that
bFGF increased the number of ciliated cells compared with the
control group (Fig. 7D and E).
Discussion
The IFT protein complex is essential for the
formation and maintenance of primary cilia in eukaryotic cells
(9,11,12);
however, the molecular mechanisms involved in the effects of IFT
complexes on cilia remain to be elucidated. In addition, little is
known regarding the regulation of IFT proteins by cytokines in
vivo or in vitro, and the effects of cytokines on cilium
formation.
In the present study, exogenous bFGF was
demonstrated to increase the mRNA and protein expression of IFT88
in ATDC5 chondrocytes in vitro. It is possible that the
upregulated expression of IFT88 may potentiate the function of the
IFT system in chondrocytes, through the amplification of mechanical
stimulation and sensory perception of the extracellular
microenvironment (6–8). These processes have been reported to
contribute to the regulation of cartilage development (19,20).
A depletion of primary cilia has been demonstrated in the articular
cartilage of Col2αCre; IFT88fl/fl mice, which resulted
in abnormal articular cartilage development (21). The cartilage of Col2αCre;
IFT88fl/fl mice was thicker, had increased cell density
and exhibited enhanced expression of osteoarthritic markers,
including matrix metalloproteinase-13, disintegrin-like and
metalloprotease with thrombospondin type 1 motif 5, collagen X and
runt-related transcription factor 2 (21).
The findings of the present study suggested that
bFGF may upregulate the protein expression of IFT88 through the
MAPK/ERK signaling pathway in chondrocytes. Following treatment
with the ERK inhibitor PD0325901, the FGFR inhibitor BGJ398, or
IFT88-targeting siRNA, the protein expression levels of p-ERK
appeared to be downregulated. These findings suggested that the
IFT88 and MAPK/ERK pathways may be closely associated in
chondrocytes; however, further studies are required to investigate
the specific targets of the MAPK/ERK pathway and its downstream
effects in primary cilia.
Murine chondrocytes express all FGFR subtypes
(FGFR1-4); however, bFGF treatment has been reported to
significantly induce the expression of FGFR3 (22,23),
which exerted anabolic effects in murine chondrocytes. In the
present study, the FGFR inhibitor BGJ398 suppressed the expression
of IFT88 in murine chondrocytes, thus suggesting that the IFT88
expression regulation process may involve FGFR regulation. It is of
note that bFGF in human articular cartilage has been suggested to
exert opposite roles compared with in murine cartilage, and FGF has
been reported to activate catabolic processes primarily via FGFR1
signaling in human cartilage (24,25).
Considered together, these findings suggested that the effects of
bFGF may be species-dependent.
Primary cilia have a microtubule-based
infrastructure, which consists of 9 pairs of peripheral
microtubules without a central microtubule, and can detect
alterations in mechanical and biochemical stimulation from the
extracellular milieu (26,27). In addition, it has been suggested
that cilia may be essential for the development and progression of
tumors (28). In the present
study, IFT88 expression was downregulated in ATDC5 chondrocytes
in vitro, through the inhibition of ERK and FGFR signaling.
Following siRNA-mediated knockdown, IFT88 downregulation resulted
in the suppression of primary cilia development in chondrocytes.
These findings suggested that IFT88 may be an essential factor for
the formation and maintenance of primary cilia. To the best of our
knowledge, the present study demonstrated for the first time that
bFGF enhanced the mRNA and protein expression of IFT88, which in
turn may promote primary cilia formation in chondrocytes. These
findings propose a novel function for bFGF in chondrocytes.
Acknowledgements
The present study was supported by the National
Natural Science Foundations of China (grant nos. 81371915 and
81572094).
References
|
1
|
Seeley ES and Nachury MV: The perennial
organelle: Assembly and disassembly of the primary cilium. J Cell
Sci. 123:511–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim S and Dynlacht BD: Assembling a
primary cilium. Curr Opin Cell Biol. 25:506–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satir P and Christensen ST: Overview of
structure and function of mammalian cilia. Annu Rev Physiol.
69:377–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enuka Y, Hanukoglu I, Edelheit O, Vaknine
H and Hanukoglu A: Epithelial sodium channels (ENaC) are uniformly
distributed on motile cilia in the oviduct and the respiratory
airways. Histochem Cell Biol. 137:339–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tobin JL and Beales PL: The nonmotile
ciliopathies. Genet Med. 11:386–402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoey DA, Tormey S, Ramcharan S, O'Brien FJ
and Jacobs CR: Primary cilia-mediated mechanotransduction in human
mesenchymal stem cells. Stem Cells. 30:2561–2570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa H and Marshall WF: Ciliogenesis:
Building the cell's antenna. Nat Rev Mol Cell Biol. 12:222–234.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muhammad H, Rais Y, Miosge N and Ornan EM:
The primary cilium as a dual sensor of mechanochemical signals in
chondrocytes. Cell Mol Life Sci. 69:2101–2107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenbaum JL and Witman GB: Intraflagellar
transport. Nat Rev Mol Cell Biol. 3:813–825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuno N, Taschner M, Engel BD and
Lorentzen E: Structural studies of ciliary components. J Mol Biol.
422:163–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sung CH and Leroux MR: The roles of
evolutionarily conserved functional modules in cilia-related
trafficking. Nat Cell Biol. 15:1389–1397. 2013. View Article : Google Scholar
|
|
12
|
Pazour GJ, Baker SA, Deane JA, Cole DG,
Dickert BL, Rosenbaum JL, Witman GB and Besharse JC: The
intraflagellar transport protein, IFT88, is essential for
vertebrate photoreceptor assembly and maintenance. J Cell Biol.
157:103–113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pazour GJ, Dickert BL, Vucica Y, Seeley
ES, Rosenbaum JL, Witman GB and Cole DG: Chlamydomonas IFT88 and
its mouse homologue, polycystic kidney disease gene tg737, are
required for assembly of cilia and flagella. J Cell Biol.
151:709–718. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedl A, Chang Z, Tierney A and Rapraeger
AC: Differential binding of fibroblast growth factor-2 and −7 to
basement membrane heparin sulfate: Comparison of normal and
abnormal human tissues. Am J Pathol. 150:1443–1455. 1997.PubMed/NCBI
|
|
15
|
Okada-Ban M, Thiery JP and Jouanneau J:
Fibroblast growth factor-2. Int J Biochem Cell Biol. 32:263–267.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song H, Kwon K, Lim S, Kang SM, Ko YG, Xu
Z, Chung JH, Kim BS, Lee H, Joung B, et al: Transfection of
mesenchymal stem cells with the FGF-2 gene improves their survival
under hypoxic conditions. Mol Cells. 19:402–407. 2005.PubMed/NCBI
|
|
17
|
Ng EW and Adamis AP: Targeting
angiogenesis, the underlying disorder in neovascular age-related
macular degeneration. Can J Ophthalmol. 40:352–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McGlashan SR, Cluett EC, Jensen CG and
Poole CA: Primary cilia in osteoarthritic chondrocytes: From
chondrons to clusters. Dev Dyn. 237:2013–2020. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wann AK, Zuo N, Haycraft CJ, Jensen CG,
Poole CA, McGlashan SR and Knight MM: Primary cilia mediate
mechanotransduction through control of ATP-induced Ca2+
signaling in compressed chondrocytes. FASEB J. 26:1663–1671. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang CF, Ramaswamy G and Serra R:
Depletion of primary cilia in articular chondrocytes result in
reduced Gli3 repressor to activator ratio, increased Hedgehog
signaling, and symptoms of early osteoarthritis. Osteoarthritis
Cartilage. 20:152–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Ellman MB, Kroin JS, Chen D, Yan D,
Mikecz K, Ranjan KC, Xiao G, Stein GS, Kim SG, et al:
Species-specific biological effects of FGF-2 in articular
cartilage: Implication for distinct roles within the FGF receptor
family. J Cell Biochem. 113:2532–2542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chia SL, Sawaji Y, Burleigh A, McLean C,
Inglis J, Saklatvala J and Vincent T: Fibroblast growth factor 2 is
an intrinsic chondroprotective agent that suppresses ADAMTS-5 and
delays cartilage degradation in murine osteoarthritis. Arthritis
Rheum. 60:2019–2027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan D, Chen D, Cool SM, Van Wijnen AJ,
Mikecz K, Murphy G and Im HJ: Fibroblast growth factor receptor 1
is principally responsible for fibroblast growth factor 2-induced
catabolic activities in human articular chondrocytes. Arthritis Res
Ther. 13:R1302011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan D, Chen D and Im HJ: Fibroblast growth
factor-2 promotes catabolism via FGFR1-Ras-Raf-MEK1/2-ERK1/2 axis
that coordinates with the PKCδ pathway in human articular
chondrocytes. J Cell Biochem. 113:2856–2865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pazour GJ and Witman GB: The vertebrate
primary cilium is a sensory organelle. Curr Opin Cell Biol.
15:105–110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bloodgood RA: Sensory reception is an
attribute of both primary cilia and motile cilia. J Cell Sci.
123:505–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiang W, Jiang T, Guo F, Gong C, Yang K,
Wu Y, Huang X, Cheng W and Xu K: Hedgehog pathway inhibitor-4
suppresses malignant properties of chondrosarcoma cells by
disturbing tumor ciliogenesis. Oncol Rep. 32:1622–1630. 2014.
View Article : Google Scholar : PubMed/NCBI
|