Introduction
Apolipoprotein A5 (apoA5), first described in 2001,
has been demonstrated to be an important modulator of triglyceride
(TG) metabolism (1). ApoA5 is
synthesized and secreted exclusively by the liver and is present in
plasma associated with chylomicrons, very low density lipoprotein
(LDL) and high density lipoprotein (1). Increasing evidence suggests that a
number of variants of the gene encoding human apoA5 (APOA5)
alter plasma TG levels and is associated with obesity (2–4).
Lower plasma levels of apoA5 were observed in obese subjects and
were inversely associated with body mass index (BMI) in humans,
suggesting that decreased apoA5 levels may have importance in the
pathophysiology of obesity in humans (5,6).
However, the underlying mechanisms of apoA5-associated obesity
remain to be understood and cannot be fully explained by its impact
on plasma TG levels (7,8).
An intracellular role of apoA5 has been suggested,
as apoA5 is associated with cytoplasmic lipid droplets (9,10)
and affects intrahepatic TG accumulation (10,11).
Given that adipocytes provide the largest storage depot for energy
in the form of TG within lipid droplets, and serve an important
role in the development of obesity, the present study hypothesized
that apoA5 may additionally target adipocytes and regulate
adipocyte lipid storage. Our previous data demonstrated that apoA5
may be internalized by human adipocytes, as treatment of adipocytes
with receptor associated protein (RAP) or heparin, both of which
interrupt binding of apoA5 to low-density lipoprotein receptor
(LDLR) family members, resulted in a decrease in the quantity of
apoA5 internalized by adipocytes (12). LDLR-related protein 1 (LRP1), which
belongs to the LDLR family and is abundantly expressed in
adipocytes, has been reported to interact with apoA5 with high
affinity and mediates receptor-mediated endocytosis of apoA5
(13). This suggests that LRP1 may
serve a role in uptake of apoA5 by adipocytes. In addition, it was
revealed that internalized apoA5 surrounded lipid droplets,
co-localized with the known lipid droplet protein perilipin and
significantly decreased cellular TG content in adipocytes (12).
Lipid droplets are dynamic organelles that contain a
neutral lipid core surrounded by a phospholipid monolayer, and is
coated with specific proteins (14,15).
Numerous studies on the formation of lipid droplets and regulation
of lipid accumulation have demonstrated the importance of lipid
droplet-associated proteins in these processes. Based on shared
sequence homology, one set of lipid droplet proteins, the
perilipin-adipophilin-TIP47 (PAT) family of proteins, are well
characterized (16). Within
adipocytes, perilipin is the most abundant protein on adipocyte
lipid droplets and is an important regulator of lipid storage and
release. Under basal conditions, perilipin is a gatekeeper that
prevents lipases from gaining access to neutral lipids in the
droplet core, and therefore reduces TG hydrolysis (17). By contrast, once phosphorylated in
response to catecholamine stimulation, perilipin actively
facilitates lipase action, partially by recruiting
hormone-sensitive lipase (HSL, officially known as LIPE) to the
droplet surface. Ablation of perilipin from white adipose tissue
(WAT) causes dysregulation of adipocyte lipid storage characterized
by increased basal lipolysis and decreased stimulated lipolysis and
results in a dramatic reduction in WAT mass (18,19).
Cidec, a human homolog of rodent fat-specific
protein 27 (Fsp27), has been identified as a novel lipid
droplet-associated protein in adipocytes and serves an important
role in controlling diverse metabolic processes (20–24).
Cidec/Fsp27 belong to the cell death-inducing DNA fragmentation
factor-α-like effector (Cide) family that shares a common CIDE-N
domain in the N-terminus and CIDE-C domain in the C-terminus
(25). In humans, cidec is
predominantly expressed in subcutaneous adipose tissue (26). Nishino et al (22) demonstrated that depletion of Fsp27
in murine white adipocytes resulted in the formation of numerous
small lipid droplets, increased lipolysis and decreased TG storage,
whereas expression of Fsp27 in COS monkey fibroblast cells promoted
the formation of large lipid droplets. This suggested that Fsp27
contributes to efficient energy storage in WAT by promoting the
formation of unilocular lipid droplets, thereby restricting
lipolysis. In addition Fsp27-knockout mice have been described to
exhibit a phenotype of obesity-resistance, elevated oxygen
consumption, reduced WAT mass and smaller white adipocytes with
multilocular lipid droplets (22,23).
In addition, expression of genes associated with fatty acid
β-oxidation, mitochondrial biosynthesis and brown adipose tissue
(BAT)-special genes were significantly increased in Fsp27 knockout
mice. These alterations suggest that WAT tissue of Fsp27-knockout
mice may have acquired certain BAT-like properties, and therefore
was transformed from a typical energy storage tissue into an energy
consuming tissue.
In the present study, expression of apoA5 by
adipocytes under different conditions, the effect of apoA5 on the
expression of the genes encoding perilipin and cidec and the
mechanism responsible for apoA5 negative modulation in adipocyte TG
accumulation was investigated.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM)/F-12,
DMEM, fetal bovine serum (FBS) and human insulin were all purchased
from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Dexamethasone (Dex), 3-isobutyl-1-methylxanthine (IBMX),
rosiglitazone, isoproterenol and recombinant murine tumor necrosis
factor-α (TNF-α) were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Mouse anti-human apoA5 antibody was obtained
from Novus Biologicals, LLC (cat. no. NB400-139; Littleton, CO,
USA) (6). Rabbit anti-mouse LRP1
antibody (cat. no. ab92544), mouse anti-human cidec antibody (cat.
no. ab77115) and rabbit anti-human perilipin antibody (cat. no.
ab50291) were all purchased from Abcam (Cambridge, UK). Goat
anti-rabbit IgG antibody (bs-0295G) and rabbit anti-mouse IgG
antibody (bs-0296R) were purchased from Bioss (Beijing, China).
Recombinant human apoA5 was produced in Escherichia coli and
isolated, as previously described (27).
Samples of human tissues and cell
lines
Subcutaneous adipose tissue samples were obtained
from 19 patients (7 male and 12 female) undergoing elective
open-abdominal surgical procedures in the Second Xiangya Hospital,
Central South University, China between December 2014 and November
2015. None of the patients had known diabetes or severe systemic
illness, and were not taking medications known to affect adipose
tissue mass or metabolism. The mean BMI was 22.9 kg/m2
(range, 16.3–31.1 kg/m2), and the mean age was 40 years
(range, 15–55). In particular, 3 obese patients (BMI ≥30) were
recruited together with 3 matched non-obese patients (BMI <25)
to obtain the subcutaneous adipose tissues for comparing the
endocytosis of apoA5 by adipocytes in vivo. Tissue samples
were immediately either frozen in liquid nitrogen and stored at
−80°C, or digested with collagenase (Gibco; Thermo Fisher
Scientific, Inc.) to isolate preadipocytes, as previously described
(28). The protocol was approved
by the Ethics Committee of Central South University (Hunan, China)
and all patients provided written informed consent. The 3T3-L1
preadipocyte murine cell line was obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA).
Cell culture
Human preadipocytes were differentiated into
adipocytes as previously described, with a few modifications
(29). Briefly, human
preadipocytes were cultured in DMEM/F-12 medium supplemented with
10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin (complete
medium) and incubated at 37°C in a humidified 5% CO2/95%
air atmosphere. At 100% confluence, cells were treated with
differentiation medium containing DMEM/F-12, 100 U/ml penicillin,
100 U/ml streptomycin, 5% FBS, 1 µM Dex, 500 µM IBMX, 10 µg/ml
insulin and 1 µM rosiglitazone for 12 days. Cells continued to be
cultured in differentiation medium, but without rosiglitazone for a
further 9 days. Cells were then cultured in complete medium for 2
days. These cells were used as differentiated adipocytes in all
experiments. Each medium was replaced with fresh medium every 3
days.
3T3-L1 fibroblasts were cultured and differentiated
into adipocytes as previously described (30,31).
Cells at day 8 and day 21 post-induction of differentiation were
used as non-hypertrophied and hypertrophied adipocytes,
respectively (30). To render
adipocytes insulin resistant, fully differentiated adipocytes were
treated with 3 ng/ml recombinant murine TNF-α for 3 days, with
daily replacement of medium.
Knockdown of LRP1 in adipocytes
Differentiated 3T3-L1 adipocytes were transfected
with 5 nM small interfering RNA (siRNA) targeting LRP1 (CGC TGA CCC
TAT TTG AAGA) or non-silencing control siRNA (TTC TCC GAA CGT GTC
ACGT) using HiPerFect transfection reagent (Qiagen GmbH, Hilden,
Germany) for up to 48 h at 37°C, according to the manufacturer's
instructions. The silencing effect of LRP1 siRNA transfection was
quantified by western blot analysis.
Pulse-chase experiment
For expression studies, apoA5 was labeled with
125I using Iodogen (Sigma-Aldrich; Merck KGaA) according
to the manufacturer's instructions. Differentiated 3T3-L1
adipocytes were pulsed with 125I-labeled apoA5
(~2×106 cpm/well) in serum-free medium for 2 h at 37°C.
At the end of the pulse, the medium was aspirated and cells were
washed three times with ice-cold PBS, and incubated with heparin
(10 mg/ml in PBS; Sigma-Aldrich; Merck KGaA) for 3 min at room
temperature, and then washed three times with PBS. Fresh medium was
added, and cells were chased for various time periods (0, 1, 2, 4,
6 and 24 h) at 37°C. At the end of the chase period, the medium was
collected and cells were treated with heparin as described above.
This heparin wash was added to the collected medium which was then
precipitated with 13% trichloroacetic acid to estimate degradation
of apoA5. Following heparin treatment, cells were collected in 0.1
M NaOH. Total cell protein was determined using the bicinchoninic
acid assay (Novagen; Merck KGaA). Radioactivity (cpm) measured by
gamma-ray counter was normalized to cell protein, and data are
expressed as total cpm/mg protein at each time-point.
Measurement of intracellular TG
content and glycerol release
Differentiated human adipocytes were incubated in
serum-free DMEM/F-12 in the presence of 200 ng/ml recombinant apoA5
for 48 h. The TG content of adipocytes was determined using a
triglyceride quantification kit (BioVision, Inc., Milpitas, CA,
USA) according to the manufacturer's instructions. For lipolysis
analysis, adipocytes were washed with PBS once to remove the phenol
red. Then 200 ng/ml apoA5 was added in phenol red free DMEM/F-12
for 24 h. In some experiments, after apoA5 treatment, adipocytes
were subsequently incubated with 10 µM isoproterenol for 3 h. At
the end of incubation, the medium was collected and glycerol
content was measured using a colorimetric assay at 412 nm
(BioVision, Inc.).
Oil Red O staining for lipid
droplets
Mature adipocytes grown on coverslips were washed
three times with cold PBS, fixed in 4% paraformaldehyde for 20 min
and permeabilized with 0.2% Triton X-100 in PBS for 10 min at room
temperature. Oil Red O (0.5% in isopropyl alcohol; Sigma-Aldrich;
Merck KGaA) was mixed with distilled water in the ratio of 60:40
and filtered through 0.45 µm filters. The freshly prepared Oil Red
O solution was added to permeabilized cells for 30 min. These cells
were then washed with PBS three times, incubated with 0.2 µg/ml of
4′,6-diamidino-2-phenylindole for 10 min at room temperature and
washed with PBS three times. Cells were mounted on slides with
ProLong Gold antifade reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and visualized under a confocal microscope
(model TCS SP5; Leica Microsystems, Inc., Buffalo Grove, IL, USA).
The size of Oil Red O-stained lipid droplets was calculated using
ImageJ software version 1.48s (National Institutes of Health,
Bethesda, MD, USA).
Western blot analysis
Total protein lysate from frozen tissues or cultured
cells was prepared using lysis buffer containing 50 mM Tris (pH
7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% sodium
deoxycholate and protease inhibitor (Invitrogen; Thermo Fisher
Scientific, Inc.) on ice for 0.5 h. The cell lysates were
centrifuged at 13,000 × g for 10 min at 4°C. The supernatants were
separated by 12% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. The membranes were blocked for 2 h with 5%
skim milk in TBS containing 0.1% Tween-20 at room temperature and
incubated overnight at 4°C with mouse anti-human apoA5 (1:1,000),
mouse anti-LRP1 (1:1,000), human anti-Cidec (1:1,000) or human
anti-perilipin (1:1,000). The blots were then incubated with a
corresponding horseradish peroxidase-conjugated secondary antibody
(goat anti-rabbit IgG, 1:5,000 or rabbit anti-mouse IgG, 1:5,000)
for 1 h at room temperature. The immunoblot signals were visualized
using an enhanced chemiluminescence substrate (Invitrogen; Thermo
Fisher Scientific, Inc.) and quantified by ImageJ software version
1.48s (National Institutes of Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from human differentiated adipocytes was
isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
First strand cDNA was synthesized using a cDNA synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. qPCR was performed on an ABI 7300
Real-Time PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.) with SYBR-Green mix (Takara Bio Inc., Otsu,
Japan) and specific primers to amplify the genes. An initial
denaturation step at 95°C for 30 min; 40 cycles at 95°C for 5 sec
and 60°C for 31 sec. Gene expression levels of the genes encoding
cidec (CIDEC), perilipin (PLIN1), diacylglycerol
acyltransferase (DGAT) 1, DGAT2, stearoyl-CoA
desaturase 1 (SCD1), HSL, adipose triglyceride lipase
(ATGL), nuclear respiratory factor 1 (NRF1), subunit
IV of cytochrome c oxidase (COXIV, officially known
as COX4I1), PPARγ coactivator 1α (PGC1α), uncoupling
protein 1 (UCP1) and forkhead box C2 (FOXC2), were
normalized to 18S rRNA, and presented as the fold change relative
to the control. The following primer sequences were used:
CIDEC sense, 5′-AAGTCCCTTAGCCTTCTCTACC-3′ and antisense,
5′-CCTTCCTCACGCTTCGATCC-3′; PLIN1 sense,
5′-GACCTCCCTGAGCAGGAGAAT-3′ and antisense,
5′-GTGGGCTTCCTTAGTGCTGG-3′; DGAT1 sense,
5′-AACCTCATCAAGTATGGCATCCT-3′ and antisense,
5′-CATTGGCCGCAATAACCAGG-3′; DGAT2 sense,
5′-AAGACCCTCATAGCCGCCTA-3′ and antisense,
5′-TTGGACCTATTGAGCCAGGTG-3′; SCD1 sense,
5′-TCTAGCTCCTATACCACCACCA-3′ and antisense,
5′-TGTCGTCTTCCAAGTAGAGGG-3′; HSL sense,
5′-TCAGTGTCTAGGTCAGACTGG-3′ and antisense,
5′-AGGCTTCTGTTGGGTATTGGA-3′; ATGL sense,
5′-ATGGTGGCATTTCAGACAACC-3′ and antisense,
5′-CGGACAGATGTCACTCTCGC-3′; NRF1 sense,
5′-AACAAAATTGGGCCACGTTACA-3′ and antisense,
5′-TCTGGACCAGGCCATTAGCA-3′; COXIV sense,
5′-CAGGGTATTTAGCCTAGTTGGC-3′ and antisense,
5′-GCCGATCCATATAAGCTGGGA-3′; PGC1α sense,
5′-TCTGAGTCTGTATGGAGTGACAT-3′ and antisense,
5′-CCAAGTCGTTCACATCTAGTTCA-3′; UCP1 sense,
5′-AGGTCCAAGGTGAATGCCC-3′ and antisense, 5′-GCGGTGATTGTTCCCAGGA-3′;
FOXC2 sense, 5′-CCTCCTGGTATCTCAACCACA-3′ and antisense,
5′-GAGGGTCGAGTTCTCAATCCC-3′; 18S rRNA sense,
5′-GCTTAATTTGACTCAACACGGGA-3′ and antisense,
5′-AGCTATCAATCTGTCAATCCTGTC-3′. Gene expression levels were
quantified using the 2−ΔΔCq method and normalized to the
internal reference gene 18S rRNA (32).
Statistical analysis
Statistical comparisons between 2 groups were
assessed by a two-tailed, unpaired Student's t-test. Comparisons
between ≥3 groups were analyzed by one-way analysis of variance
followed by a Newman-Keuls post hoc test. P<0.05 was
considered to indicate a statistically significant difference. Data
are expressed as the mean ± standard error of ≥3 independent
experiments.
Results
Decreased endocytosis of apoA5 by
adipocytes in obese and insulin resistant states
3T3-L1 fibroblasts were completely differentiated
into adipocytes after 8 days incubation with induction media. For
generating hypertrophied adipocytes, 3T3-L1 adipocytes were
cultured up to 21 days after the induction of differentiation. Oil
red O staining exhibited a gradual increase in lipid accumulation
from day 8 to day 21 (Fig. 1). At
4 h after the addition of apoA5, internalized apoA5 was quantified
by immunoblotting. During the course of adipocyte hypertrophy,
apoA5 internalization was significantly lower on day 21 (15%) in
hypertrophied cells than that on day 8 in non-hypertrophied control
cells (Fig. 2A). Using a
pulse-chase protocol in which cells were incubated with
[125I]-labeled apoA5 and chased for various time-points
up to 24 h after removal of the radiolabeled sample, internalized,
labeled apoA5 in hypertrophied adipocytes was significantly
decreased by 66% compared with control cells (Fig. 2B). The quantity of degraded apoA5
in the medium was increased in a time-dependent manner during the
chase period, reaching 63% of total apoA5 at the 24 h time-point
both in hypertrophied and non-hypertrophied adipocytes (data not
shown).
Similar results were observed in the case of the
insulin-resistant adipocytes. An in vitro model of metabolic
insulin resistance was established using TNF-α-treated 3T3-L1
adipocytes, as described previously (33). Western blot analysis revealed that
TNF-α treatment decreased apoA5 internalization by 52% (P<0.05;
Fig. 3A). Similar data was also
confirmed by a pulse-chase experiment (Fig. 3B).
To determine the impact of obesity on apoA5
expression in human adipose tissue, we obtained subcutaneous
abdominal adipose tissue biopsies from 6 patients with a wide range
of BMI. The apoA5 protein was detected in these human adipose
tissue samples. The amount of apoA5 was significantly reduced by
69% in the obese group as compared with the non-obese group
(Fig. 4).
Silencing of LRP1 expression inhibits
apoA5 expression by adipocytes
It was investigated whether knockdown of LRP1
expression in adipocytes prevents apoA5 internalization. After 48 h
transfection of differentiated 3T3-L1 adipocytes with LRP1 siRNA,
knockdown efficiency was 87% (P<0.05) as determined by western
blot analysis (Fig. 5A). The
reduction of LRP1 expression resulted in decreased apoA5 uptake by
adipocytes (~70%), as determined by western blot analysis and the
pulse-chase experiment (Fig. 5B and
C).
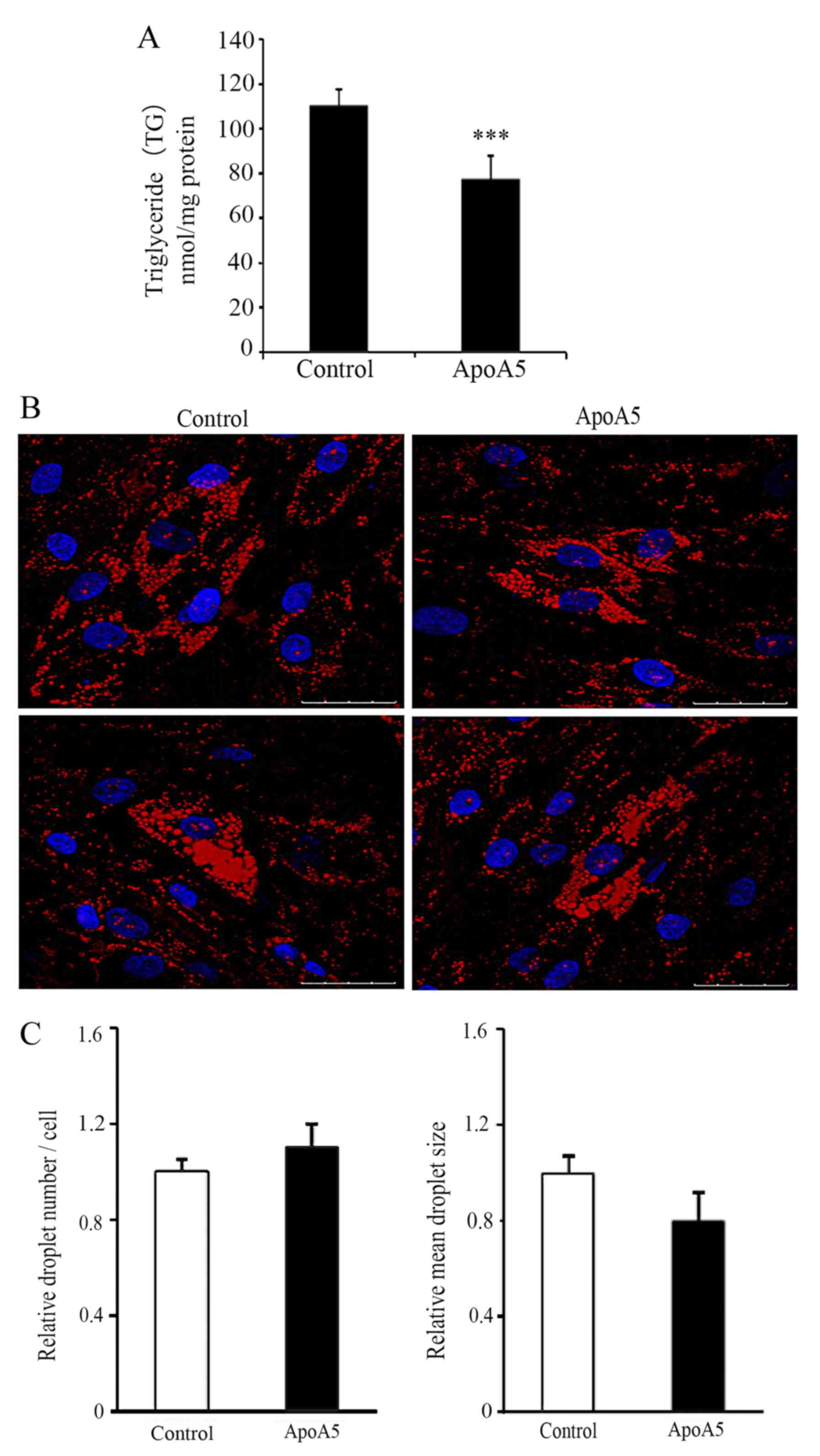
ApoA5 significantly decreases TG
accumulation in adipocytes but does not affect lipid droplet
formation
Our previous study revealed that treatment with 200
and 600 ng/ml apoA5 for 48 h significantly decreased intracellular
TG levels in human adipocytes (12). As 200 ng/ml is within the normal
range of human plasma levels of apoA5 (5.4–455.6 ng/ml) (6), this concentration was used in the
present study. ApoA5 treatment for 48 h led to a significant
decrease in TG content in adipocytes, which was consistent with our
previous findings (6) (P<0.001;
Fig. 6A). Next, the effect of
apoA5 on the morphological changes of lipid droplets was
investigated. Oil Red O staining and confocal microscopy revealed
that apoA5 treatment did not affect lipid droplet formation in
adipocytes; however, the lipid droplets in apoA5-treated cells were
generally smaller than those in control cells (Fig. 6B and C).
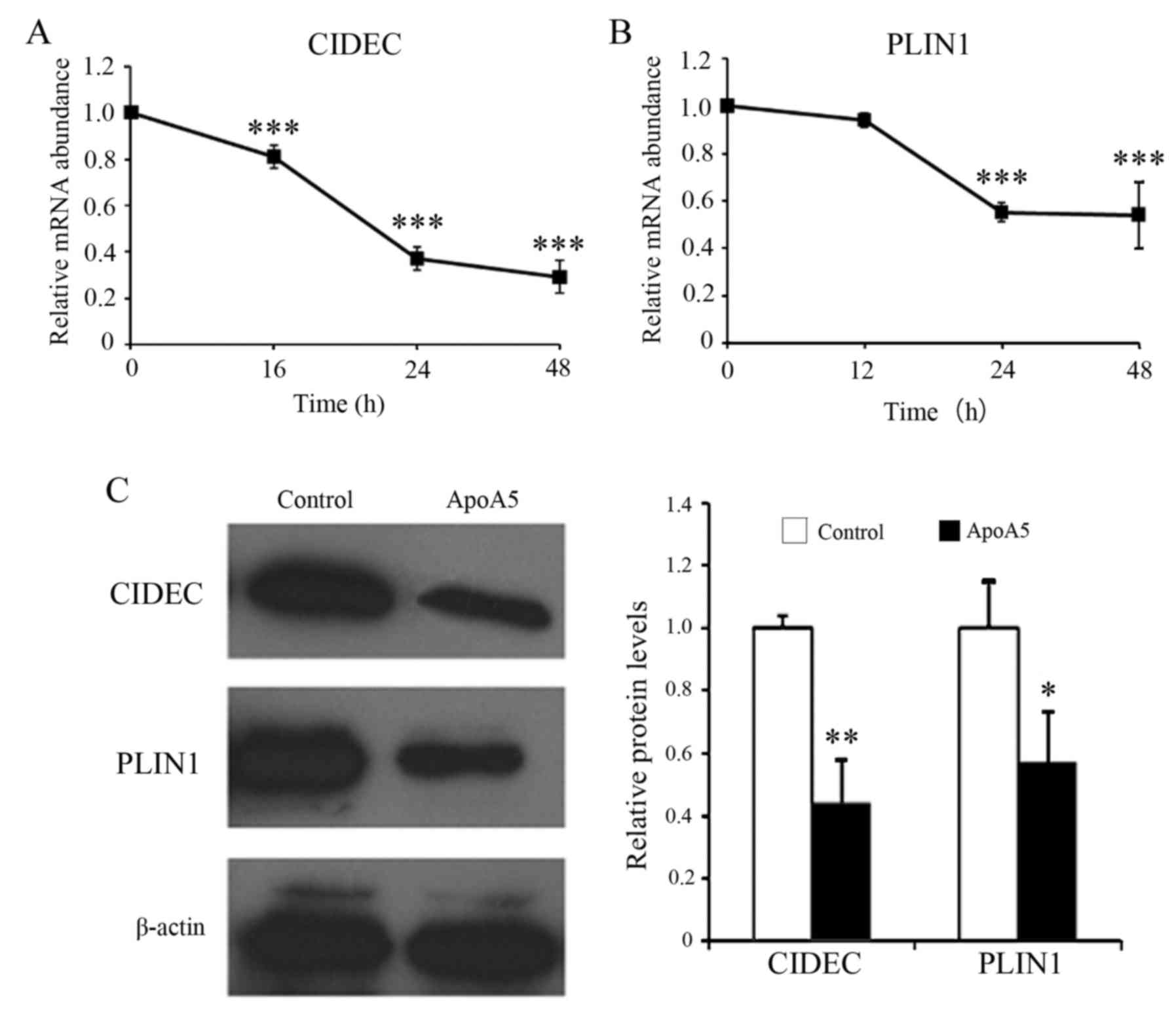
ApoA5 markedly decreases the
expression of the genes encoding cidec and perilipin in
adipocytes
RT-qPCR analysis revealed that apoA5 decreased
CIDEC mRNA expression levels in a time-dependent manner with
maximal effect at 48 h (Fig. 7A).
Treatment with apoA5 for 48 h decreased CIDEC mRNA
expression levels by 71%. Similarly, apoA5 significantly decreased
PLIN1 mRNA expression levels by 45%, with maximal effect at
24 h (Fig. 7B). Western blot
analysis further confirmed that apoA5 reduced the protein
expression levels of cidec and perilipin in human adipocytes
(Fig. 7C).
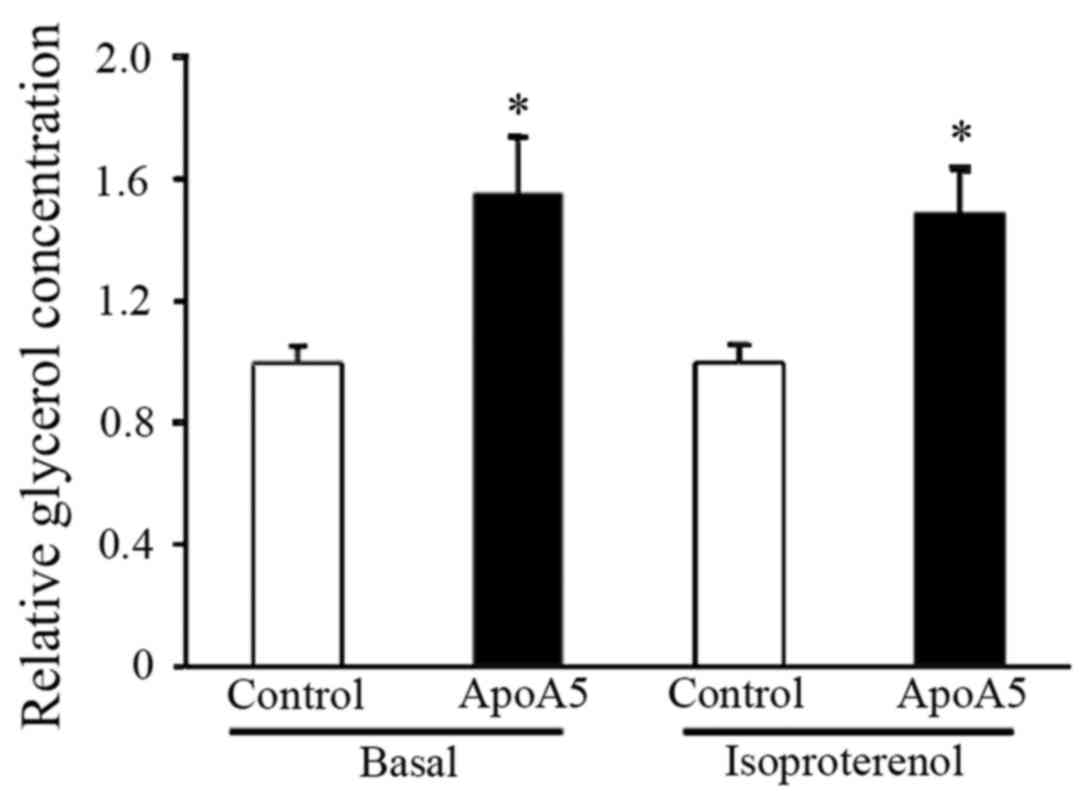
ApoA5 treatment results in enhanced
lipolysis and upregulated expression of the brown fat-specific gene
UCP1
To further address the potential regulation of
TG-associated metabolic processes by apoA5, lipolysis in adipocytes
was measured following apoA5 treatment. Measurement of glycerol
released into the medium during culture of cells under basal
conditions for 24 h revealed that lipolysis was significantly
increased in apoA5-treated adipocytes. Examination of lipolysis in
response to β-adrenergic stimulation revealed that treatment with
apoA5 also enhanced isoproterenol-induced lipolysis in human
adipocytes (Fig. 8).
Next, the effects of apoA5 on expression of genes
associated with lipid synthesis and hydrolysis in adipocytes was
investigated. RT-qPCR analysis was performed 48 h after
intervention of apoA5 and revealed that the mRNA expression levels
of DGAT1, DGAT2 and SCD1, all of which
participate in lipid synthesis, did not significantly differ
between apoA5-treated and control adipocytes (Fig. 9A). In addition, mRNA expression
levels of HSL and ATGL, both of which contribute to
TG hydrolysis in adipocytes, were not significantly different
between the apoA5-treated and control cells (Fig. 9B). In addition, the expression of
genes associated with mitochondrial biogenesis or oxidative
phosphorylation were measured. Treatment of apoA5 for 48 h did not
affect the mRNA expression levels of NRF1, COXIV and
PGC1α in adipocytes (Fig.
9C). Finally, the mRNA expression levels of brown fat-specific
gene UCP1 were measured, which serves a role in uncoupling
oxidative phosphorylation and converts this proton gradient energy
into heat to maintain normal body temperature (34). UCP1 mRNA expression levels
were significantly increased (~1.87-fold; P<0.05) by apoA5
treatment for 48 h (Fig. 9D). The
mRNA expression levels of FOXC2, which is an important
regulator of UCP1 (35),
were also significantly elevated (P<0.05; Fig. D). These results suggested that the
decrease in TG accumulation in human adipocytes after apoA5
treatment may be accompanied by increased lipolysis, uncoupling or
thermogenesis, but not by increased mitochondrial biogenesis or
oxidative phosphorylation.
 | Figure 9.Effects of apoA5 treatment on the
expression of lipid synthesis-associated genes in human adipocytes.
mRNA expression levels of (A) lipolysis-associated genes, (B) TG
hydrolysis-associated genes, (C) mitochondrial biogenesis or
oxidative phosphorylation-associated genes and (D) BAT-specific
genes in human adipocytes, 48 h after 200 ng/ml apoA5 treatment.
Data were normalized to 18S rRNA mRNA expression levels, and are
expressed as the mean ± standard error of 5 independent
experiments. *P<0.05 vs. control. ApoA5, apolipoprotein A5;
DGAT, diacylglycerol acyltransferase; SCD1, stearoyl-CoA desaturase
1; TG, triglyceride; HSL, hormone-sensitive lipase; ATGL, adipose
triglyceride lipase; NRF1, nuclear respiratory factor 1; COXIV,
subunit IV of cytochrome c oxidase; PGC1a, PPARγ coactivator
1α; UCP1, uncoupling protein 1; FOXC2, forkhead box C2; BAT, brown
adipose tissue. |
Discussion
The present study revealed that apoA5 protein was
detected in human subcutaneous abdominal adipose tissues ex
vivo, indicating that circulating apoA5 may be internalized by
adipocytes in vivo. This finding concurs with our previous
results in vitro (12). It
was demonstrated that apoA5 expression was significantly decreased
in both adipose tissue from obese patients and hypertrophied or
insulin resistant 3T3-L1 adipocytes. The underlying mechanism for
this phenomenon is unknown but it is possibly due to reduced uptake
of apoA5, as the degraded rates of the apoA5 protein was similar in
adipocytes under different conditions, as revealed by the
pulse-chase experiment. The present study demonstrated that
knockdown of LRP1 by siRNA transfection in adipocytes resulted in
decreased apoA5 expression, suggesting that LRP1 serves a role in
apoA5 uptake by adipocytes. As LRP1 has been demonstrated to be
upregulated in obese mouse adipocytes and obese human adipose
tissues (36), the decreased
uptake of apoA5 is possibly due to reduced endocytic activity of
LRP1. Hypertrophied adipocytes usually exhibit modified metabolic
properties and develop insulin resistance (37). Under physiological conditions,
insulin mediates recycling of LRP1 from an endosomal pool to the
plasma membrane in differentiated adipocytes, resulting in an
increase in the cell surface presentation of LRP1 and a concomitant
increased endocytic activity (38,39).
Taken together, it may be hypothesized that, during insulin
resistance, the movement of LRP1 from intracellular structures to
the cell surface is attenuated, leading to decreased apoA5
endocytosis by adipocytes.
It was previously demonstrated that internalized
apoA5 by human adipocytes was associated with lipid droplets and
decreased cellular TG content (12). In the present study, the underlying
mechanism of adipocytes regulation and TG storage was investigated.
The results revealed that treatment with recombinant apoA5
significantly decreased the expression of the genes encoding cidec
and perilipin, both of which localize to the surface of lipid
droplets and serve roles in the negative regulation of lipolysis
and promotion of TG accumulation in adipocytes. ApoA5-treated
adipocytes exhibited increased lipolysis activity. This data
concurred with observations of Nishino et al (22), who revealed that depletion of cidec
or perilipin by siRNA transfection in cultured adipocytes enhanced
basal and isoproterenol-induced lipolysis, whereas depletion of
cidec and perilipin increased basal and stimulated lipolysis in an
approximately additive manner. These results suggested that the
reduced expression of cidec and perilipin induced by apoA5 may
contribute individually to enhanced lipolysis activity. However,
isolated adipocytes of perilipin null mice have been demonstrated
to exhibit attenuated stimulated lipolytic activity and elevated
basal lipolysis (18,19). This discrepancy may be due to
incomplete depletion of perilipin in the present study and the
study by Nishino et al (22), as the remaining perilipin may still
facilitate lipase action in response to catecholamine
stimulation.
In addition to alterations in lipid accumulation and
enhanced lipolysis, cidec/Fsp27 deficient adipocytes acquire a
BAT-like property, including the formation of multiple small lipid
droplets, an elevated rate of lipid oxidation, upregulated
mitochondrial activity and expression of BAT-specific gene UCP1
(22,23), which is not observed in perilipin
depleted adipocytes. FOXC2, a crucial regulator of the cyclin
adenosine monophosphate pathway that promotes BAT differentiation,
has also been demonstrated to increase in the WAT of Fsp27-knockout
mice (23). Additionally, Sawada
et al (40) revealed that
perilipin overexpression causes a significant reduction in Fsp27
(82% reduction) which is associated with decreased adipocyte size,
upregulation of UCP1 and genes involved in fatty acid oxidation and
a decrease in lipogenic genes, suggesting that attenuation of Fsp27
is crucial in mediating these metabolic processes. Consistent with
these previous observations, the present study revealed that the
downregulation of cidec by apoA5 treatment was accompanied by
increased gene expression levels of UCP1 and FOXC2 in
human adipocytes, suggesting that apoA5 may upregulate these genes
by decreasing cidec expression. Enhanced FOXC2 expression
may contribute to the increased expression of UCP1. However,
alterations in expression of genes associated with fatty acid
oxidation, mitochondrial biosynthesis and lipid droplet formation,
including DGAT1, DGAT2, SCD1, HSL,
ATGL, NRF1, COXIV and PGC1α, was not
observed. As the present study only observed a 71% reduction of
cidec induced by apoA5, it is possible that this may be due to
incomplete depletion of cidec, which requires clarification with
future research.
UCP1 is an inner mitochondrial membrane protein that
may dissipate mitochondrial membrane potential, partially uncouple
substrate oxidation and oxidative phosphorylation and promote the
dissipation of cellular biochemical energy as heat to maintain body
temperature (41). In particular,
UCP1 is enriched in BAT and is minimally present in WAT (42). Strategies aimed at increasing UCP1
expression in adipose cells are of great interest in view of
evidence that ectopic expression of UCP1 is significantly
associated with a reduction in TG content in adipocytes or adipose
tissue (43–45), and that a reduced brown
adipose-like phenotype in WAT depots may contribute to obesity and
type 2 diabetes in humans (46,47).
Therefore, alteration of energy balance via an increased
utilization of fat in WAT may be a promising target for the
treatment of obesity and metabolic syndrome. In the present study,
in the presence of apoA5, the mRNA expression levels of UCP1
were significantly increased in differentiated human adipocytes.
Although ~2-fold increase in UCP1 induced by apoA5 is
relatively mild, this small alteration in UCP1 expression may
contribute to efficient energy dissipation and decreased TG
accumulation. Toh et al (23) demonstrated that depletion of Fsp27
in differentiated mouse embryonic fibroblasts causes increased
expression levels of UCP1 by ~2-fold, and is accompanied by reduced
intracellular TG content. Consistent with this in vitro
data, a clinical study by Oberkofler et al (46) also revealed that lean subjects have
~2-fold increased UCP1 mRNA expression levels in the
intraperitoneal adipose tissue compared with that of obese groups.
The present study revealed that apoA5 may behave as a regulator to
promote UCP1 gene expression in human adipocytes, suggesting
that apoA5 may exert its protection against obesity by inducing
white adipocytes to acquire certain characteristics of brown
adipocytes and increasing energy consumption.
In conclusion, the present study suggested that
apoA5 may be internalized by adipocytes both in vivo and in
vitro, primarily via binding to LRP1. The uptake of apoA5 was
attenuated in human obese adipose tissues and in cultured
adipocytes with hypertrophy or insulin resistance. In addition,
decreased TG accumulation in human adipocytes induced by apoA5
intervention may be associated with enhanced lipolysis and energy
expenditure, which may result from reduced expression of cidec and
perilipin. These findings may provide a greater understanding of
the roles of apoA5 in regulating the intracellular TG metabolism of
adipocytes. Under hypertrophied and insulin resistant conditions,
attenuated endocytosis of apoA5 by adipocytes may lead to excessive
augmentation of TG storage and abnormal metabolism of adipocytes,
which promotes the development of obesity. As a novel regulator of
lipid storage in adipocytes, apoA5 may serve an important role in
whole body energy homeostasis and may be a potential therapeutic
target for the treatment of obesity and obesity-associated
disorders.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81170262 and
81300205) and Specialized Research Fund for the Doctoral Program of
Higher Education (grant no. 20130162120057).
References
|
1
|
Wong K and Ryan RO: Characterization of
apolipoprotein A-V structure and mode of plasma triacylglycerol
regulation. Curr Opin Lipidol. 18:319–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horvatovich K, Bokor S, Baráth A, Maász A,
Kisfali P, Járomi L, Polgár N, Tóth D, Répásy J, Endreffy E, et al:
Haplotype analysis of the apolipoprotein A5 gene in obese pediatric
patients. Int J Pediatr Obes. 6:e318–e325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niculescu LS, Fruchart-Najib J, Fruchart
JC and Sima A: Apolipoprotein A-V gene polymorphisms in subjects
with metabolic syndrome. Clin Chem Lab Med. 45:1133–1139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng XY, Zhao SP and Yan H: The role of
apolipoprotein A5 in obesity and the metabolic syndrome. Biol Rev
Camb Philos Soc. 88:490–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang XS, Zhao SP, Hu M, Bai L, Zhang Q
and Zhao W: Decreased apolipoprotein A5 is implicated in insulin
resistance-related hypertriglyceridemia in obesity.
Atherosclerosis. 210:563–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao SP, Hu S, Li J, Hu M, Liu Q, Wu LJ
and Zhang T: Association of human serum apolipoprotein A5 with
lipid profiles affected by gender. Clin Chim Acta. 376:68–71. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corella D, Lai CQ, Demissie S, Cupples LA,
Manning AK, Tucker KL and Ordovas JM: APOA5 gene variation
modulates the effects of dietary fat intake on body mass index and
obesity risk in the Framingham Heart Study. J Mol Med (Berl).
85:119–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin S, Nicaud V, Humphries SE and
Talmud PJ; EARS group, : Contribution of APOA5 gene variants to
plasma triglyceride determination and to the response to both fat
and glucose tolerance challenges. Biochim Biophys Acta.
1637:217–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu X, Chan J, Ryan RO and Forte TM:
Apolipoprotein A-V association with intracellular lipid droplets. J
Lipid Res. 48:1445–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shu X, Nelbach L, Ryan RO and Forte TM:
Apolipoprotein A-V associates with intrahepatic lipid droplets and
influences triglyceride accumulation. Biochim Biophys Acta.
1801:605–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ress C, Moschen AR, Sausgruber N, Tschoner
A, Graziadei I, Weiss H, Schgoer W, Ebenbichler CF, Konrad RJ,
Patsch JR, et al: The role of apolipoprotein A5 in non-alcoholic
fatty liver disease. Gut. 60:985–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng XY, Zhao SP, Yu BL, Wu CL and Liu L:
Apolipoprotein A5 internalized by human adipocytes modulates
cellular triglyceride content. Biol Chem. 393:161–167. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nilsson SK, Christensen S, Raarup MK, Ryan
RO, Nielsen MS and Olivecrona G: Endocytosis of apolipoprotein A-V
by members of the low density lipoprotein receptor and the VPS10p
domain receptor families. J Biol Chem. 283:25920–25927. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farese RV Jr and Walther TC: Lipid
droplets finally get a little R-E-S-P-E-C-T. Cell. 139:855–860.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolins NE, Brasaemle DL and Bickel PE: A
proposed model of fat packaging by exchangeable lipid droplet
proteins. FEBS Lett. 580:5484–5491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brasaemle DL: Thematic review series:
Adipocyte biology. The perilipin family of structural lipid droplet
proteins: Stabilization of lipid droplets and control of lipolysis.
J Lipid Res. 48:2547–2559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Souza SC, Muliro KV, Liscum L, Lien P,
Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M and
Greenberg AS: Modulation of hormone-sensitive lipase and protein
kinase A-mediated lipolysis by perilipin A in an adenoviral
reconstituted system. J Biol Chem. 277:8267–8272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tansey JT, Sztalryd C, Gruia-Gray J, Roush
DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR and
Londos C: Perilipin ablation results in a lean mouse with aberrant
adipocyte lipolysis, enhanced leptin production, and resistance to
diet-induced obesity. Proc Natl Acad Sci USA. 98:6494–6499. 2001;
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinez-Botas J, Anderson JB, Tessier D,
Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH and Chan L:
Absence of perilipin results in leanness and reverses obesity in
Lepr(db/db) mice. Nat Genet. 26:474–479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keller P, Petrie JT, De Rose P, Gerin I,
Wright WS, Chiang SH, Nielsen AR, Fischer CP, Pedersen BK and
MacDougald OA: Fat-specific protein 27 regulates storage of
triacylglycerol. J Biol Chem. 283:14355–14365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Puri V, Konda S, Ranjit S, Aouadi M,
Chawla A, Chouinard M, Chakladar A and Czech MP: Fat-specific
protein 27, a novel lipid droplet protein that enhances
triglyceride storage. J Biol Chem. 282:34213–34218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishino N, Tamori Y, Tateya S, Kawaguchi
T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S,
Matsuki Y, et al: FSP27 contributes to efficient energy storage in
murine white adipocytes by promoting the formation of unilocular
lipid droplets. J Clin Invest. 118:2808–2821. 2008.PubMed/NCBI
|
|
23
|
Toh SY, Gong J, Du G, Li JZ, Yang S, Ye J,
Yao H, Zhang Y, Xue B, Li Q, et al: Up-regulation of mitochondrial
activity and acquirement of brown adipose tissue-like property in
the white adipose tissue of fsp27 deficient mice. PLoS One.
3:e28902008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsusue K: A physiological role for fat
specific protein 27/cell death-inducing DFF45-like effector C in
adipose and liver. Biol Pharm Bull. 33:346–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inohara N, Koseki T, Chen S, Wu X and
Núñez G: CIDE, a novel family of cell death activators with
homology to the 45 kDa subunit of the DNA fragmentation factor.
EMBO J. 17:2526–2533. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Magnusson B, Gummesson A, Glad CA,
Goedecke JH, Jernås M, Lystig TC, Carlsson B, Fagerberg B, Carlsson
LM and Svensson PA: Cell death-inducing DFF45-like effector C is
reduced by caloric restriction and regulates adipocyte lipid
metabolism. Metabolism. 57:1307–1313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beckstead JA, Oda MN, Martin DD, Forte TM,
Bielicki JK, Berger T, Luty R, Kay CM and Ryan RO:
Structure-function studies of human apolipoprotein A-V: A regulator
of plasma lipid homeostasis. Biochemistry. 42:9416–9423. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gauthier B, Robb M and McPherson R:
Cholesteryl ester transfer protein gene expression during
differentiation of human preadipocytes to adipocytes in primary
culture. Atherosclerosis. 142:301–307. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prawitt J, Niemeier A, Kassem M, Beisiegel
U and Heeren J: Characterization of lipid metabolism in
insulin-sensitive adipocytes differentiated from immortalized human
mesenchymal stem cells. Exp Cell Res. 314:814–824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suganami T, Nishida J and Ogawa Y: A
paracrine loop between adipocytes and macrophages aggravates
inflammatory changes: Role of free fatty acids and tumor necrosis
factor alpha. Arterioscler Thromb Vasc Biol. 25:2062–2068. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suganami T, Tanimoto-Koyama K, Nishida J,
Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S,
et al: Role of the Toll-like receptor 4/NF-kappaB pathway in
saturated fatty acid-induced inflammatory changes in the
interaction between adipocytes and macrophages. Arterioscler Thromb
Vasc Biol. 27:84–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samad F, Pandey M, Bell PA and Loskutoff
DJ: Insulin continues to induce plasminogen activator inhibitor 1
gene expression in insulin-resistant mice and adipocytes. Mol Med.
6:680–692. 2000.PubMed/NCBI
|
|
34
|
Rousset S, Alves-Guerra MC, Mozo J, Miroux
B, Cassard-Doulcier AM, Bouillaud F and Ricquier D: The biology of
mitochondrial uncoupling proteins. Diabetes. 53 Suppl 1:S130–S135.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cederberg A, Grønning LM, Ahrén B, Taskén
K, Carlsson P and Enerbäck S: FOXC2 is a winged helix gene that
counteracts obesity, hypertriglyceridemia, and diet-induced insulin
resistance. Cell. 106:563–573. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Masson O, Chavey C, Dray C, Meulle A,
Daviaud D, Quilliot D, Muller C, Valet P and Liaudet-Coopman E:
LRP1 receptor controls adipogenesis and is up-regulated in human
and mouse obese adipose tissue. PLoS One. 4:e74222009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Ferranti S and Mozaffarian D: The
perfect storm: Obesity, adipocyte dysfunction, and metabolic
consequences. Clin Chem. 54:945–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ko KW, Avramoglu RK, McLeod RS, Vukmirica
J and Yao Z: The insulin-stimulated cell surface presentation of
low density lipoprotein receptor-related protein in 3T3-L1
adipocytes is sensitive to phosphatidylinositide 3-kinase
inhibition. Biochemistry. 40:752–759. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Corvera S, Graver DF and Smith RM: Insulin
increases the cell surface concentration of alpha 2-macroglobulin
receptors in 3T3-L1 adipocytes. Altered transit of the receptor
among intracellular endocytic compartments. J Biol Chem.
264:10133–10138. 1989.PubMed/NCBI
|
|
40
|
Sawada T, Miyoshi H, Shimada K, Suzuki A,
Okamatsu-Ogura Y, Perfield JW II, Kondo T, Nagai S, Shimizu C,
Yoshioka N, et al: Perilipin overexpression in white adipose tissue
induces a brown fat-like phenotype. PLoS One. 5:e140062010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Busiello RA, Savarese S and Lombardi A:
Mitochondrial uncoupling proteins and energy metabolism. Front
Physiol. 6:362015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nicholls DG and Locke RM: Thermogenic
mechanisms in brown fat. Physiol Rev. 64:1–64. 1984.PubMed/NCBI
|
|
43
|
Kopecky J, Clarke G, Enerbäck S,
Spiegelman B and Kozak LP: Expression of the mitochondrial
uncoupling protein gene from the aP2 gene promoter prevents genetic
obesity. J Clin Invest. 96:2914–2923. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tiraby C, Tavernier G, Lefort C, Larrouy
D, Bouillaud F, Ricquier D and Langin D: Acquirement of brown fat
cell features by human white adipocytes. J Biol Chem.
278:33370–33376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Si Y, Palani S, Jayaraman A and Lee K:
Effects of forced uncoupling protein 1 expression in 3T3-L1 cells
on mitochondrial function and lipid metabolism. J Lipid Res.
48:826–836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oberkofler H, Dallinger G, Liu YM, Hell E,
Krempler F and Patsch W: Uncoupling protein gene: Quantification of
expression levels in adipose tissues of obese and non-obese humans.
J Lipid Res. 38:2125–2133. 1997.PubMed/NCBI
|
|
47
|
Yang X, Enerbäck S and Smith U: Reduced
expression of FOXC2 and brown adipogenic genes in human subjects
with insulin resistance. Obes Res. 11:1182–1191. 2003. View Article : Google Scholar : PubMed/NCBI
|