Introduction
Dendritic cells (DCs) are bone marrow-derived
professional antigen-presenting cells, which are critical in the
regulation of adaptive immune responses. Immature DCs usually
reside in peripheral tissues, expressing low levels of
co-stimulatory molecules (CD80, CD86 and CD40), which disable them
from activating naïve T cells. Immature DCs begin maturation
following uptake of antigens and exposure to microbial agents or
inflammatory mediators in peripheral tissues (1,2). The
common characteristics of DC maturation include the upregulation of
co-stimulatory molecules and major histocompatibility complex (MHC)
class II on the cell surface, reduced capacity for antigen uptake,
production of various inflammatory cytokines, and trafficking to
secondary lymphoid organs through afferent lymphatic vessels.
Subsequently, DCs present antigenic peptides and stimulate naïve
antigen-specific T cells in lymphoid organs (3). Overall, the DCs are a feasible
therapeutic target for the pharmacological modulation of immune
responses, and the inhibition of DC maturation represents a
strategy for modulating immune responses (4).
Natural compounds derived from marine organisms
serve as potentially valuable sources for the identification of
immunomodulatory drugs for application in the biotechnology and
pharmaceutical industries. Cembrane diterpenoids and their cyclized
derivatives are the most abundant metabolites of soft corals and
vary substantially in structural complexity (5–7).
These cembranes have a defensive function in resisting natural
predators, including other corals and fish, and settlement by a
variety of microorganisms (8,9). In
addition, cembranes have been shown to possess different
pharmacological activities in anti-inflammatory (10,11)
and antitumor effects (12,13).
Sinulariolide, a cembrane-type diterpenoids, is an
active compound isolated from the cultured soft coral Sinularia
flexibilis. This compound has been reported to exhibit
biological activities, which include antimicrobial (14) and anticancer activities (15–18).
However, its effect on normal immune function remains to be fully
elucidated. In the present study, whether sinulariolide can affect
the maturation and functional properties of murine bone
marrow-derived dendritic cells was investigated, and their
underlying signaling pathways were examined.
Materials and methods
Mice and preparation of bone
marrow-derived murine DCs
Female C57BL/6 mice (n=20; 6–8-weeks-old; 20–25 g)
were purchased from the National Laboratory Animal Center (Taipei,
Taiwan). The mice were housed under a controlled temperature
(22±2°C) and humidity (45–65%) with a 12-h light/dark cycle, and
free access food and water. Procedures governing the use and care
of animals were performed according to the Institutional Animal
Care and Use Committee guidelines and approved by the Bioethics
Committee of National Chung-Hsing University (Taichung, Taiwan).
The murine bone marrow-derived DCs were prepared as previously
described (19).
Chemicals
Sinulariolide was isolated from the cultured soft
coral Sinularia flexibilis according to previously reported
procedures (20) and was provided
by Dr Jui-Hsin Su (National Museum of Marine Biology and Aquarium,
Pingtung, Taiwan). The stock solution was prepared at a
concentration of 50 mg/ml in dimethyl sulfoxide (DMSO; Sigma
Aldrich; Merck Millipore, Darmstadt, Germany). The working solution
was freshly prepared by diluting with medium to desired
concentrations.
Cell viability and apoptosis
assay
The DCs (1×106) were prepared by treating
with different concentrations of sinulariolide with or without 100
ng/ml LPS at 37°C for 24 h. Cytotoxicity was examined using a Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol. The
absorbance was recorded on a microplate reader (Tecan Group Ltd.,
Männedorf, Switzerland) at a wavelength of 450 nm. For the analysis
of apoptosis, the cells were stained with an Annexin V kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol, and determined on an
Accuri 5 flow cytometer (BD Biosciences, San
Jose, CA, USA). The mean fluorescence intensity was calculated
using C6 Accuri system software (BD
Biosciences).
Flow cytometric analysis of surface
molecules
The DCs (1×106) were treated with DMSO
(0.1%) or sinulariolide for 1 h at 37°C, followed by LPS (100
ng/ml) stimulation at 37°C for 24 h. The expression of
co-stimulatory markers CD40, CD80, and CD86 were detected on an
Accuri 5 flow cytometer. Immunofluorescence staining
for flow analysis was performed using mouse IgG anti-mouse CD11
mAb-FITC conjugated (1:100 dilution; cat. no. 553,801; BD
Biosciences), mouse IgG anti-mouse CD80 mAb-phycoerythrin (PE)
conjugated (1:100 dilution; cat. no. 104,708; BioLegend, Inc., San
Diego, CA, USA), mouse IgG anti-mouse CD86 mAb-PE conjugated (1:100
dilution; cat. no. 105,008; BioLegend, Inc.), mouse IgG anti-mouse
CD40 mAb-PE conjugated (1:100 dilution; cat. no. 12-0401-82;
eBioscience; Thermo Fisher Scientific, Inc.) for 1 h at 4°C. The
mean fluorescence intensity was calculated using C6 Accuri™
system software (BD Biosciences).
Cytokine and nitric oxide (NO)
assay
Centrifugation was performed at 1,000 × g for 15 min
at 4°C, then the levels of tumor necrosis factor (TNF)-α,
interleukin (IL)-6 and IL-12p70 in the culture supernatants were
determined using murine ELISA kits for TNF-α (cat. no. 900-K54),
IL-6 (cat. no. 900-K50), and IL-12p70 (cat. no. 900-K97; PeproTech,
Inc., London, UK) according to the manufacturer's protocol. The
production of NO was assayed indirectly by measuring the levels of
nitrite (NO2−) in the culture supernatants
using a colorimeter assay based on the Griess reaction.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA isolation was performed using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (2 µg; 10
µl) was reverse transcribed using M-MLV reverse transcriptase
(Promega Corporation, Madison, WI, USA) to cDNA in a 20 µl final
reaction volume containing M-MLV 5X Reaction Buffer (5 µl), 10
mM dNTP (1 µl), 500 µg/ml oligo dT15 primers (1
µl) and nuclease-free water (3 µl; all Promega Corporation), which
were incubated at 42°C for 15 min. A total of 100 pg of cDNA was
used to initiate qPCR, which was performed using the SYBR-Green PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) in
the ABI 7500 Fast Real-Time system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions were as
follows: 95°C for 5 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. The primer pairs used were as follows:
Inducible nitric oxide synthase (iNOS), forward
5′-ACATCGACCCGTCCACAGTAT-3′ and reverse 5′-CAGAGGGGTAGGCTTGTCTC-3′;
GAPDH, forward 5′-CGTGTTCCTACCCCCAATGT-3′ and reverse
5′-TGTCATCATACTTGGCAGGTTTCT-3′. The 2−ΔΔCq method
(21) was used to normalize
transcription to GAPDH and calculate the fold induction relative to
controls, which were without sinulariolide treatment.
DC-induced mixed lymphocyte
reaction
The process of determining allogeneic mixed
lymphocytes was performed as described previously (22). Briefly, enriched CD4+ T
cells were negatively purified from the spleen of C57BL/6 mice
using a CD4+ T-cell Isolation kit (Miltenyi Biotech,
Bisley, UK). The DCs were treated with DMSO (0.1%) or sinulariolide
(10 µM) in the presence or absence of LPS (100 ng/ml) for 18 h,
which was added in graded doses to 2.5×105 allogeneic T
cells in round-bottom 96-well plates. The plates were incubated at
37°C for 48 h and T cell proliferation was determined using a CCK-8
assay.
Western blot analysis
The DCs were seeded at a density of 2×106
cells per six-well plate and pretreated with DMSO (0.1%) or
sinulariolide for 1 h, followed by stimulation with LPS (100 ng/ml)
for the indicated durations. Western blot analysis was performed as
described previously (22). In
brief, the total cell lysates were extracted in RIPA lysis and
extraction buffer (Thermo Fisher Scientific, Inc.). The protein
concentrations were measured using a Bio-Rad protein assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA USA), following which 20
µg of protein was subjected to 10% SDS-PAGE and transferred
onto nitrocellulose membranes. The membranes were incubated with
antibodies against phosphorylated (p-)extracellular
signal-regulated kinase (ERK; 1:1,000 dilution; cat. no. 4370), ERK
(1:1,000 dilution; cat. no. 3192;), p-p38 (1:1,000 dilution; cat.
no. 4631), p38 (1:1,000 dilution; cat. no. 8690), p-AKT (1:1,000
dilution; cat. no. 4060), AKT (1:1,000 dilution; cat. no. 4685) and
inhibitor of NF-κB IκBα (1:1,000 dilution; cat. no. 4812; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The
membranes were then incubated with horseradish peroxidase-labeled
secondary antibody (1:2,000 dilution; cat. no. 111-035-003; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) overnight
at 4°C. The protein-antibody complexes were detected by enhanced
chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK),
performed using a Hansor Luminescence Image system (Hansor,
Taichung, Taiwan). The blots were quantified by densitometric
analysis using ImageJ software version 1.47 (National
Institutes of Health, Bethesda, MD USA).
Preparation of nuclear extracts and
NF-κB activity assay
Nuclear extracts were prepared using NE-PER Nuclear
and Cytoplasmic Extraction reagents (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
samples were stored at −80°C for the analysis of NF-κB activity.
The activity of NF-κB was measured in the nuclear protein extracts
(15 µg) using the TransAM™ NF-κB p65 ELISA-based assay kit (Active
Motif, Carlsbad, CA, USA), which is an ELISA-based method designed
to detect NF-κB p65 subunit activation. The assay was performed
according to the manufacturer's protocol and analyzed using a
microplate reader at 450 nm, with a reference wavelength of 655 nm
(Tecan Group Ltd.).
Statistical analysis
Data are expressed as the mean ± standard deviation
of the indicated number of experiments. The statistical
significance of differences between groups were examined using one
way analysis of variables followed by Tukey's test or
Student's t-test (GraphPad version 5 for Windows; GraphPad
Software, Inc., San Diego, CA USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
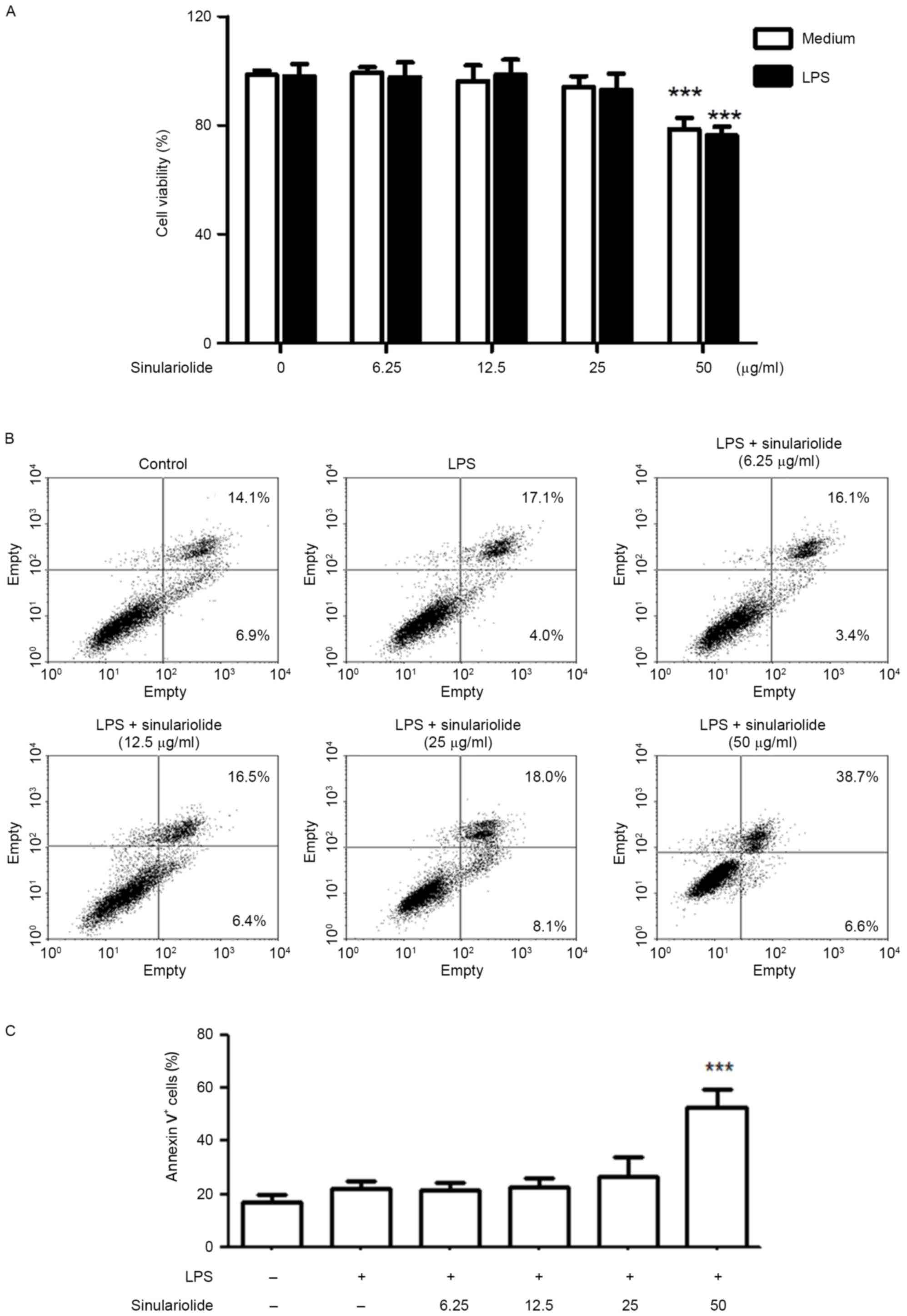
Sinulariolide has no significant
cytotoxic or apoptotic effects on DCs
The present study first evaluated the cytotoxicity
of sinulariolide on DCs by treating DCs with different
concentrations of sinulariolide in the presence or absence of LPS
(100 ng/ml) for 24 h. No significant toxic effects were observed
following treatment with sinulariolide in the concentration range
of 6.25–25 µg/ml, however, 50 µg/ml sinulariolide significantly
decreased cell viability (Fig.
1A). The expression of Annexin V and CD11c+ were
then analyzed, in which sinulariolide (6.25–25 µg/ml) exerted
minimal or no apoptotic effects on DCs (Fig. 1B and C). Therefore, sinulariolide
concentrations <25 µg/ml were used in the following
experiments.
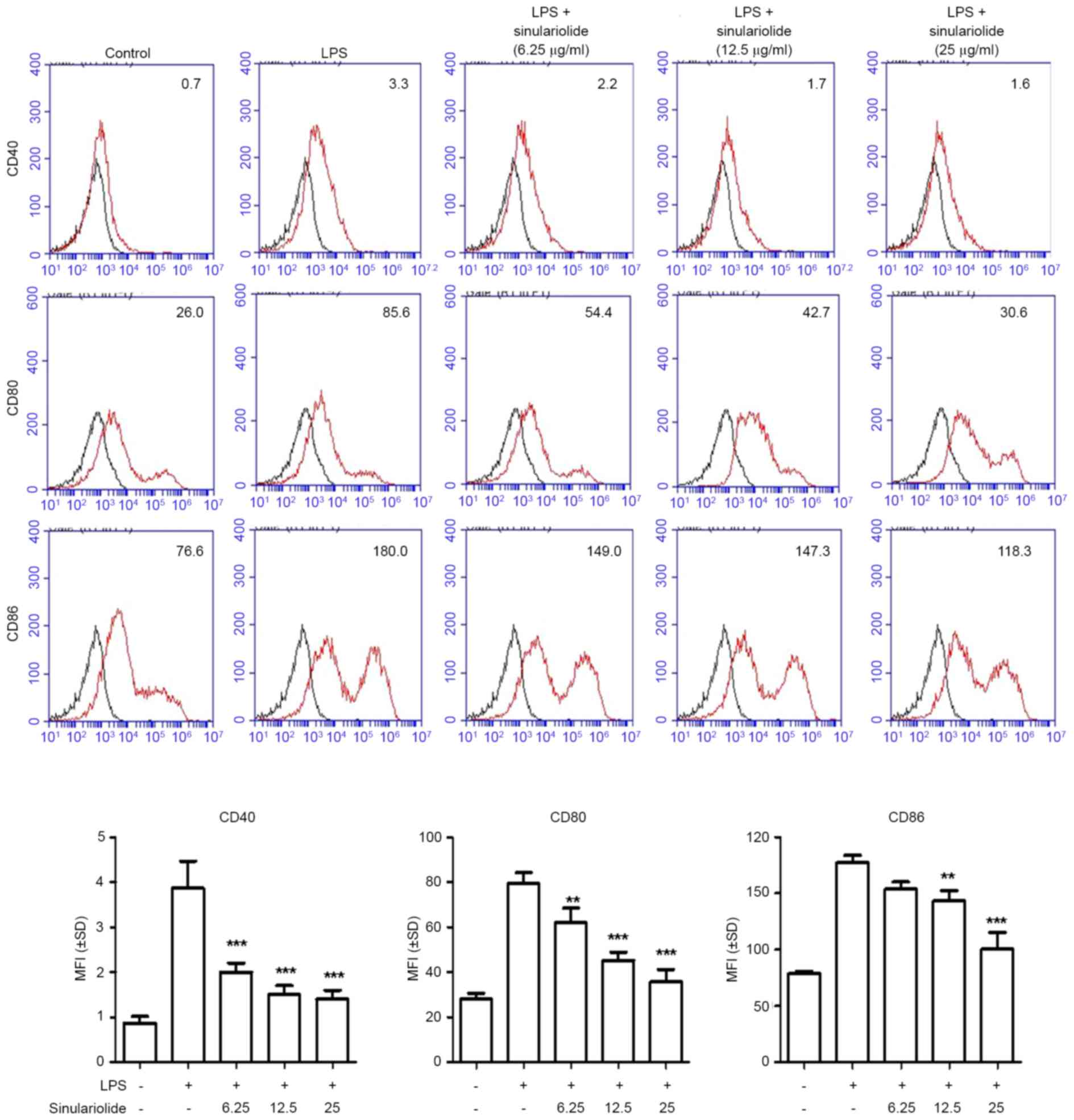
Reduction in the expression of
co-stimulatory molecules by sinulariolide
As co-stimulatory molecules are markers of the
maturation of DCs (1,2), the present study investigated whether
sinulariolide can alter the expression levels of CD40, CD80 and
CD86 in LPS-treated DCs. As shown in Fig. 2, the expression levels of CD40,
CD80 and CD86 were increased in immature DC following LPS treatment
(100 ng/ml) for 24 h, and sinulariolide treatment effectively
decreased the induction of co-stimulatory molecules in a
dose-dependent manner.
Sinulariolide inhibits the secretion
of proinflammatory mediators in LPS-induced maturation
Subsequently, the present study evaluated the
inhibitory effect of sinulariolide on the production of
proinflammatory cytokines and NO by LPS-stimulated DCs. The levels
of TNF-α, IL-6, IL-12 and NO in the medium were measured following
treatment of the immature DCs with sinulariolide for 1 h, followed
by LPS (100 ng/ml) stimulation for 24 h. Despite the fact that the
LPS-treated DCs showed markedly increased production of IL-12 p70,
TNF-α, IL-6 and NO, compared with the untreated DCs, the results
showed that sinulariolide suppressed this LPS-induced release of
cytokines and NO in a dose-dependent manner (Fig. 3A-D). The present study also
measured the mRNA levels of iNOS using RT-qPCR analysis to quantify
the inhibitory effect of sinulariolide on NO, which is regulated by
iNOS. The data, as shown in Fig.
3E, revealed that sinulariolide markedly decreased the mRNA
level of iNOS in the LPS-stimulated DCs (Fig. 3E).
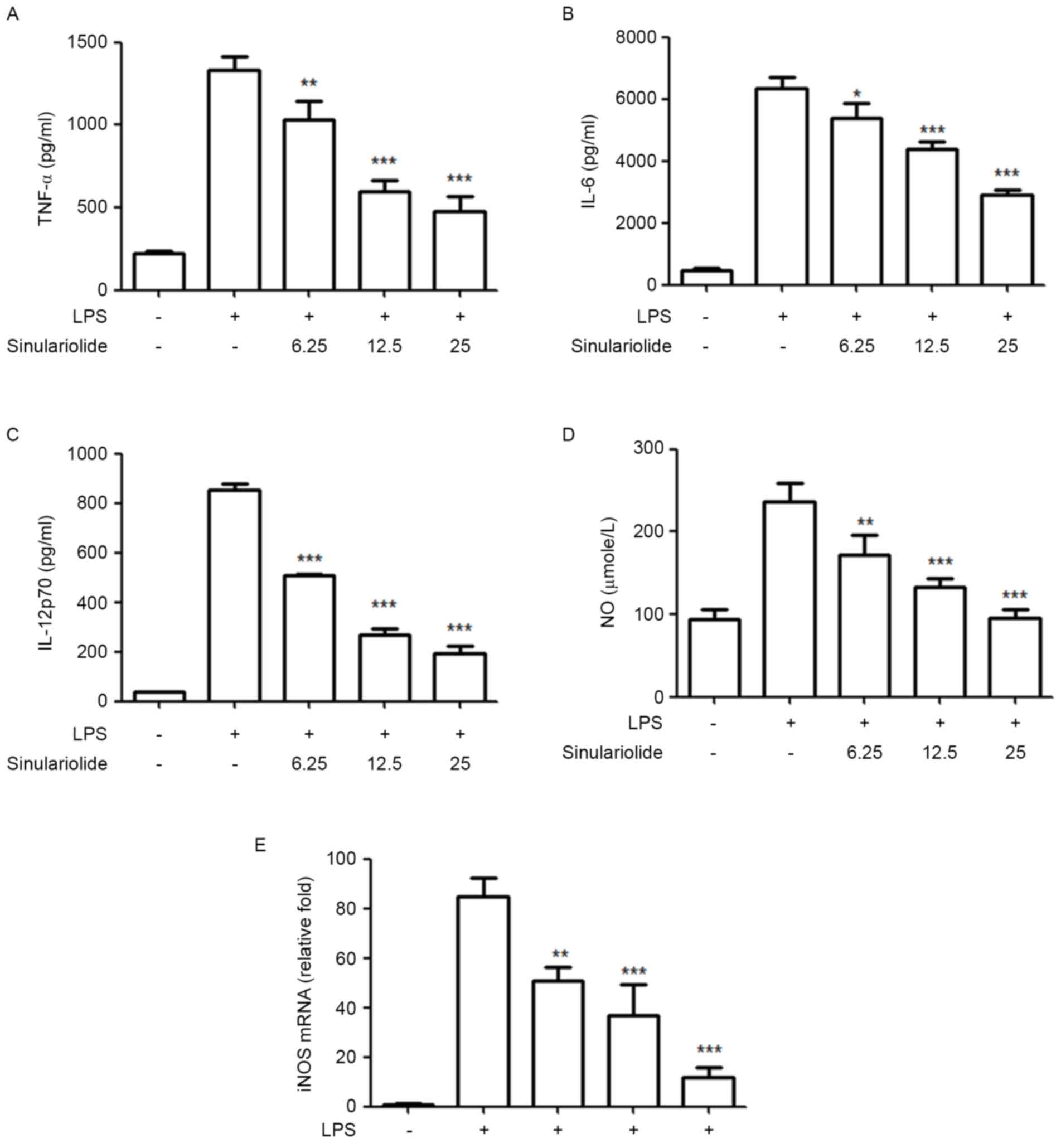 | Figure 3.Sinulariolide suppresses the release
of IL-12p70, TNF-α, IL-6 and NO in LPS-stimulated DCs. Immature DCs
were treated with sinulariolide for 1 h and then stimulated with
LPS (100 ng/ml) for an additional 24 h. Cytokine levels of (A)
TNF-α, (B) IL-6 and (C) IL-12 p70 in the culture supernatant were
measured using ELISA. (D) NO was measured using Griess reagent.
Data are expressed as the mean ± standard deviation of triplicate
values. (E) mRNA expression levels of iNOS were assessed using
reverse transcription-quantitative polymerase chain reaction
analysis using the 2−ΔΔCq method with GAPDH mRNA as a
reference. Quantitative data are expressed as the mean ± standard
deviation of triplicate values. All data are representative of
three independent experiments with similar results. *P<0.05,
**P<0.01 and ***P<0.001 vs. dimethyl sulfoxide-treated
control group. DCs, dendritic cells; LPS, lipopolysaccharide;
TNF-α, tumor necrosis factor-α; IL-, interleukin; NO, nitric oxide;
iNOS, inducible nitric oxide synthase. |
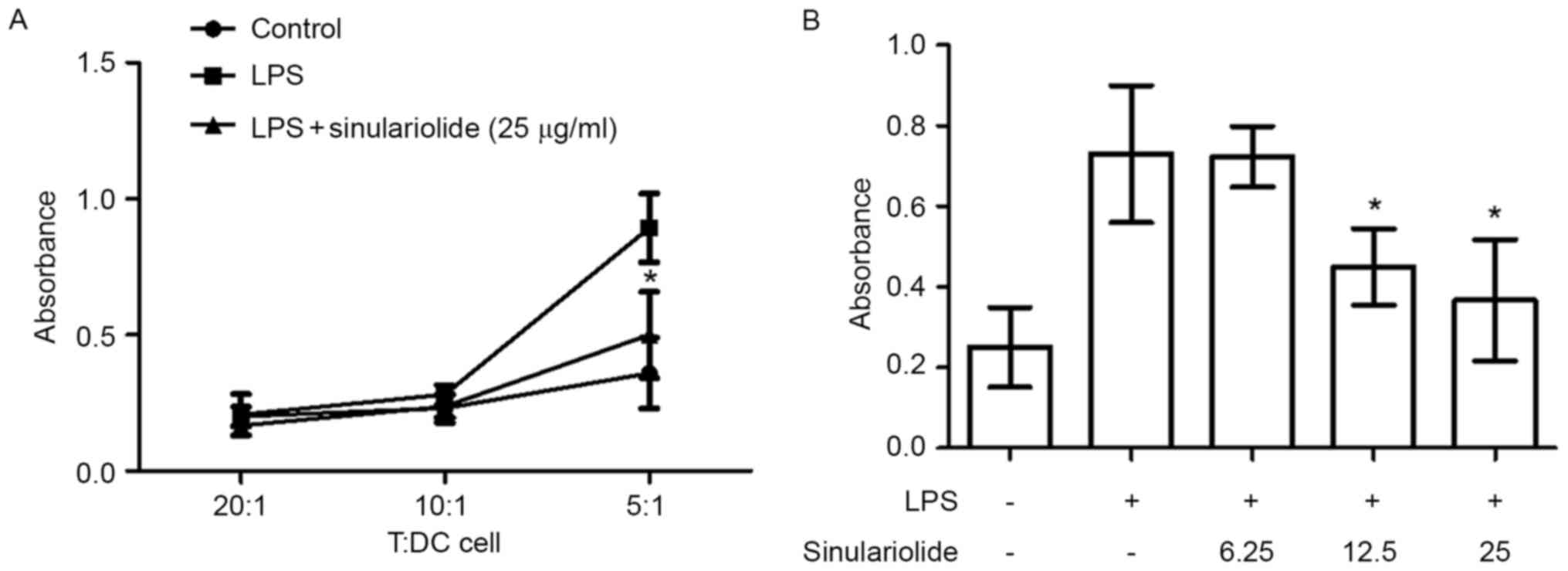
Sinulariolide suppresses the ability
of LPS-stimulated DCs to activate allogeneic T cells
The effect of sinulariolide on DC-mediated
allogeneic T cell proliferation was then examined. The
LPS-stimulated DCs were pretreated with or without sinulariolide
for 24 h and co-cultured with allogeneic spleen CD4+ T
cells for 48 h prior to measuring T cell proliferation. As shown in
Fig. 4A, the DCs treated with LPS
induced more marked proliferative responses in allogeneic T cells,
compared with the untreated DCs, when the T/DC cell ratio was 5:1.
This effect was reduced by sinulariolide in the dose range of
6.25–25 µg/ml (Fig. 4B).
Sinulariolide represses the activation
of NF-κB in LPS-stimulated DCs
To further elucidate the possible suppressive
molecular mechanism of sinulariolide, the present study examined
and identified the signaling pathways, which may be affected by
sinulariolide in the LPS-stimulated DCs. As shown in Fig. 5A, ERK, c-Jun N-terminal kinase
(JNK), p38 mitogen-activated protein kinases (MAPKs) and AKT were
phosphorylated in the DCs upon stimulation, and sinulariolide
appeared to partially reduce the LPS-induced phosphorylation of AKT
at 30 min (P=0.08) and 60 min (P=0.07), compared with
p-ERK, p-p38 and p-JNK. The expression of non-phosphorylated
proteins were not affected by sinulariolide. The present study also
analyzed the protein levels of IκB and the binding activity of
NF-κB using a TransAM™ NF-κB assay. The results showed that
sinulariolide decreased the degradation of IκB induced by LPS
(Fig. 5B) and significantly
inhibited the LPS-induced NF-κB binding activity of p65 (Fig. 5C). This suggested that
sinulariolide repressed LPS-induced DC maturation via inhibition of
the NF-κB pathway, which may further explain the inhibitory effect
of sinulariolide on DC activation.
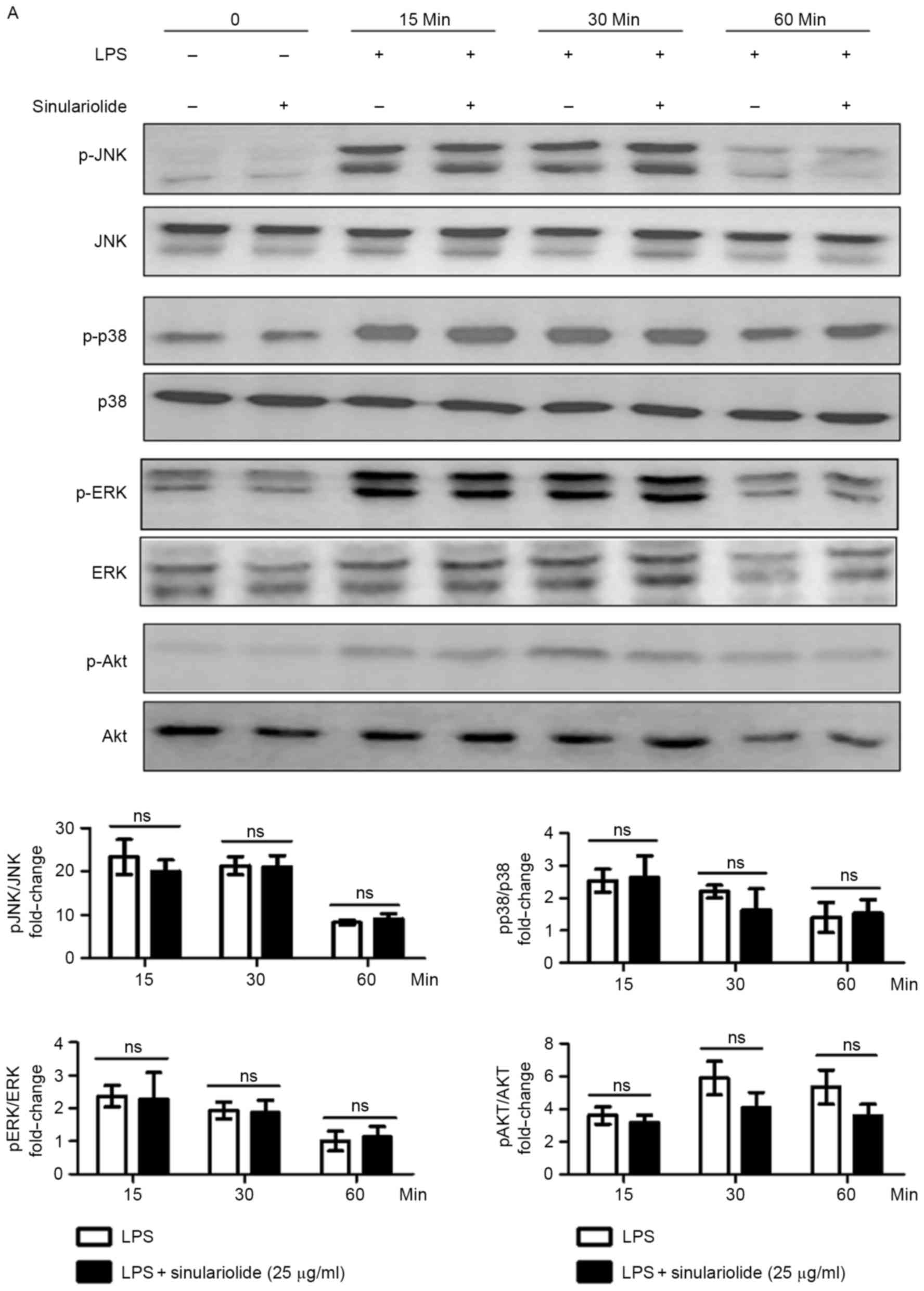 | Figure 5.Inhibition of LPS-induced NF-κB
signaling pathway in DCs by sinulariolide. Sinulariolide was used
to treat immature DCs for 1 h, stimulated with LPS (100 ng/ml) for
the indicated durations and lysed for protein extraction. Levels of
(A) ERK, JNK, p38 MPAK, AKT (phosphorylated and non-phosphorylated)
and (B) IκB were examined by western blot assay. GAPDH was used as
a loading control. Densitometric analysis was performed using Image
J software. (C) NF-κB p65 DNA binding activity in nuclear extracts
of DCs was determined using the TransAM kit. Data are expressed as
the mean OD450 values of triplicate values. All data presented are
representative of three independent experiments with similar
results. *P<0.05, **P<0.01 and ***P<0.001 vs. dimethyl
sulfoxide-treated control group. DCs, dendritic cells; LPS,
lipopolysaccharide; JNK, c-Jun N-terminal kinase; ERK,
extracellular signal-regulated kinase; NF-κB, nuclear factor κB;
IκB, inhibitor of NF-κB; p-, phosphorylated; ns, not significant
(P>0.05); OD450, optical density at 450 nm. |
Discussion
The inhibitory effects of sinulariolide on the
phenotype and functional activation of DCs were investigated in the
present study. Sinulariolide has been shown to exert anticancer
effects in various cancer cell types. In contrast to anticancer
effects, the present study is the first, to the best of our
knowledge, to report that sinulariolide is an immunomodulator,
which can suppress the activation of DCs. The data suggested that
sinulariolide had an inhibitory effect on the modulation of harmful
and undesirable immune responses.
The immunogenic phenotype of mature DCs is
functionally characterized by the upregulation of co-stimulatory
markers (CD40, CD80 and CD86) and surface MHC molecules
(23,24). These distinct molecules regulate
the stimulatory capacity of DCs to activate allogeneic T cell
proliferation. In the present study, sinulariolide significantly
decreased the LPS-induced expression levels of CD40, CD80 and CD86
in a dose-dependent manner, indicating that DCs treated with
sinulariolide were resistant to phenotypic maturation.
Another distinctive feature of DC maturation is the
increased production of pro-inflammatory cytokines and NO, which
have critical regulatory functions in inflammation, T cell
differentiation and expansion. TNF-α, IL-6 and IL-12 are key
pro-inflammatory cytokines involved in the pathogenesis of chronic
inflammatory diseases. DC maturation induced by TNF-α augments Th1
cell differentiation, whereas IL-6 induces the differentiation of
Th2 cells (25). The production of
IL-12 by mature DCs is important in priming naïve CD4+ T
cells to undergo Th1 differentiation (26). NO, synthesized by the enzyme NOS
from L-arginine, is a potent signaling molecule, which is crucial
in various physiological and pathophysiological processes (27). NO is also produced by DCs upon
activation, thus providing a negative feedback mechanism to
suppress lymphocyte proliferation and induce apoptosis of DCs
(28,29). As a result, inhibiting the
production of NO with the NOS inhibitor, NG-monomethyl
arginine, can reduce this apoptotic process (28). Furthermore, iNOS is activated by
bacterial infection and various immunogenic stimuli, including LPS,
interferon-γ or TNF-α, enabling the generation of NO from DCs to
regulate DC responsiveness in an autoregulatory feedback loop. The
results of the present study showed that LPS significantly
increased the expression of TNF-α, IL-6 and IL-12, and the release
of NO/iNOS by DCs, and this increase was inhibited by
sinulariolide, indicating the immunomodulatory role of
sinulariolide in DC functions.
As the MAPK, phosphoinositide-3 kinase/Akt and NF-κB
signaling pathways in DCs can be activated by LPS stimulation, it
has been revealed that these signaling pathways are distinct, but
overlapping, in the phenotypic maturation, cytokine production and
functional activation of DCs. Among these pathways, NF-κB has a
more important role, compared with that of JNK, in mediating
LPS-stimulated DC phenotypic maturation (30,31).
The differentiation of human monocytes into immature DCs has been
determined by the activation of p38 MAPK (32). ERK and PI3 kinase/Akt are essential
in LPS-stimulated DC survival (33). In the present study, it was
observed that activation of the MAPK (ERK, p38 and JNK), AKT and
NF-κB pathways were affected by LPS treatment, which was consistent
with previous studies. However, pretreatment with sinulariolide
exerted marked inhibitory effects on the degradation of IκB and
activation of NF-κB in response to LPS stimuli, without inhibiting
the LPS-induced phosphorylation of MAPK and AKT. These data
suggested that the attenuation of LPS-stimulated maturation and
inflammatory responses of DCs by sinulariolide were associated with
downregulation of the NF-κB signaling pathway. However, the exact
mechanisms underlying the suppressive effect of sinulariolide on
LPS-stimulated DC activation requires further investigation.
Previous studies have shown that the bioactivity of
sinulariolide can be enhanced by conjugating hyaluronan
nanoparticles (34) and that
hyaluronan nanoparticle/sinulariolide aggregates exert more potent
anticancer effects on lung cancer cells, compared with
sinulariolide alone. Conceivably, these techniques are likely to
further facilitate the development of clinical applications of
sinulariolide.
In conclusion, the results of the present study
demonstrated that sinulariolide suppressed LPS-stimulated DC
phenotypic maturation, cytokine and NO production, and
co-stimulatory molecule expression. Therefore, sinulariolide may be
utilized in the treatment of autoimmune and inflammatory disorders.
These findings provide novel insight into the immunopharmacological
functions of sinulariolide.
Acknowledgements
The present study was supported by Changhua
Christian Hospital (grant nos. 103-CCH-IRP-003 and
103-CCH-ICO-001).
References
|
1
|
Li X, He X, Liu B, Xu L, Lu C, Zhao H, Niu
X, Chen S and Lu A: Maturation of murine bone marrow-derived
dendritic cells induced by Radix Glycyrrhizae polysaccharide.
Molecules. 17:6557–6568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim GY, Lee MY, Lee HJ, Moon DO, Lee CM,
Jin CY, Choi YH, Jeong YK, Chung KT, Lee JY, et al: Effect of
water-soluble proteoglycan isolated from Agaricus blazei on the
maturation of murine bone marrow-derived dendritic cells. Int
Immunopharmacol. 5:1523–1532. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
English K, Barry FP and Mahon BP: Murine
mesenchymal stem cells suppress dendritic cell migration,
maturation and antigen presentation. Immunol Lett. 115:50–58. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hackstein H and Thomson AW: Dendritic
cells: Emerging pharmacological targets of immunosuppressive drugs.
Nat Rev Immunol. 4:24–34. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li G, Zhang Y, Deng Z, van Ofwegen L,
Proksch P and Lin W: Cytotoxic cembranoid diterpenes from a soft
coral Sinularia gibberosa. J Nat Prod. 68:649–652. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang B, Zhou XF, Lin XP, Liu J, Peng Y,
Yang XW and Liu YH: Cembrane diterpenes chemistry and biological
properties. Curr Org Chem. 16:1512–1539. 2012. View Article : Google Scholar
|
|
7
|
Chao CH, Chou KJ, Huang CY, Wen ZH, Hsu
CH, Wu YC, Dai CF and Sheu JH: Bioactive cembranoids from the soft
coral Sinularia crassa. Mar Drugs. 9:1955–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Gao AH, Huang H, Li J, Mollo E,
Gavagnin M, Cimino G, Gu YC and Guo YW: Diterpenoids from the
hainan soft coral sinularia parva. Helvetica Chim Acta.
92:1341–1348. 2009. View Article : Google Scholar
|
|
9
|
Coll JC, Price IR, Konig GM and Bowden BF:
Algal overgrowth of alcyonacean soft corals. Mar Biol. 96:129–135.
1987. View Article : Google Scholar
|
|
10
|
Lu Y, Huang CY, Lin YF, Wen ZH, Su JH, Kuo
YH, Chiang MY and Sheu JH: Anti-inflammatory cembranoids from the
soft corals Sinularia querciformis and Sinularia granosa. J Nat
Prod. 71:1754–1759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y, Su JH, Huang CY, Liu YC, Kuo YH, Wen
ZH, Hsu CH and Sheu JH: Cembranoids from the soft corals Sinularia
granosa and Sinularia querciformis. Chem Pharm Bull (Tokyo).
58:464–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu CI, Chen CC, Chen JC, Su JH, Huang HH,
Chen JY and Wu YJ: Proteomic analysis of anti-tumor effects of
11-dehydrosinulariolide on CAL-27 cells. Mar Drugs. 9:1254–1272.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YS, Chen CH, Liaw CC, Chen YC, Kuo YH
and Shen YC: Cembrane diterpenoids from the Taiwanese soft coral
Sinularia flexibilis. Tetrahedron. 65:9157–9164. 2009. View Article : Google Scholar
|
|
14
|
Aceret TL, Coll JC, Uchio Y and Sammarco
PW: Antimicrobial activity of the diterpenes flexibilide and
sinulariolide derived from Sinularia flexibilis Quoy and Gaimard
1833 (Coelenterata: Alcyonacea, Octocorallia). Comp Biochem Physiol
C Pharmacol Toxicol Endocrinol. 120:121–126. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu YJ, Neoh CA, Tsao CY, Su JH and Li HH:
Sinulariolide suppresses human hepatocellular carcinoma cell
migration and invasion by inhibiting matrix metalloproteinase-2/-9
through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci.
16:16469–16482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YJ, Su JH, Tsao CY, Hung CT, Chao HH,
Lin JJ, Liao MH, Yang ZY, Huang HH, Tsai FJ, et al: Sinulariolide
induced hepatocellular carcinoma apoptosis through activation of
mitochondrial-related apoptotic and PERK/eIF2α/ATF4/CHOP pathway.
Molecules. 18:10146–10161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li HH, Su JH, Chiu CC, Lin JJ, Yang ZY,
Hwang WI, Chen YK, Lo YH and Wu YJ: Proteomic investigation of the
sinulariolide-treated melanoma cells A375: Effects on the cell
apoptosis through mitochondrial-related pathway and activation of
caspase cascade. Mar Drugs. 11:2625–2642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neoh CA, Wang RY, Din ZH, Su JH, Chen YK,
Tsai FJ, Weng SH and Wu YJ: Induction of apoptosis by sinulariolide
from soft coral through mitochondrial-related and p38MAPK pathways
on human bladder carcinoma cells. Mar Drugs. 10:2893–2911. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li S, Lin YC, Ho CT, Lin PY, Suzawa M,
Wang HC, Chu CL, Chen DY and Lin CC: Formulated extract from
multiple citrus peels impairs dendritic cell functions and
attenuates allergic contact hypersensitivity. Int Immunopharmacol.
20:12–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsieh PW, Chang FR, McPhail AT, Lee KH and
Wu YC: New cembranolide analogues from the formosan soft coral
Sinularia flexibilis and their cytotoxicity. Nat Prod Res.
17:409–418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin CC, Chu CL, Ng CS, Lin CY, Chen DY,
Pan IH and Huang KJ: Immunomodulation of phloretin by impairing
dendritic cell activation and function. Food Funct. 5:997–1006.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caux C, Massacrier C, Vanbervliet B,
Dubois B, Van Kooten C, Durand I and Banchereau J: Activation of
human dendritic cells through CD40 cross-linking. J Exp Med.
180:1263–1272. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinchuk LM, Polacino PS, Agy MB, Klaus SJ
and Clark EA: The role of CD40 and CD80 accessory cell molecules in
dendritic cell-dependent HIV-1 infection. Immunity. 1:317–325.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diehl S and Rincón M: The two faces of
IL-6 on Th1/Th2 differentiation. Mol Immunol. 39:531–536. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heufler C, Koch F, Stanzl U, Topar G,
Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N and Schuler
G: Interleukin-12 is produced by dendritic cells and mediates T
helper 1 development as well as interferon-gamma production by T
helper 1 cells. Eur J Immunol. 26:659–668. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: Physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.PubMed/NCBI
|
|
28
|
Bonham CA, Lu L, Li Y, Hoffman RA, Simmons
RL and Thomson AW: Nitric oxide production by mouse bone
marrow-derived dendritic cells: Implications for the regulation of
allogeneic T cell responses. Transplantation. 62:1871–1877. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu L, Bonham CA, Chambers FG, Watkins SC,
Hoffman RA, Simmons RL and Thomson AW: Induction of nitric oxide
synthase in mouse dendritic cells by IFN-gamma, endotoxin, and
interaction with allogenic T cells: Nitric oxide production is
associated with dendritic cell apoptosis. J Immunol. 157:3577–3586.
1996.PubMed/NCBI
|
|
30
|
Neves BM, Cruz MT, Francisco V,
Garcia-Rodriguez C, Silvestre R, Cordeiro-da-Silva A, Dinis AM,
Batista MT, Duarte CB and Lopes MC: Differential roles of
PI3-kinase, MAPKs and NF-kappaB on the manipulation of dendritic
cell T(h)1/T(h)2 cytokine/chemokine polarizing profile. Mol
Immunol. 46:2481–2492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rescigno M, Martino M, Sutherland CL, Gold
MR and Ricciardi-Castagnoli P: Dendritic cell survival and
maturation are regulated by different signaling pathways. J Exp
Med. 188:2175–2180. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ardeshna KM, Pizzey AR, Devereux S and
Khwaja A: The PI3 kinase, p38 SAP kinase, and NF-kappaB signal
transduction pathways are involved in the survival and maturation
of lipopolysaccharide-stimulated human monocyte-derived dendritic
cells. Blood. 96:1039–1046. 2000.PubMed/NCBI
|
|
33
|
Xie J, Qian J, Yang J, Wang S, Freeman ME
III and Yi Q: Critical roles of Raf/MEK/ERK and PI3K/AKT signaling
and inactivation of p38 MAP kinase in the differentiation and
survival of monocyte-derived immature dendritic cells. Exp Hematol.
33:564–572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsiao KY, Wu YJ, Liu ZN, Chuang CW, Huang
HH and Kuo SM: Anticancer effects of sinulariolide-conjugated
hyaluronan nanoparticles on lung adenocarcinoma cells. Molecules.
21:2972016. View Article : Google Scholar : PubMed/NCBI
|