Introduction
Lower back pain is one the most common
musculoskeletal disorders, inflicting a large economic burden on
the healthcare system worldwide (1–3). The
causes of lower back pain are complex, and intervertebral disc
degeneration (IDD) is considered to contribute substantially to the
development of this pain (4,5).
Although various etiological factors have been suggested to
influence the development of IDD, including aging, genetic
predisposition and environmental factors (6,7), the
underlying cellular mechanisms remain largely unknown. Several
previous reports have demonstrated that the formation of clusters
of nucleus pulposus (NP) cells and fibrocartilaginous tissue
proliferation have important roles in IDD (8–10).
However, the precise cause of enhanced proliferation of NP cells in
IDD remains unclear.
MicroRNAs (miRNAs) are a category of small (~22
nucleotides), single-stranded, noncoding RNAs that exert their
biological functions through recognition and binding to specific
sequences within the 3′-untranslated region (3′-UTR) of target
genes. miRNAs binding to these regions in mRNA leads to mRNA
degradation or represses protein translation (11,12).
There is increasing evidence that indicates that miRNAs have
important functions in various cellular processes, including cell
proliferation, differentiation and apoptosis (13–15).
In addition, a number of studies have demonstrated that
dysregulated miRNAs participate in various human cancers and act as
oncogenes or suppressor genes, depending the function of their
targets (16,17). Additionally, several miRNAs were
reported to regulate various target genes, pathways and processes
essential for the pathogenesis of IDD (18–20).
However, although miRNAs have been investigated extensively in
recent years, their roles in IDD development, and as potential
markers for diagnosis and prognosis, remain unclear. As an
important miRNA, miRNA-96 (miR-96) is involved in regulating the
proliferation of various tumor types, including urothelial
carcinoma, bladder cancer and colorectal cancer (21–23).
As miR-96 is a crucial regulator of cell proliferation in various
pathologies, and IDD is characterized by the abnormal proliferation
of NP cells, we hypothesized that miR-96 may be involved in the
development of IDD. Therefore, the aim of the present study was to
evaluate the functional role of miR-96 in IDD and to investigate
the underlying molecular mechanism.
Materials and methods
Ethics statement
All experimental protocols were approved by the
Clinical Research Ethics Committee of The Affiliated Hospital of
Guilin Medical University (Guilin, China). Written informed consent
was obtained from all patients.
Patients and samples
NP tissue samples from patients with IDD or
traumatic lumbar fracture were collected from patients undergoing
discectomy in the Department of Orthopedics of the Affiliated
Hospital of Guilin Medical University between June 2015 and June
2016. A total of 30 patients (mean age, 50±11.7 years; age range,
40–63 years; 16 males, 14 females) with IDD, including patients
with lumbar (L)4/5 (n=22) and L5/sacral 1 (n=8) disk herniations,
and 5 patients with a recent traumatic lumbar fracture (mean age,
21.6±3.8 years; age range, 18–26 years; 3 males, 2 females) were
enrolled in the present study. Patients with infections, previous
lumbar disc surgery or idiopathic scoliosis were excluded. Routine
magnetic resonance imaging scans of the lumbar spine were taken of
each patient prior to surgery. The degree of disc degeneration was
classified using a modified Pfirrmann grading system (24).
Isolation and culture of human NP
cells
NP cells were isolated as described previously
(25). NP tissue specimens were
first washed twice with PBS. NP tissue was separated from annulus
fibrosus tissue by visualizing under a stereotaxic microscope and
was cut into pieces (2–3 mm3). NP cells were released
from NP tissues by incubation with 0.25 mg/ml type II collagenase
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for
12 h at 37°C in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.). Following isolation, NP cells were
resuspended in DMEM containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin, 100 U/ml
penicillin and 1% L-glutamine, and incubated at 37°C in a
humidified atmosphere with 95% air and 5% CO2. Fully
confluent cells were detached by trypsinization, seeded into 35-mm
tissue culture dishes in complete culture medium (DMEM supplemented
with 10% FBS, 100 µg/ml streptomycin and 100 U/ml penicillin) and
incubated at 37°C in a humidified atmosphere with 95% air and 5%
CO2. The medium was changed every 3 days. Second passage
cells were used for experiments.
Oligonucleotides, constructs and
transfections
A total of 24 h prior to transfection, cells were
seeded into 6-well dishes in 2 ml DMEM containing 1%
penicillin/streptomycin. The medium used was discarded after 24 h
and cells were washed twice with Opti-MEM I medium (Thermo Fisher
Scientific, Inc.). Opti-MEM (11.5 ml) was added to each well.
miR-96 mimic, miR-96 control, miR-96 inhibitor and miR-96 control
inhibitor (5 µl; Guangzhou RiboBio Co., Ltd., Guangzhou, China)
were diluted in Opti-MEM I (250 µl). The sequences of the
oligonucleotides used were as follows: miR-96 mimic, forward
5′-CAGUAGGAAUAGACUUUCG-3′, reverse 5′-GUCAUCCUUAUCUGAAAGC-3′;
miR-96 control, forward 5′-UAAAGUGCUUAUAGUGCAGGUAG-3′, reverse
5′-CUACCUGCACUAUAAGCACUUUA-3′; miR-96 inhibitor, forward
5′-AGCUAUAGACAAAAGCACG-3′, reverse 5′-GUTCTGGCGCACGAAAGC-3′; and
miR-96 inhibitor control, forward 5′-CAGUAGCUUAUACAGUAG-3′ and
reverse 5′-GCAGTACACACUUUATTGCT-3′. AT-rich interaction domain 2
(ARID2) plasmid, control siRNA and ARID2 small interfering (si)RNA
were purchased from Guangzhou RiboBio Co., Ltd. The sequences of
the siRNAs that were used were as follows: Control siRNA, forward
5′-GCTTGAGGGTCTGAATCTTGCT-3′, reverse 5′-GTCCGCAGTCTTACGAGGAG-3′;
siARID2, forward 5′-CCAGCTCGAGGGATTCAGGAATTGCTCCACCA-3′, reverse
5′-CCAGGCGGCCGCCTCCTCTGGCAGTAATGGTCCT-3′. Cells were seeded at a
density of 5×106 cells/well 16 h prior to transfection
in DMEM with 1% penicillin/streptomycin. Briefly, 10 nM miRNAs, 100
nM siRNAs, 800 ng ARID2 plasmid, 800 ng control vector and were
diluted in the serum-free medium at room temperature for 5 min
using Lipofectamine 2000 as the transfection reagent (Thermo
Fischer Scientific, Inc.). Then, the mixture was added to the cells
(500 µl/well) and incubated in 5% CO2 incubator at 37°C
for 6 h. Subsequently, the medium was replaced with fresh DMEM
containing 10% FBS and cells were cultured for 48–72 h. A total of
2×105 cells that were transfected with miRNAs were
incubated for 24 h at 37°C, and then in the presence or absence of
15 µM of the Akt inhibitor LY294002 (Thermo Fisher Scientific,
Inc.) for an additional 6 h at 37°C.
Bioinformatics analysis
The miRWalk database (http://www.ma.uni-heidelberg.de/apps/zmf/mirwalk/) and
Targetscan online software version 3.1 (targetscan.org/vert_71/)) were used to predict
potential target genes for miR-96.
Luciferase reporter assays
The 3′-UTR sequence of ARID2 that was predicted to
interact with miR-96, or the mutated sequence within the predicted
target sites, was synthesized and inserted into the pGL3 control
vector (Promega Corporation, Madison, WI, USA). These constructs
were referred to as wild-type or mutant ARID2-3′-UTR, respectively.
A total of 24 h prior to transfection, NP cells were seeded in
24-well plates at a density of 2×105 cells/well. Cells
were co-transfected with 200 ng wild-type ARID2-3′-UTR vector or
mutant ARID2-3′-UTR vector along with 500 nM miR-96 mimic, miR-96
inhibitor, or control miRNA and 10 ng pRL-SV40 Renilla
plasmid (Promega Corporation) using Lipofectamine 2000. A total of
48 h post-transfection, cells were harvested and luciferase
activity was measured using the Dual-Luciferase Reporter Assay
system (Promega Corporation) with a luminometer (Promega
Corporation) according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity and the firefly/Renilla ratio was reported. Results
were obtained from three independent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 2×105 NP
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using the RevertAid RT Reverse
Transcription kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The temperature protocol was as follows:
Incubation at 37°C for 60 min, followed by enzymatic inactivation
by incubation at 85°C for 5 min. qPCR was performed on cDNA using
the Primescript RT-PCR kit (Takara Bio, Inc., Otsu, Japan) on a
LightCycler 480 instrument (Roche Diagnostics, Basel, Switzerland).
GAPDH was used as a housekeeping gene for ARID2 mRNA expression.
miRNA was extracted from 2×105 NP cells using the
mirVana miRNA Isolation kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. miR-96 expression was
determined using the miScript SYBR-Green PCR kit (Qiagen, Inc.,
Valencia, CA, USA) on a LightCycler 480 instrument. U6 was used as
a housekeeping gene for miR-96 expression. Thermocycling conditions
were as follows: Initial denaturation at 95°C for 3 min, followed
by 40 cycles of denaturation at 95°C for 15 sec and
annealing/elongation for 34 sec at 60°C. The specific primers used
were as follows: ARID2, 5′-AGCTCTTGGCAGCTAATCGT-3′ (forward),
5′-ACAGGGTCCAGTAAAGCTCAG-3′ (reverse); GAPDH
5′-CTGGGCTACACTGAGCACC-3′ (forward), 5′-AAGTGGTCGTTGAGGGCAATG-3′
(reverse); miR-96, 5′-CAUCUGAAUAGACGATTAG-3′ (forward),
5′-GUAAGCACUAUAAGCCGC-3′ (reverse); and U6,
5′-CCCACTCAACAGCGTGTCTC-3′ and 5′-CGTCGATTATCTGAATTTGGCCT-3′
(reverse). Primers were designed by Applied Biosystems; Thermo
Fisher Scientific, Inc. Relative mRNA and miRNA expression was
quantified according to the relative Cq method (26). Experiments were performed in
triplicate.
Western blotting
Cells were lysed using radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China), followed by 5–10 min boiling and centrifugation at 12,000 ×
g for 15 min at 4°C to obtain the protein supernatants. Protein
concentration was quantified using a bicinchoninic acid protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts
of extracted protein samples (50 µg) were separated by 10% SDS-PAGE
(Beyotime Institute of Biotechnology) and transferred onto
polyvinylidene difluoride membranes (Thermo Fisher Scientific,
Inc.), which were then blocked with 5% non-fat dried milk in PBS
containing 0.1% Tween-20 (PBST; Beyotime Institute of
Biotechnology) overnight at 4°C. The membrane was then incubated
with the following primary antibodies at room temperature for 3 h:
Anti-ARID2 (cat no. 13594; 1:100; Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-Akt (cat no. 4691; 1:100; Cell Signaling
Technology, Inc.), anti-phosphorylated (phospho) 473-Akt (cat no.
4060; 1:100; Cell Signaling Technology, Inc.), anti-cyclin D1 (cat
no. 2978; 1:100; Cell Signaling Technology, Inc.) and anti-GAPDH
(cat no. ab9485; 1:100; Abcam, Cambridge, UK). Following washing
with PBST 3 times, the membrane was incubated with goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (cat no.
ab7090; 1:5,000; Abcam) for 40 min at room temperature. Protein
bands were visualized using an enhanced chemiluminescence kit (GE
Healthcare Life Sciences, Little Chalfont, UK). Blots were
semi-quantified by densitometry using Image-Pro Plus software
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) and
normalized to GAPDH.
Cell proliferation
NP cells were seeded into 96-well plates at a
density of 1×104 cells/well and cultured at 37°C for 24
h after transfection. Cell viability was measured every 24 h for 3
days by adding 10% Cell Counting Kit (CCK)-8 reagent (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). After incubation at
room temperature for 10 min, the absorbance at 450 nm was measured
using a spectrophotometer. Cell proliferation was estimated using
predefined absorbance values. All experiments were performed in
triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 independent experiments. Statistical analysis was performed
using SPSS statistical software version 17.0 (SPSS, Inc., Chicago,
IL, USA). The statistical significance of the differences between
groups was assessed using Student's t-test for air-wise comparisons
or one-way analysis of variance followed by a post hoc Bonferoni or
Fisher's exact test for multiple comparisons. The correlation
between the expression of miR-96 and ARID2 mRNA was estimated using
Spearman's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-96 expression is increased in
patients with IDD
In order to understand the role of miR-96 in the
development of IDD, the expression of miR-96 in human degenerated
NP tissues and normal controls was investigated. The expression of
miR-96 in patients with IDD was significantly higher compared with
healthy controls (P<0.01; Fig.
1A). miR-96 expression was increased progressively in patients
with Pfirrmann grades III, IV and V disc degeneration, while the
expression of ARID2 was decreased as the grade increased.
(P<0.05; Fig. 1B). In patients
with IDD, the expression of ARID2 was significantly decreased
compared with healthy controls (P<0.01; Fig. 1C). Correlation analysis
demonstrated that there was a negative correlation between miR-96
expression and ARID2 mRNA levels in patients with IDD (r=−0.8681;
P<0.01; Fig. 1D).
miR-96 induces NP cell
proliferation
As miR-96 expression was demonstrated to be
associated with the grade of disc degeneration in patients, the
effects of miR-96 expression on NP cell proliferation were
subsequently investigated. NP cells were transfected with an miR-96
mimic or miR-96 inhibitor. As determined by RT-qPCR, the miR-96
mimic significantly increased the level of miR-96 in NP cells,
while the miR-96 inhibitor significantly decreased its expression
(P<0.001; Fig. 2A and B).
Furthermore, the CCK-8 assay demonstrated that overexpression of
miR-96 increased NP cell proliferation compared with
miR-control-transfected cells (Fig.
2C). By contrast, downregulation of miR-96 significantly
reduced the proliferation of NP cells compared with cells
transfected with anti-miR-NC (P<0.05; Fig. 2D). These results indicate that
miR-96 promoted the proliferation of NP cells.
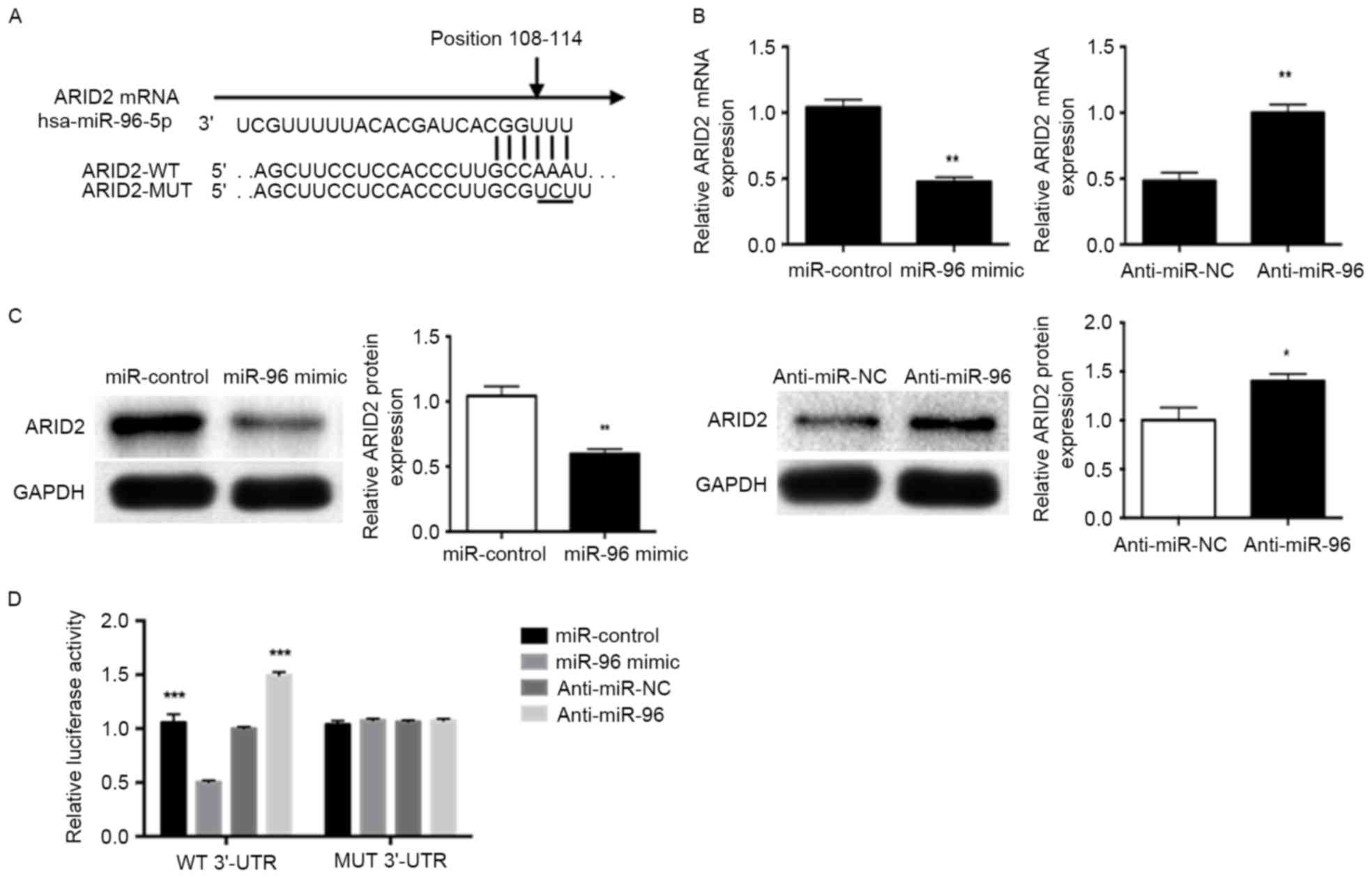
ARID2 is a direct target of miR-96 in
IDD
To investigate the mechanism of miR-96 regulation in
IDD, bioinformatics analysis was performed using miRWalk and
TargetScan to predict the putative target genes of miR-96. Among
the target genes identified, the binding sites for miR-96 in the
3′-UTR of ARID2 were conserved across species (Fig. 3A). To confirm the regulatory role
of miR-96 on ARID2, RT-qPCR and western blotting were performed to
determine the effect of miR-96 on ARID2 mRNA and protein levels,
respectively. miR-96 mimic significantly decreased, while
inhibition of miR-96 significantly increased, ARID2 mRNA
(P<0.01; Fig. 3B) and protein
(P<0.05; Fig. 3C) levels,
compared with the respective controls. In addition, miR-96 mimic
inhibited the luciferase reporter activity of ARID2 containing a
wild-type 3′-UTR, but did not suppress the activity of ARID2 with a
mutant 3′-UTR. Suppression of miR-96 by anti-miR-96 increased the
luciferase reporter activity of wild-type ARID2 3′-UTR (P<0.01;
Fig. 3D). However, with the mutant
ARID2 3′-UTR constructs, no relative increase in reporter activity
was observed (Fig. 3D). These
results indicate that ARID2 is a direct target gene for miR-96 and
that miR-96 downregulates ARID2 expression.
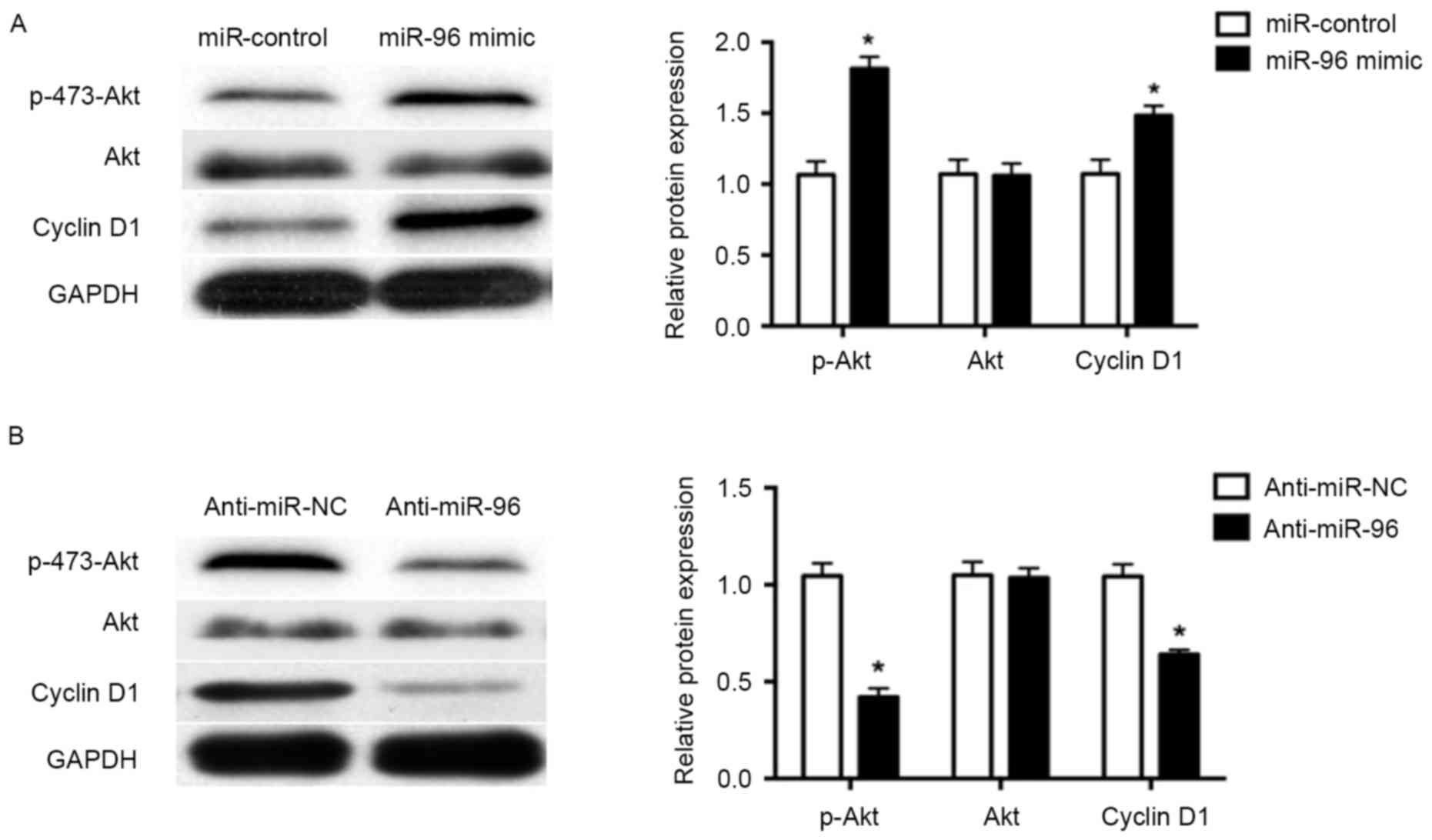
miR-96 promotes cell proliferation by
activating Akt phosphorylation
Previous studies have demonstrated that activation
of Akt signaling by phosphorylation has an important role in cell
proliferation (27,28). Therefore, the presents study
investigated the molecular mechanism underlying the miR-96-mediated
promotion of NP cell proliferation. Overexpression of miR-96 using
miR-96 mimic led to significantly increased Akt phosphorylation in
NP cells (P<0.05; Fig. 4A),
while inhibition of miR-96 significantly decreased Akt
phosphorylation (P<0.05; Fig.
4B), compared with the respective controls. Additionally, the
expression of cyclin D1, a downstream effector of Akt signaling and
a key regulator of cell cycle progression and proliferation, was
increased by miR-96 mimic and decreased by miR-96 inhibitor
treatment in NP cells (P<0.05; Fig.
4A and B).
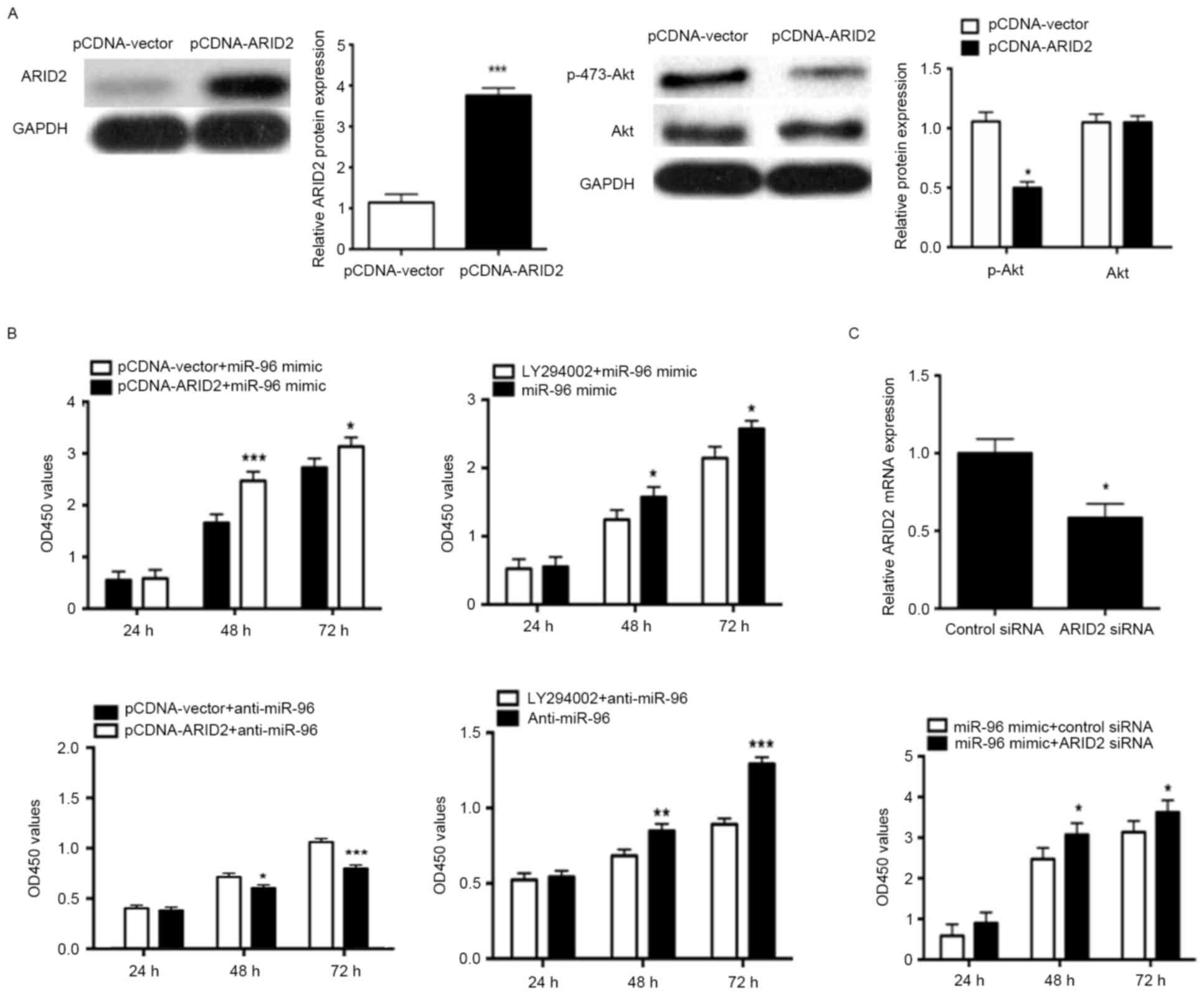
ARID2 is involved in the effect of
miR-96 on NP cell proliferation
To confirm that ARID2 was a functional target of
miR-96, the present study used an ARID2 expression plasmid to
significantly increase ARID2 protein expression in NP cells,
compared with NP cells treated with control vector (P<0.001;
Fig. 5A). In addition, the results
demonstrated that overexpression of ARID2 significantly inhibited
Akt phosphorylation in NP cells, compared with control
vector-transfected cells (P<0.05; Fig. 5A). Furthermore, overexpression of
ARID2 in NP cells, and treatment with the Akt inhibitor LY294002,
decreased the proliferative effect of miR-96, while ARID2
overexpression and Akt inhibitor LY294002 enhanced the
antiproliferative effect of miR-96 downregulation (P<0.05;
Fig. 5B). In order to further
confirm that ARID2 mediates the proliferation effects of miR-96,
ARID2 was knocked down using siRNA, and the results demonstrated
that ARID2 siRNA increased the miR-96 mimic-induced proliferation
of NP cells (P<0.05; Fig.
5C).
Discussion
miRNAs are key modulators of a variety of biological
and pathological processes, which include cell growth,
differentiation, apoptosis and carcinogenesis, through the direct
regulation of gene expression. However, the molecular mechanisms of
miRNAs in disc degeneration remain largely unknown. In the present
study, the expression of miR-96 was significantly increased in
degenerated NP tissues compared with control NP tissues, and
positively associated with the disc degeneration grade. These
results indicate that miR-96 may have a critical role in IDD.
Furthermore, the results demonstrated that overexpression of miR-96
increased the proliferation of NP cells, and ARID2 was identified
as a direct novel target of miR-96. In addition, ARID2 mRNA
expression was downregulated in degenerated NP tissues compared
with control NP tissues, and ARID2 mRNA expression was inversely
correlated with the expression of miR-96. Mechanistically, the
present study demonstrated that overexpression of miR-96, using an
miR-96 mimic, activated Akt signaling by directly targeting ARID2.
Furthermore, the proliferative effect of miR-96 was decreased by
overexpressing ARID2. These results indicated that miR-96, and the
downstream ARID2/Akt pathway, may serve as potential novel
therapeutic targets in the treatment of IDD.
Previous reports have demonstrated that miR-96
expression is frequently increased in various types of human
cancer, and is involved in regulating various developmental and
cellular processes, including cell proliferation, apoptosis,
migration and invasion (29,30).
In addition, upregulation of miR-96 was reported to be closely
associated with the progression of various tumor types and their
pathological grade, including lung, esophageal, hepatocellular and
breast cancers (30–33). However, the role of miR-96 in
degenerated NP tissues, and its importance in the pathogenesis of
IDD, are yet to be established. To investigate the mechanisms
underlying the role of miR-96 in the development of IDD, the
present study transfected NP cells with miR-96 mimic in order to
overexpress miR-96. Overexpression of miR-96 led to a significant
increase in NP cell proliferation. Previous studies have reported
that the formation of NP cell clusters and the proliferation of
fibrocartilaginous tissue have important roles in the development
of IDD (34–36). The results of the current study
indicated that increased NP cell proliferation, which was promoted
by miR-96 upregulation, may be a potential mechanism in the
development of IDD.
ARID2, a novel tumor suppressor gene, was initially
identified in the polybromo-associated BRG1-associated factor
complex, which is a SWI/SNF chromatin-remodeling complex that
functions in ligand-dependent activation of transcription by
nuclear receptors (37). Previous
studies have demonstrated that ARID2 is involved in various
biological processes and suppression of ARID2 promoted cell
proliferation by inducing G1/S transition in hepatoma cells
(38,39). Furthermore, a number of studies
have reported that ARID2 expression is downregulated in certain
cancer types, including hepatocellular carcinoma and gastric cancer
(39,40). In the current study, miR-96
negatively regulated ARID2 mRNA and protein levels in NP cells. In
addition, the vector expressing the mutant form of the ARID2 3′-UTR
was resistant to miR-96 inhibition in NP cells. Therefore, the
results indicate that ARID2 is a direct target of miR-96.
Akt phosphorylation promotes cell cycle progression
and proliferation via cyclin D1 upregulation. Loss of ARID2 is
reported to enhance Akt activation, upregulation of cyclin D1 and
downregulation of p27, which subsequently increases growth and
survival in various cell types (39–41).
In the present study, overexpression of miR-96 increased Akt
activation in NP cells. Furthermore, the effect of altered miR-96
expression on the proliferation of NP cells was reduced by
overexpressing ARID2 or by the Akt inhibitor LY294002. These
results indicate that miR-96 may promote the proliferation of NP
cells by directly targeting the ARID2/Akt signaling pathway.
In conclusion, the results of the present study
demonstrated that miR-96 is upregulated in human degenerated NP
tissues and its expression level is positively associated with the
disc degeneration grade. Overexpression of miR-96 promoted NP cell
proliferation by targeting ARID2 to activate Akt signaling.
Combined, these results demonstrate an important role for miR-96
and the downstream ARID2/Akt pathway in the pathogenesis of IDD.
Importantly, these results have identified a potential novel
therapeutic target for the treatment of IDD.
Acknowledgements
The authors would like to thank members of the
laboratory of Dr. Yu (Y Department of Institute for Nutritional
Sciences, Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences) for technical advice and critical reviews of
the manuscript. The present study was supported by the National
Natural Science Foundation of P.R. China (grant nos. 31260233 and
81460198).
References
|
1
|
Feuerstein M, Marcus SC and Huang GD:
National trends in nonoperative care for nonspecific back pain.
Spine J. 4:56–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katz JN: Lumbar disc disorders and
low-back pain: Socioeconomic factors and consequences. J Bone Joint
Surg Am. 88 Suppl 2:S21–S24. 2006. View Article : Google Scholar
|
|
3
|
Lee HJ, Seo JC, Kwak MA, Park SH, Min BM,
Cho MS, Shin I, Jung JY and Roh WS: Acupuncture for low back pain
due to spondylolisthesis: Study protocol for a randomized
controlled pilot trial. Trials. 15:1052014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freemont TJ, LeMaitre C, Watkins A and
Hoyland JA: Degeneration of intervertebral discs: Current
understanding of cellular and molecular events, and implications
for novel therapies. Expert Rev Mol Med. 2001:1–10. 2001.PubMed/NCBI
|
|
5
|
Mooney V: The classification of low back
pain. Ann Med. 21:321–325. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Liang J, Wu WK, Yu X, Yu J, Weng X
and Shen J: Leptin activates RhoA/ROCK pathway to induce
cytoskeleton remodeling in nucleus pulposus cells. Int J Mol Sci.
15:1176–1188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu G, Cao P, Chen H, Yuan W, Wang J and
Tang X: miR-27a regulates apoptosis in nucleus pulposus cells by
targeting PI3K. PLoS One. 8:e752512013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G
and Liu J: Leptin induces cyclin D1 expression and proliferation of
human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK
pathways. PLoS One. 7:e531762012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson WE, Eisenstein SM and Roberts S:
Cell cluster formation in degenerate lumbar intervertebral discs is
associated with increased disc cell proliferation. Connect Tissue
Res. 42:197–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Huang X, Liu X, Xiao S, Zhang Y,
Xiang T, Shen X, Wang G and Sheng B: miR-21 promotes human nucleus
pulposus cell proliferation through PTEN/AKT signaling. Int J Mol
Sci. 15:4007–4018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brennecke J and Cohen SM: Towards a
complete description of the microRNA complement of animal genomes.
Genome Biol. 4:2282003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soifer HS, Rossi JJ and Saetrom P:
microRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu X and Li Z: microRNAs regulate vascular
smooth muscle cell functions in atherosclerosis (review). Int J Mol
Med. 34:923–933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: microRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Wang P, Zhang Z, Wang W, Liu Y and
Qi Q: miR-184 regulates proliferation in nucleus pulposus cells by
targeting GAS1. World Neurosurg. 97:710–715.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen B, Huang SG, Ju L, Li M, Nie FF,
Zhang Y, Zhang YH, Chen X and Gao F: Effect of microRNA-21 on the
proliferation of human degenerated nucleus pulposus by targeting
programmed cell death 4. Braz J Med Biol. 49:pii2016.
|
|
20
|
Jing W and Jiang W: microRNA-93 regulates
collagen loss by targeting MMP3 in human nucleus pulposus cells.
Cell Prolif. 48:284–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada Y, Enokida H, Kojima S, Kawakami K,
Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N
and Nakagawa M: miR-96 and miR-183 detection in urine serve as
potential tumor markers of urothelial carcinoma: Correlation with
stage and grade, and comparison with urinary cytology. Cancer Sci.
102:522–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu XM, Qian JC, Deng ZL, Cai Z, Tang T,
Wang P, Zhang KH and Cai JP: Expression of miR-21, miR-31, miR-96
and miR-135b is correlated with the clinical parameters of
colorectal cancer. Oncol Lett. 4:339–345. 2012.PubMed/NCBI
|
|
23
|
Liu Y, Han Y, Zhang H, Nie L, Jiang Z, Fa
P, Gui Y and Cai Z: Synthetic miRNA-mowers targeting miR-183-96-182
cluster or miR-210 inhibit growth and migration and induce
apoptosis in bladder cancer cells. PLoS One. 7:e522802012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Shen J, Wu WK, Yu X, Liang J, Qiu G
and Liu J: The role of leptin on the organization and expression of
cytoskeleton elements in nucleus pulposus cells. J Orthop Res.
31:847–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–528. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Liao W, Yuan Q, Ou Y and Huang J:
TTK activates Akt and promotes proliferation and migration of
hepatocellular carcinoma cells. Oncotarget. 6:34309–34320.
2015.PubMed/NCBI
|
|
28
|
Wang ZQ, Cai Q, Hu L, He CY, Li JF, Quan
ZW, Liu BY, Li C and Zhu ZG: Long noncoding RNA UCA1 induced by SP1
promotes cell proliferation via recruiting EZH2 and activating AKT
pathway in gastric cancer. Cell Death Dis. 8:e28392017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Li P, Chen T, Gao G, Chen X, Du Y,
Zhang R, Yang R, Zhao W, Dun S, et al: Expression of microRNA-96
and its potential functions by targeting FOXO3 in non-small cell
lung cancer. Tumour Biol. 36:685–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Kong X, Li J, Luo Q, Li X, Shen
L, Chen L and Fang L: miR-96 promotes tumor proliferation and
invasion by targeting RECK in breast cancer. Oncol Rep.
31:1357–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu L, Pu X, Wang Q, Cao J, Xu F, Xu LI and
Li K: miR-96 induces cisplatin chemoresistance in non-small cell
lung cancer cells by downregulating SAMD9. Oncol Lett. 11:945–952.
2016.PubMed/NCBI
|
|
32
|
Xia H, Chen S, Chen K, Huang H and Ma H:
miR-96 promotes proliferation and chemo-or radioresistance by
down-regulating RECK in esophageal cancer. Biomed Pharmacother.
68:951–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu D, He X, Chang Y, Xu C, Jiang X, Sun S
and Lin J: Inhibition of miR-96 expression reduces cell
proliferation and clonogenicity of HepG2 hepatoma cells. Oncol Rep.
29:653–661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai P, Yang T, Jiang X, Zheng M, Xu G and
Xia J: Role of miR-15a in intervertebral disc degeneration through
targeting MAP3K9. Biomed Pharmacother. 87:568–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Lv G, Li J, Wang B, Zheng Q and Lu
C: LncRNA-RP11-296A18.3/miR-138/HIF1A pathway regulates the
proliferation ECM synthesis of human nucleus pulposus cells
(HNPCs). J Cell Biochem. May 24–2017.doi: 10.1002/jcb.26166. (Epub
ahead of print).
|
|
36
|
Li Z, Yu X, Shen J, Chan MT and Wu WK:
microRNA in intervertebral disc degeneration. Cell Prolif.
48:278–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Madden HP, Breslin RJ, Wasserkrug HL,
Efron G and Barbul A: Stimulation of T cell immunity by arginine
enhances survival in peritonitis. J Surg Res. 44:658–663. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu P, Wu D, You Y, Sun J, Lu L, Tan J and
Bie P: miR-208-3p promotes hepatocellular carcinoma cell
proliferation and invasion through regulating ARID2 expression. Exp
Cell Res. 336:232–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang L, Wang W, Li X, He S, Yao J, Wang
X, Zhang D and Sun X: microRNA-155 promotes tumor growth of human
hepatocellular carcinoma by targeting ARID2. Int J Oncol.
48:2425–2434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aso T, Uozaki H, Morita S, Kumagai A and
Watanabe M: Loss of ARID1A, ARID1B, and ARID2 expression during
progression of gastric cancer. Anticancer Res. 35:6819–6827.
2015.PubMed/NCBI
|
|
41
|
Duan Y, Tian L, Gao Q, Liang L, Zhang W,
Yang Y, Zheng Y, Pan E, Li S and Tang N: Chromatin remodeling gene
ARID2 targets cyclin D1 and cyclin E1 to suppress hepatoma cell
progression. Oncotarget. 7:45863–45875. 2016. View Article : Google Scholar : PubMed/NCBI
|