Introduction
Venous thromboembolism is a severe life-threatening
disease that comprises deep vein thrombosis and pulmonary embolism
that significantly affects the life quality of patients with venous
thrombosis (1,2). Venous thrombosis is the most common
disorder disease, which associates with various molecular
mechanisms and diverse processes (3). A previous study indicated that
anticoagulation treatments are the most common used and efficient
drugs for the prevention and treatment adults and children
undergone catheter-related thrombosis in clinical (4). Many factors can induce the pathology
of venous thromboembolism main including vascular endothelial cell
injury, blood flow state changes and the increase of blood
clotting, which can easily cause venous thrombosis (5). Importantly, venous thromboembolism
can lead to a large number diseases, such as pulmonary embolism,
neurologic diseases and skin disease (6–8).
Previously, changes in hydrogen sulfide and homocysteine expression
levels lead to significant clinical directive significance for the
patients with vein thrombosis (9,10).
Currently, hydrogen sulfide can impact function of
platelets that is enzymatically generated by platelets (11). Evidence has suggested that
platelets serve important roles in hemostasis and thrombosis via
circulating blood elements through activated by various stimuli
(12). Hydrogen sulfide from
slow-release compounds inhibits aggregation and exerts
anti-thrombotic effects of platelets to in vivo (13). Theoretically, hydrogen sulfide acts
as a potentially negative regulator of thrombosis through
regulation of platelet activation (14). Research has also indicated that
hydrogen sulfide is identified as an indicator of physiology of
venous thromboembolism, myocardial infarction and stroke (15). In addition, the association of
cystathionine γ-lyase (CGL) enzyme can be used to evaluate the risk
of retinal vein thrombosis (16).
Moreover, a previous study suggested that hydrogen sulfide
generation in the endothelium may be a potential target for the
treatment of arterial thrombosis (17). Therefore, the authors assumed that
hydrogen sulfide generation may contribute to underlying the
pathological characteristics of vein thrombosis.
The functions of hemostasis as an independent risk
factor for venous thrombosis still remain controversial. Previous
studies have presented the relationships between homocysteine and
deep vein thrombosis (18,19). The correlation of homocysteine and
vascular thrombosis disease has been investigated in several
clinical studies that demonstrated an increasing plasma total
homocysteine (20,21). Ravari et al (22) indicated that serum homocysteine in
deep venous thrombosis is higher than in healthy volunteers.
Potential mechanisms of homocysteine-mediated thrombosis and
angiostasis in vascular pathobiology have been reviewed in a
previous study (23). Therefore, a
large number of drug targets for the regulation of homocysteine
plasma concentration and its regulatory enzyme were put forward to
improve the treatment of pathological characteristics of vein
thrombosis.
In the present study, the authors investigated the
efficacies and molecular mechanism of edoxaban-mediated
differentiation changes of hydrogen sulfide and homocysteine in
vein endothelial cells and in rats with venous thrombosis. The data
suggested that edoxaban can significantly improve pathological
characteristics of venous thrombosis by decreasing hydrogen sulfide
and homocysteine through the phosphoinositide 3-kinase
(PI3K)/protein kinase B (AKT) signaling pathway. These findings
indicated that edoxaban may be a potential anti-thrombosis drug for
the treatment of venous thrombosis.
Materials and methods
Ethics statement
The rat experiments were implemented legitimately
according to the Guide for the Care and Use of Laboratory Animals,
and were approved by the People's Hospital of Xinjiang Uygur
Autonomous Region (Urumqi, China). All patients were recruited
voluntarily and signed an agreement in a written form. All surgical
operations and euthanasia were made to minimize suffering.
Cells culture and reagents
Vein endothelial cells (VECs) were isolated from
Sprague-Dawley rats (n=40; male; age, 12 months; weight, 250–300 g)
with ferric chloride-induced liver fibrosis. VECs were cultured in
Dulbecco's modified Eagle's medium (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) supplemented with 10% FBS (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). All cells were cultured
in a 37°C humidified atmosphere of 5% CO2.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from VECs was extracted using RNAeasy Mini
kit (Qiagen GmbH, Hilden, Germany). A total of 1 µg total RNA was
used to synthesize cDNA by performing the SuperScript II
First-Strand Synthesis system (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Gene expression levels were measured by RT-qPCR. All the forward
and reverse primers were synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. All mRNA was quantified by using Power SYBR Green
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Primers were designed as follows: Hydrogen sulfide, forward,
5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse,
5′-GGTGAGCATTATCACCCAGAA-3′; homocysteine, forward,
5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse,
5′-GGTGAGCATTATCACCCAGAA-3′; β-actin, forward,
5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse,
5′-GGTGAGCATTATCACCCAGAA-3′. The PCR thermocycling conditions were
as follows: 95°C for 5 min, then 35 cycles of 95°C for 20 sec, 58°C
for 20 sec and 72°C for 20 sec, and a final extension at 72°C for 5
min. β-actin was used as the internal reference gene. Relative mRNA
expression levels were calculated by 2−ΔΔCq (24). The results analyzed in triplicate
according to the 2−ΔΔCq method as the n-fold way
compared to control.
Western blotting
Vein endothelial cells were isolated from
experimental mice and homogenized in lysate buffer containing
protease-inhibitor and were centrifuged at 7,104 × g at 4°C for 10
min. The supernatant of mixture was used for analysis of purpose
protein. Proteins were extracted using NP40 lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) and quantified using the
standard bicinchoninic acid method (Thermo Fisher Scientific,
Inc.). SDS-PAGE assays were performed as previously described
(25). For western blotting,
primary antibodies against cystathionine β-synthase (CBS; 1:1,000;
cat no. ab96252), CGL (1:1,000; cat no. ab131052), E26
transformation-specific (ETS; 1:1,000; cat no. ab26096), PI3K
(1:1,000; cat no. ab74136), AKT (1:1,000; cat no. ab38449),
phospho-AKT (1:1,000; cat no. ab8805), matrix metalloproteinase-9
(MMP-9; 1:5,000; cat no. ab38898) (all from Abcam, Cambridge, UK),
ADP-dependent glucokinase precursor (1:1,000; cat no. ABP56684;
Abbkine Scientific Co., Ltd., Wuhan, China), plasminogen activator
inhibitors (PAIs; 1:1,000; cat no. ab169550), von Willebrand factor
(vWf; 1:500; cat no. ab6994), thromboxane-A2 (TH-A2; 1:1,000; cat
no. ab137607) and β-actin (1:1,000; cat no. ab8226) (all from
Abcam) were added after blocking (5% skimmed milk) for 1 h at 37°C
and then incubated with horseradish peroxidase-conjugated rabbit
anti-rat IgG secondary antibodies (1:1,000; cat no. ab6734; Abcam)
for 24 h at 4°C. The results were visualized using the ChemiDoc XRS
system with Quantity One software v2.9 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Protein expression was analyzed using BandScan
5.0 software (Glyko, Inc., Novato, CA, USA) and all experiments
were repeated at least three times.
Animals study
The Sprague-Dawley male rats (n=40; male; age, 12
months; weight, 250–300 g) were purchased from Vital River
Laboratory Animal Technology Co., Ltd. (Shanghai, China). All mice
were free to access food and water, and housed with a 12 h
light-dark artificial cycle. To develop venous thrombosis, ferric
chloride was used to induce Sprague-Dawley rat venous thrombosis
according to a previous study (25). Rats with venous thrombosis were
divided into two groups and received treatment with edoxaban (10
mg/kg) or the same dose of PBS. The treatments were continued for
60 days and received drugs once a day. On day 60, rats were
sacrificed and the venous samples were obtained for further
analysis.
CBS and CGL activities
Activities of CBS and CGL were analyzed in
microvascular endothelial cells following treatment with edoxaban
(10 mg/l) according previous reports (26,27).
Histological analysis
The cerebral vein was isolated from experimental
rats and then fixed with 4% paraformaldehyde and permeabilized by
incubating with absolute ethanol. Antigen retrieval was performed
before performing immunofluorescent staining. The cerebral vein
sections were incubated with goat anti-murine primary antibodies
(Cell Signaling Technology, Inc., Danvers, MA, USA) for 12 h at
4°C. Fluorescent labeled Alexa Fluor 488 (Molecular Probes; Thermo
Fisher Scientific, Inc.) rabbit anti-goat secondary antibodies
(1:1,000; cat no. ab6741; Abcam) were used to stain the proteins.
The histological sections were subsequently scanned by a confocal
microscope (Zeiss 510; Zeiss GmbH, Jena, Germany).
ELISA
The plasma concentration levels of hydrogen sulfide
(cat no. 0-009160) and homocysteine (cat no. 0-011940) (Jianglai
Biotechnology Co., Ltd., Shanghai, China) in patients with venous
thrombosis and rat model of venous thrombosis were analyzed by
commercialized ELISA kits. The operational procedures were
performed by the manufacturer's instructions. The results were
analyzed using the ELISA reader system (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are presented as mean ± standard error of
the mean and was conducted in triplicate. Statistical differences
between experimental groups were analyzed by Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of hydrogen sulfide
and homocysteine in patients with venous thrombosis and rat model
of venous thrombosis
To investigate the molecular mechanism of hydrogen
sulfide and homocysteine in the progression of venous thrombosis, a
murine model of venous thrombosis were established and patients
with venous thrombosis were recruited to analyze the expression
levels of hydrogen sulfide and homocysteine. Plasma concentration
levels of hydrogen sulfide were downregulated in patients with
venous thrombosis compared to healthy volunteers (Fig. 1A). Expression levels of
homocysteine were higher in patients with venous thrombosis
compared to healthy volunteers (Fig.
1B). Results demonstrated expression levels of hydrogen sulfide
and homocysteine (Fig. 1C and D)
in the rat model of venous thrombosis (MMVH) presented the same
downregulation in expression as CBS and CGL (Fig. 1G). It was observed that CBS and CGL
expression levels and activities were markedly downregulated in
patients with venous thrombosis (Fig.
1E and F). Results also demonstrated that CBS and CGL
expression levels and activities were also significantly
downregulated in a rat model of venous thrombosis (Fig. 1G and H). Taken together, these data
suggested that hydrogen sulfide and homocysteine are downregulated
in patients with venous thrombosis.
Edoxaban regulates hydrogen sulfide
and homocysteine levels in vein endothelial cells
To explore the underlying molecular mechanism of
upregulation of hydrogen sulfide and homocysteine, vein endothelial
cells were used to analyze the efficacy of edoxaban in
vitro. As presented in Fig. 2A and
B, edoxaban treatment upregulated hydrogen sulfide and
downregulated homocysteine mRNA expression levels may be regulated
by methionine metabolism disorder in vein endothelial cells. In
addition, transsulfuration enzymes, CBS and CGL levels were
upregulated in edoxaban-treated murine microvascular endothelial
cells (Fig. 2C-E). Results
demonstrated that CBS and CGL activities were upregulated in rat
microvascular endothelial cells, and following treatment with
edoxaban (Fig. 2F and G).
Furthermore, edoxaban enhanced the activity of vein endothelial
cells following 48 h treatment (Fig.
2H). Taken together, these data suggested that edoxaban
upregulates hydrogen sulfide and homocysteine expression levels in
vein endothelial cells.
Edoxaban regulates the hydrogen
sulfide and homocysteine activities through MMP9-induce PI3K/AKT
signaling pathway
MMP-9 expression level and activity and
homocysteine-hydrogen sulfide metabolism were increased in murine
microvascular endothelial cells following edoxaban incubation.
Expression levels of PI3K and AKT were increased in
edoxaban-incubated microvascular endothelial cells (Fig. 3A and B). Phosphorylation of AKT
also was upregulated in microvascular endothelial cells following
edoxaban incubation (Fig. 3C).
MMP-9 expression level was also increased in edoxaban-treated
microvascular endothelial cells (Fig.
3D). In addition, the edoxaban induced increase of PI3K, AKT
and MMP-9 levels was canceled by the PI3K inhibitor LY294002
(PI3KIR) in microvascular endothelial cells (Fig. 3E-G). Notably, PI3KIR markedly
inhibited hydrogen sulfide and homocysteine activities in
microvascular endothelial cells (Fig.
3H). Taken together, these findings indicated that edoxaban can
regulate the hydrogen sulfide and homocysteine activities through
the MMP9-induced PI3K/AKT signaling pathway.
The in vivo effects of edoxaban on rat
with ferric chloride-induced venous thrombosis
In a rat glycyrrhizin-induced venous thrombosis
model, the authors examined the therapeutic effects of edoxaban
determined by changes of venous lesions. It was first demonstrated
that edoxaban significantly improved ferric chloride-induced venous
thrombosis in rat with venous thrombosis (Fig. 4A). Plasma concentration levels of
hydrogen sulfide and homocysteine were also increased in
edoxaban-treated rat with ferric chloride-induced venous thrombosis
(Fig. 4B and C). Additionally,
transsulfuration enzymes, CBS and CGL activities in microvascular
endothelial cells were upregulated in experimental mice following
edoxaban treatment (Fig. 4D-F).
Furthermore, it was observed that ADP, PAIs, vWF and TH-A2 protein
levels were markedly downregulated in microvascular endothelial
cells following edoxaban treatment compared to the control
(Fig. 4G). Importantly,
apolipoprotein and thrombomodulin levels were decreased in
microvascular endothelial cells following edoxaban treatment
compared to control (Fig. 4H).
Taken together, these results demonstrated that edoxaban is an
efficient anti-thrombosis drug for the treatment of ferric
chloride-induced venous thrombosis.
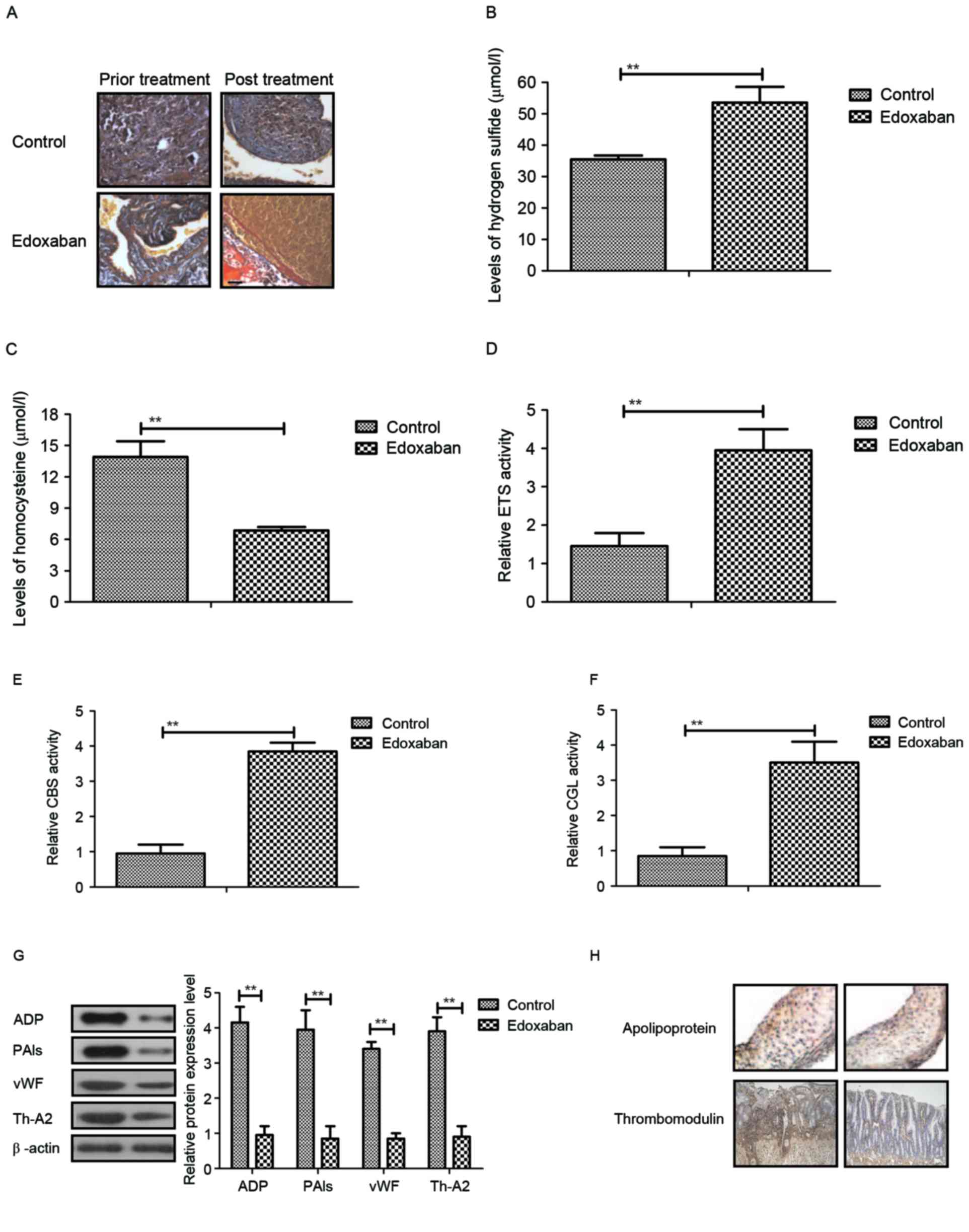 | Figure 4.In vivo effects of edoxaban in
rats with ferric chloride-induced venous thrombosis. (A) Venous
lesions in rat with ferric chloride-induced venous thrombosis
following receiving edoxaban treatment (magnification, ×40). Plasma
concentration levels of (B) hydrogen sulfide and (C) homocysteine
in rats with ferric chloride-induced venous thrombosis treated by
edoxaban. (D) ETS, (E) CBS and (F) CGL activities in microvascular
endothelial cells in rats with ferric chloride-induced venous
thrombosis treated by edoxaban. (G) ADP, PAIs, vWF and Th-A2
protein levels in microvascular endothelial cells following
edoxaban treatment. (H) Analysis of apolipoprotein and
thrombomodulin expression levels in microvascular endothelial cells
following treatment with edoxaban (magnification, ×40). The data
are presented as the mean ± standard error of the mean of three
independent experiments. **P<0.01 as indicated. ETS, E26
transformation-specific; PAIs, plasminogen activator inhibitors;
vWF, von Willebrand factor; Th-A2, thromboxane-A2; CBS,
cystathionine beta-synthase; CGL, cystathionine γ-lyase. |
Discussion
Currently, more and more new generations of
target-specific oral anticoagulants are being developed to prevent
and treat the increasing patients at risk of venous thrombosis
(28,29). These oral anticoagulants present
different functions in the treatment of patients with atrial
fibrillation (30). Like many new
generations of target-specific anticoagulants, edoxaban is a
fast-acting oral anticoagulant, which has been used to prevent
stroke in patients with nonvalvular atrial fibrillation and in
treating acute venous thrombosis through selectively inhibiting
factor Xa (31). Edoxaban is
currently approved in Japan and the USA for the prevention and
treatment of venous thromboembolism following major orthopaedic
surgery (32). These data
suggested that edoxaban can improve ferric chloride-induced venous
thrombosis via regulation of hydrogen sulfide and homocysteine
activities through the MMP-9-induced PI3K/AKT signaling
pathway.
A previous clinical trial demonstrated that hydrogen
sulfide and homocysteine activities serve a crucial role in the
progression of stroke and venous thrombosis (33). Reports have revealed that
methionine metabolism disorder could lead to the upregulation of
homocysteine in vein endothelial cells in patients with venous
thrombosis (34,35). The current investigations have
exhibited that hydrogen sulfide and homocysteine activities and
expression levels are downregulated in patients or animals with
venous thrombosis. A previous study indicated that ADP can induce
platelet aggregation and further leads to thrombin (36). Additional studies have explored the
antithrombotic therapy targeting for ADP to improve the ischemic
disorders (37,38). PAIs are another type of
pro-thrombosis factor and Lenicek Krleza et al (39) indicated that upregulation of PAIs
contribute to deep venous thrombosis with consequent pulmonary
embolism in a case report. In addition, Yaroglu Kazanci et
al (40) have indicated that
PAI concentration levels were increased in patients with cerebral
infarction and femoral venous thrombosis. Polymorphisms and
mutations in the vWF gene and its plasma levels in patients with
deep venous thrombosis have been investigated and presented an
upregulation trend in patients with venous thrombosis (41). Additionally, TH-A2 protein levels
also can affect the activation of platelet in venous thrombosis
(42). The results have indicated
that Edoxaban can improve expression levels of ADP, PAIs, vWF and
TH-A2 protein levels both in vitro and in vivo.
Notably, the data suggested that edoxaban regulates these
thrombosis factors through the MMP9-inducedPI3K/AKT signaling
pathway.
A previous report indicated that the PI3K/AKT
signaling pathway serve multiple functions in the progression of
disease occurrence (43). Edoxaban
treatment increases PI3K and AKT expression levels in microvascular
endothelial cells and rat with ferric chloride-induced venous
thrombosis. Although the safety profile of edoxaban has been
investigated in previous reports, the edoxaban-mediated PI3K/AKT
signaling pathway has not been analyzed in previous studies.
Guidetti et al (44)
suggested that PI3K/AKT is stimulated by integrin engagement,
further inhibiting activation of platelets in thrombus formation
and stabilization, which highlights the possibility of venous
thrombosis and anti-thrombotic therapeutic strategies. Su et
al (45) also indicated that
human cathelicidin LL-37 can inhibit the aggregation of platelets
and further lead to inhibition of thrombosis via Src/PI3K/Akt
signaling. Blood activity of platelets serves a crucial role in
hemostasis and formation of thrombosis (46). In the present analysis, the authors
reported that PI3K and AKT expression levels were upregulated by
edoxaban through MMP-9 expression. However, inhibition of PI3K by
its inhibitor suppresses PI3K and AKT expression levels and
inhibits hydrogen sulfide and homocysteine activities in
microvascular endothelial cells.
In conclusion, these novel findings revealed
interesting insights to explore more efficient preclinical
mechanism and therapeutic strategies of venous thrombosis. The
results not only provide preclinical and experimental evidences to
support the efficacy of edoxaban, but also elaborate the molecular
mechanism of edoxaban-mediated changes of hydrogen sulfide and
homocysteine through the PI3K/AKT signaling pathway in ferric
chloride-induced venous thrombosis. The results indicated that
edoxaban can improve the venous lesions by upregulating hydrogen
sulfide and homocysteine activities via the MMP-9-mediated PI3K/AKT
signaling pathway, suggesting edoxaban may be an efficient
anti-thrombosis agent for the treatment of venous thrombosis in the
clinic.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. U1503121).
References
|
1
|
Shlebak A: Antiphospholipid syndrome
presenting as cerebral venous sinus thrombosis: A case series and a
review. J Clin Pathol. 69:337–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Samos M, Bolek T, Ivanková J, Stančiaková
L, Kovář F, Galajda P, Kubisz P, Staško J and Mokáň M: Heparin
induced thrombocytopenia presenting with deep venous thrombosis and
pulmonary embolism successfully treated with rivaroxaban: Clinical
case report and review of current experiences. J Cardiovasc
Pharmacol. 68:391–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jaqua NT, Stratton A, Yaccobe L, Tahir U,
Kenny P and Kerns T: A review of the literature on three
extraintestinal complications of ulcerative colitis: An ulcerative
colitis flare complicated by Budd-Chiari syndrome, cerebral venous
thrombosis and idiopathic thrombocytopenia. Acta Gastroenterol
Belg. 76:311–316. 2013.PubMed/NCBI
|
|
4
|
Barco S, Atema JJ, Coppens M, Serlie MJ
and Middeldorp S: Anticoagulants for the prevention and treatment
of catheter-related thrombosis in adults and children on parenteral
nutrition: A systematic review and critical appraisal. Blood
Transfus. 15:369–377. 2017.PubMed/NCBI
|
|
5
|
Hawbaker S: Venous thromboembolism in the
cancer population: Pathology, risk, and prevention. J Adv Pract
Oncol. 3:23–33. 2012.PubMed/NCBI
|
|
6
|
Vitale C, D'Amato M, Calabrò P, Stanziola
AA, Mormile M and Molino A: Venous thromboembolism and lung cancer:
A review. Multidiscip Respir Med. 10:282015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ungprasert P, Tanratana P and Srivali N:
Autoimmune hemolytic anemia and venous thromboembolism: A
systematic review and meta-analysis. Thromb Res. 136:1013–1017.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernandez MM, Hogue S, Preblick R and
Kwong WJ: Review of the cost of venous thromboembolism. Clinicoecon
Outcomes Res. 7:451–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Corral J, Huntington JA, González-Conejero
R, Mushunje A, Navarro M, Marco P, Vicente V and Carrell RW:
Mutations in the shutter region of antithrombin result in formation
of disulfide-linked dimers and severe venous thrombosis. J Thromb
Haemost. 2:931–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaya A, Gómez I, Mira Y, Ferrando F and
Corella D: Homocysteine levels in patients with deep vein
thrombosis lacking thrombophilic defects. Thromb Haemost.
99:1132–1134. 2008.PubMed/NCBI
|
|
11
|
Xue M, Yin H, Zhang L, Guo C, Jiang Y, Wu
C, Li X and Chen K: Dynamic expression of the main related
indicators of thrombosis, inflammatory reaction and tissue damage
in a rat model of myocardial infarction. Mol Med Rep. 4:693–696.
2011.PubMed/NCBI
|
|
12
|
Cao H, Zhang L, Sun ZB, Cheng XH, Zhang Y
and Zou HB: Salvia miltiorrhiza prevents deep vein thrombosis via
antioxidative effects in endothelial cells. Mol Med Rep.
11:3593–3600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li G, Han ZL, Dong HG, Zhang X, Kong XQ
and Jin X: Platelet endothelial cell adhesion molecule-1 gene
125C/G polymorphism is associated with deep vein thrombosis. Mol
Med Rep. 12:2203–2210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye S, Mao B, Yang L, Fu W and Hou J:
Thrombosis recanalization by paeoniflorin through the upregulation
of urokinase-type plasminogen activator via the MAPK signaling
pathway. Mol Med Rep. 13:4593–4598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Emerson M: Hydrogen sulfide and platelets:
A possible role in thrombosis. Handb Exp Pharmacol. 230:153–162.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghaznavi H, Soheili Z, Samiei S and
Soltanpour MS: Plasma homocysteine levels, methylene
tetrahydrofolate reductase A1298C gene polymorphism and risk of
retinal vein thrombosis. Blood Coagul Fibrinolysis. 27:679–683.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin YR, You SJ, Zhang Y, Li Q, Wang XH,
Wang F, Hu LF and Liu CF: Hydrogen sulfide attenuates ferric
chloride-induced arterial thrombosis in rats. Free Radic Res.
50:654–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ekim M, Ekim H, Yilmaz YK, Kulah B, Polat
MF and Gocmen AY: Study on relationships among deep vein
thrombosis, homocysteine and related B group vitamins. Pak J Med
Sci. 31:398–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fay WP: Homocysteine and thrombosis: Guilt
by association? Blood. 119:2977–2978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Minno MN, Tremoli E, Coppola A, Lupoli
R and Di Minno G: Homocysteine and arterial thrombosis: Challenge
and opportunity. Thromb Haemost. 103:942–961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahadeo KM, Dhall G, Panigrahy A, Lastra C
and Ettinger LJ: Subacute methotrexate neurotoxicity and cerebral
venous sinus thrombosis in a 12-year-old with acute lymphoblastic
leukemia and methylenetetrahydrofolate reductase (MTHFR) C677T
polymorphism: Homocysteine-mediated methotrexate neurotoxicity via
direct endothelial injury. Pediatr Hematol Oncol. 27:46–52. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ravari H, Zafarghandi MR, Alvandfar D and
Saadat S: Serum homocysteine in deep venous thrombosis, peripheral
atherosclerosis and healthy Iranians: A case-control study. Pak J
Biol Sci. 12:1019–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loscalzo J: Homocysteine-mediated
thrombosis and angiostasis in vascular pathobiology. J Clin Invest.
119:3203–3205. 2009.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srivastava AK, Kalita J, Haris M, Gupta RK
and Misra UK: Radiological and histological changes following
cerebral venous sinus thrombosis in a rat model. Neurosci Res.
65:343–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weeks CL, Singh S, Madzelan P, Banerjee R
and Spiro TG: Heme regulation of human cystathionine beta-synthase
activity: Insights from fluorescence and Raman spectroscopy. J Am
Chem Soc. 131:12809–12816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai J, Shi X, Wang H, Fan J, Feng Y, Lin
X, Yang J, Cui Q, Tang C, Xu G and Geng B: Cystathionine γ
lyase-hydrogen sulfide increases peroxisome proliferator-activated
receptor γ activity by sulfhydration at C139 site thereby promoting
glucose uptake and lipid storage in adipocytes. Biochim Biophys
Acta. 1861:419–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan EY and Sobieraj DM: Considerations for
prescribing target-specific oral anticoagulants in the setting of
renal dysfunction or drug interactions. Conn Med. 80:105–111.
2016.PubMed/NCBI
|
|
29
|
Senger S, Keiner D, Hendrix P and Oertel
J: New target-specific oral anticoagulants and intracranial
bleeding: Management and outcome in a Single-Center case series.
World Neurosurg. 88:132–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kundu A, Sen P, Sardar P, Chatterjee S,
Kapoor A and McManus DD: Intracranial hemorrhage with target
specific oral anticoagulants in patients with atrial fibrillation:
An updated meta-analysis of randomized controlled trials. Int J
Cardiol. 203:1000–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hurst KV, O'Callaghan JM and Handa A: Risk
impact of edoxaban in the management of stroke and venous
thromboembolism. Vasc Health Risk Manag. 12:329–335. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Partida RA and Giugliano RP: Edoxaban:
Pharmacological principles, preclinical and early-phase clinical
testing. Future Cardiol. 7:459–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu XQ, Liu XQ, Jiang P, Huang H and Yan
Y: Plasma levels of endogenous hydrogen sulfide and homocysteine in
patients with Alzheimer's disease and vascular dementia and the
significance thereof. Zhonghua Yi Xue Za Zhi. 88:2246–2249.
2008.(In Chinese). PubMed/NCBI
|
|
34
|
Obeid OA, Johnston K and Emery PW: Plasma
taurine and cysteine levels following an oral methionine load:
Relationship with coronary heart disease. Eur J Clin Nutr.
58:105–109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klerk M, Lievers KJ, Kluijtmans LA, Blom
HJ, den Heijer M, Schouten EG, Kok FJ and Verhoef P: The 2756A>G
variant in the gene encoding methionine synthase: Its relation with
plasma homocysteine levels and risk of coronary heart disease in a
Dutch case-control study. Thromb Res. 110:87–91. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tohti I, Tursun M, Umar A, Turdi S, Imin H
and Moore N: Aqueous extracts of Ocimum basilicum L. (sweet basil)
decrease platelet aggregation induced by ADP and thrombin in vitro
and rats Arterio-venous shunt thrombosis in vivo. Thromb Res.
118:733–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Du JR, Wang J, Yu DK, Chen YS, He
Y and Wang CY: Z-ligustilide extracted from Radix Angelica sinensis
decreased platelet aggregation induced by ADP ex vivo and
Arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi.
129:855–859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bernat A, Vallee E, Maffrand JP and Gordon
JL: The role of platelets and ADP in experimental thrombosis
induced by venous stasis in the rat. Thromb Res. 52:65–70. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krleza J Lenicek, Jakovljevic G, Bronic A,
Herak D Coen, Bonevski A, Stepan-Giljevic J and Roic G:
Contraception-related deep venous thrombosis and pulmonary embolism
in a 17-year-old girl heterozygous for factor V leiden, prothrombin
G20210A mutation, MTHFR C677T and homozygous for PAI-1 mutation:
Report of a family with multiple genetic risk factors and review of
the literature. Pathophysiol Haemost Thromb. 37:24–29. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kazanci S Yaroglu, Yesilbas O, Ersoy M,
Kihtir HS, Yildirim HM and Sevketoglu E: Cerebral infarction and
femoral venous thrombosis detected in a patient with diabetic
ketoacidosis and heterozygous factor V Leiden G1691A and PAI-1
4G/5G mutations. J Pediatr Endocrinol Metab. 28:1183–1186.
2015.PubMed/NCBI
|
|
41
|
Bittar LF, de Paula EV, Mello TB, Siqueira
LH, Orsi FL and Annichino-Bizzacchi JM: Polymorphisms and mutations
in vWF and ADAMTS13 genes and their correlation with plasma levels
of FVIII and vWF in patients with deep venous thrombosis. Clin Appl
Thromb Hemost. 17:514–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tarantino E, Amadio P, Squellerio I, Porro
B, Sandrini L, Turnu L, Cavalca V, Tremoli E and Barbieri SS: Role
of thromboxane-dependent platelet activation in venous thrombosis:
Aspirin effects in mouse model. Pharmacol Res. 107:415–425. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang XP, Ding H, Lu JD, Tang YH, Deng BX
and Deng CQ: Autophagy in cerebral ischemia and the effects of
traditional Chinese medicine. J Integr Med. 13:289–296. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guidetti GF, Canobbio I and Torti M:
PI3K/Akt in platelet integrin signaling and implications in
thrombosis. Adv Biol Regul. 59:36–52. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Su W, Chen Y, Wang C, Ding X, Rwibasira G
and Kong Y: Human cathelicidin LL-37 inhibits platelet aggregation
and thrombosis via Src/PI3K/Akt signaling. Biochem Biophys Res
Commun. 473:283–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McFadyen J and Peter K: Forget about
thrombosis: Platelets and Alzheimer's disease, yet another sticky
situation. Sci Signal. 9:fs92016. View Article : Google Scholar : PubMed/NCBI
|


















