Introduction
Renal ischemia-reperfusion injury (IRI) is
characterized by tubular epithelial cell (TEC) injury and is
present in numerous diseases and following certain treatments,
including shock, renal transplantation and cardiac surgery
(1). The underlying mechanism of
IRI includes immune injury, mitochondrial dysfunction, endoplasmic
reticulum (ER) stress and caspase cascade disorders (2). Preventing TECs from undergoing
apoptosis is vital for treatment of renal IRI.
During IRI, oxidative stress disturbs redox balance
and results in aberrations in various signalling pathways. ER
stress serves a role in the progression of renal IRI (3). Oxidative stress leads to ER stress
with the accumulation of misfolded and unfolded proteins in the ER
lumen (4). ER stress is initiated
by alterations in heterologous protein-protein interactions,
including the dissociation of the chaperone binding immunoglobulin
protein (BiP). The unfolded protein response (UPR) is induced under
moderate and transient ER stress. Activation of the UPR results in
reduced ER burden and the restoration of ER equilibrium (5). NF-E2-related factor 2 (Nrf2) is a
master transcriptional regulator of antioxidant proteins, including
heme oxygenase-1 (HO-1). The Nrf2 signalling pathway is activated
during adaptive UPR, and protects TECs from oxidative
stress-induced injury (6).
However, during IRI, intensive ER stress is initiated by C/EBP
homologous protein (CHOP) accumulation, inositol-requiring enzyme 1
(IRE1) phosphorylation and c-Jun N-terminal kinase (JNK)
activation, leading to the apoptotic UPR phase.
Helix B surface peptide (HBSP), a linear peptide
derived from non-erythropoietic helix B of erythropoietin, has been
demonstrated to be a protective agent against ischemic injury
(7,8). In addition, it does not interact with
erythropoietic receptors, or promote erythropoiesis and blood
viscosity (7,8). This suggests that HBSP is a better
candidate for renal protection than erythropoietin. However, the
plasma half-life of HBSP is only 2 min, which significantly
restricts its application in vivo (8). To address this problem,
thioether-cyclized helix B peptide (CHBP) was synthesized by
employing a cyclization strategy to improve its metabolic stability
(9). The present study
demonstrated that CHBP has significant metabolic stability and may
attenuate kidney injury by reducing inflammation and apoptosis
(9). In the present study, the
effect of CHBP and the underlying mechanism in the HK-2 human renal
proximal tubular cell line was investigated, under oxidative stress
induced by H2O2, to further understand the
protective role of CHBP in renal IRI.
Materials and methods
Materials and reagents
The HK-2 human renal proximal tubular cell line was
provided by Dr Honghong Chen (Institute of Radiation Medicine,
Fudan University, Shanghai, China). Dulbecco's modified Eagle's
medium (DMEM) F12 and foetal bovine serum (FBS) were purchased from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). CHBP was
synthesized as previously described (9). The Cell Counting kit 8 (CCK-8),
glutathione/glutathione disulphide (GSH/GSSG) assay kit, reactive
oxygen species (ROS) assay kit, nuclear and cytoplasmic protein
extraction kit, Annexin V apoptosis detection kit and one-step
terminal deoxynucleotidyl transferase-mediated dUTP nick-end label
(TUNEL) apoptosis assay kit were purchased from Beyotime Institute
of Biotechnology (Haimen, China). DNA oligonucleotides were
synthesized by Shanghai BoShang Biotechnology Co., Ltd. (Shanghai,
China). Antibodies against cleaved caspase-3 (cat. no. 9661;
1:1,000), BiP (cat. no. 3177; 1:1,000), CHOP (cat. no. 5554;
1:1,000), HO-1 (cat. no. 5853; 1:1,000), beclin-1 (cat. no. 3495;
1:1,000), light chain 3 (LC3) A/B (cat. no. 12741; 1:1,000),
phosphorylated (p)-mechanistic target of rapamycin (mTOR) Ser2481
(cat. no. 2974; 1:1,000), p-mTOR Ser2448 (cat. no. 5536; 1:1,000),
p62 (cat. no. 5114; 1:1,000), mTOR (cat. no. 2972; 1:1,000), and
β-actin (cat. no. 3700; 1:1,000) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies against Nrf2
(rabbit anti-human monoclonal; cat. no. sc-722; 1:200) and lamin B
(goat anti-human monoclonal; cat. no. sc-6216; 1:200) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The secondary antibody (cat. no. P0186; 1:1,000) used in the
immunocytochemistry assay (Goat anti-Rabbit) were purchased from
Beyotime Institute of Biotechnology (Haimen, China).
Cell culture and
H2O2 treatment
HK-2 cells were cultured in DMEM F12 medium
supplemented with 10% FBS at 37°C and 5% CO2 for 24 h.
Confluent monolayers (80%) were cultures in 6-well plates and
pretreated with or without 20 nmol/l CHBP for 1 h prior to
treatment with 500 µmol/l H2O2 diluted in
serum-free media, for 4 h.
Cell viability analysis
Cell proliferation and viability were measured using
a CCK-8 assay kit. Briefly, HK-2 cells were seeded in 96-well
tissue culture plates for 24 h (8,000/well). Subsequently, cells
were pretreated with or without 10, 20 and 40 nmol/l CHBP for 1 h
prior treatment with 500 µmol/l H2O2 diluted
in serum-free media for 4 h. Control cells were treated with media
alone. Subsequently, cells were pretreated with or without 10, 20
and 40 nmol/l CHBP for 1 h prior to treatment with 500 µmol/l
H2O2 diluted in serum-free media, for 4 h.
Cells were washed twice with PBS and incubated with culture medium
containing 10% CCK-8 solution at 37°C for 1 h. The absorbance of
the wells was detected using a microplate reader at a wavelength of
450 nm, generating an optical density (OD) value. Culture medium
containing 10% CCK-8 solution was utilized as a negative
control.
Measurement of oxidative stress
ROS activity levels were determined using a
dichloro-dihydro-fluorescein diacetate assay kit. The GSH/GSSG
ratio was measured using a GSH/GSSG assay kit. HK-2 cells were
washed twice with PBS and suspended in ice-cold 5% metaphosphoric
acid. Cells were homogenized with a TissueLyser LT (Qiagen GmbH,
Hilden, Germany), and suspensions were transferred to a microtube
and centrifuged at 10,000 × g for 10 min at 4°C. The collected
supernatant was utilized to analyse GSH and GSSG concentrations in
addition to ROS levels, according to the manufacturer's
protocol.
Western blot analysis
HK-2 cells were washed twice in PBS and harvested.
Extra-nuclear and intra-nuclear proteins were isolated using a
protein extraction kit (Beyotime Institute of Biotechnology),
according to the manufacturer's protocol. Western blotting was
performed according to a previously published procedure (10). Expression levels of cleaved
caspase-3, BiP, CHOP, Nrf2, HO-1, Beclin-1, LC3 A/B, p-mTOR
Ser2448, p-mTOR Ser2481, p62 and mTOR were quantified using
Image-Pro plus software version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). Extra-nuclear proteins were normalized to
β-actin and intra-nuclear proteins were normalized to lamin B.
TUNEL assay
Apoptosis of HK-2 cells was determined using a
one-step TUNEL assay kit according to the manufacturer's protocol.
Briefly, cells were washed with PBS and fixed in 4%
paraformaldehyde for 30 min. Cells were washed with PBS and
incubated with cold PBS containing 0.1% Triton X-100 for 2 min in a
light-proof container. Cells were washed with PBS and incubated
with TUNEL reaction buffer for 1 h at 37°C in a humidified
light-proof chamber. Following a final wash with PBS, cells were
visualized using a laser scanning confocal microscope (Leica
Microsystems, Inc., Wetzlar, Germany) at a wavelength of 530/485
nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the HK-2 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. cDNA was synthesized from
3–5 µg total RNA in a 20 µl reaction mixture using a RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
The specific primers for the genes that encode human BiP, CHOP and
β-actin are listed in Table I.
RT-qPCR was performed using ABsolute qPCR SYBR Green mix (Thermo
Fisher Scientific, Inc.) on an Eppendorf Mastercycler®
ep realplex system as follows: Incubation for 2 min at 50°C and 10
min at 95°C. This was followed by a 2-step PCR program of 95°C for
15 sec and 60°C for 20 sec for 45 cycles. (Eppendorf, Hamburg,
Germany). mRNA expression levels were normalized to those of
β-actin in the same samples using the 2−ΔΔCq method
(11).
 | Table I.Primers for quantitative polymerase
chain reaction. |
Table I.
Primers for quantitative polymerase
chain reaction.
| Target | Primer (5′-3′) |
|---|
| BiP | F:
AAAGAAGACGGGCAAAGATGT |
|
| R:
TGCTTGATGCTGAGAAGACAG |
| CHOP | F:
ACCACTCTTGACCCTGCTTCT |
|
| R:
CTCTGGGAGGTGCTTGTGAC |
| β-actin | F:
GTTGTCGACGACGAGCG |
|
| R:
GCACAGAGCCTCGCCTT |
Immunocytochemistry assay
Experimental HK-2 cells were cultured in 12-well
plate (105/well) after H2O2
stimulation for 4 h were fixed in 4% paraformaldehyde for 10 min.
Following a wash with PBS for 5 min at room temperature, cells were
permeabilized with Triton X 100 for 5 min followed by PBS for 5 min
and then incubated in blocking solution (5% BSA in PBS; Beyotime
Institute of Biotechnology) for 1 h at room temperature. Cells were
incubated with primary antibodies against Nrf2 (1:200) and LC3A/B
(1:100) for 60 min. Following three rinses with PBS, cells were
incubated with a secondary antibody conjugated to fluorescein
isothiocyanate for 30 min at room temperature and washed with PBS.
To visualize cell nuclei, slides were counterstained with DAPI
(Beyotime Institute of Biotechnology). Samples were examined under
a phase contrast microscope equipped with the appropriate
fluorescence filters in a total of 10 fields per specimen.
Statistical analysis
Data was analysed using SPSS software version 13.0
(SPSS, Inc., Chicago, IL, USA). The results in two groups were
compared using two-tailed independent t-tests, and the results
among three or more groups were compared by one-way analysis of
variance. Data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
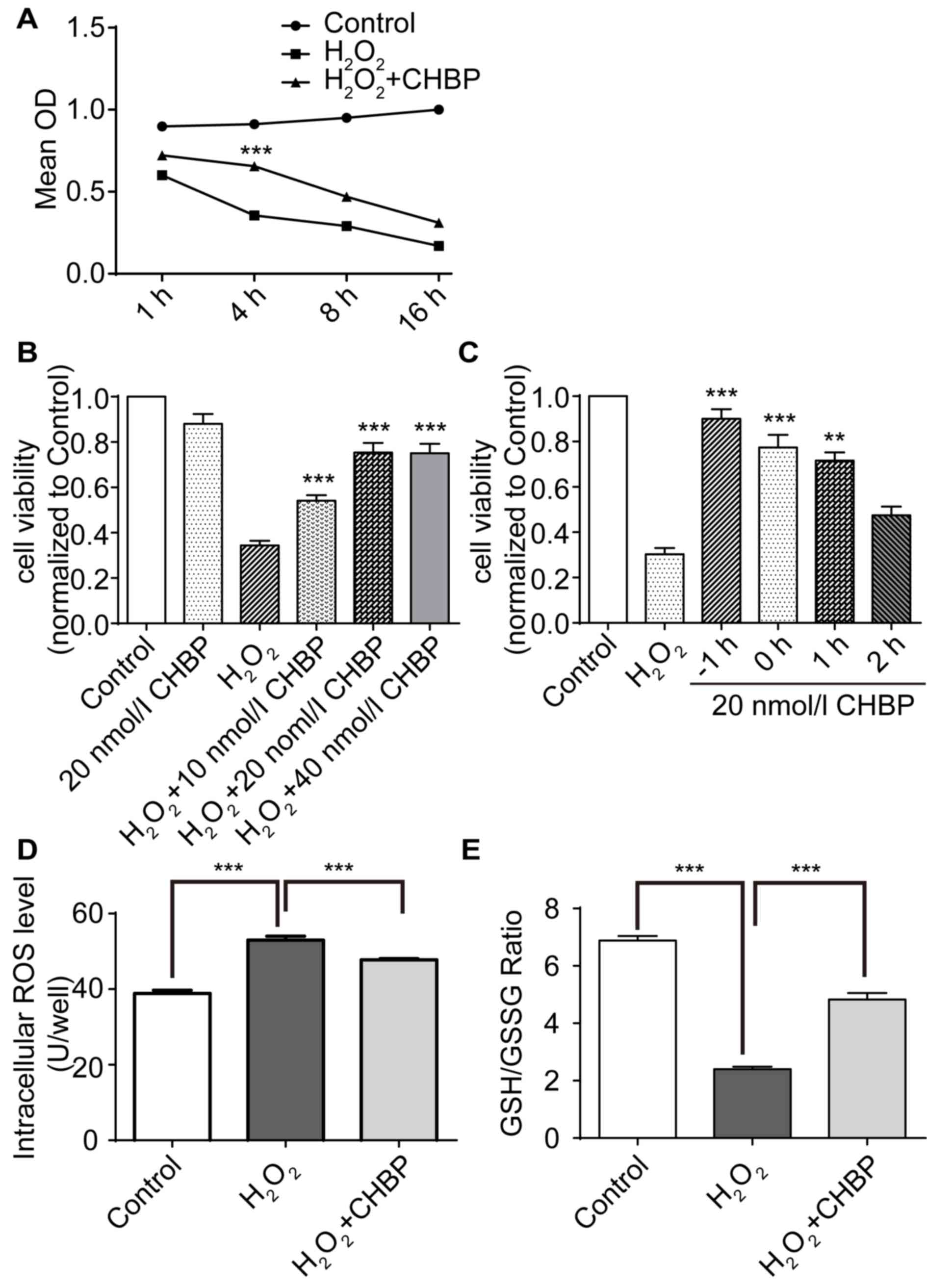
CHBP pretreatment increases HK-2 cell
viability and reduces oxidative stress levels
The effect of CHBP on viability in HK-2 cells
following oxidative stress induced by H2O2
was determined. Cells were treated with H2O2
for 1, 4, 8 and 16 h prior to measurement of cell viability via the
CCK-8 assay. Results revealed that pretreatment with CHBP
significantly reduced the decrease in cell viability caused by
H2O2 alone, and the greatest protection
observed at 4 h (Fig. 1A).
Treatment with H2O2 alone reduced the
viability of HK-2 cells by ~3-fold; however, viability of cells
treated with 20 nmol/l CHBP alone was not significantly altered
compared with the control (Fig.
1B). Pretreatment with CHBP resulted in a significant and
dose-dependent increase in the viability of HK-2 cells in the
presence of H2O2 compared with cells treated
with H2O2 alone (P<0.001; Fig. 1B). The protective effect of CHBP
peaked at 20 nmol/l (Fig. 1B). In
addition, cell viability was greatest following pretreatment with
CHBP for 1 h prior to H2O2 exposure for 4 h
(Fig. 1C). To determine whether
CHBP reduces oxidative stress induced by
H2O2, ROS activity levels and the GSH/GSSG
ratio in HK-2 cells were measured. ROS activity levels in HK-2
cells were enhanced following exposure to
H2O2 compared with the control, and this
effect was significantly reduced by CHBP pretreatment (P<0.001;
Fig. 1D). In addition, the
GSH/GSSG ratio in HK-2 cells was reduced following exposure to
H2O2 compared with the control, and this
effect was reversed by pretreatment with CHBP (P<0.001; Fig. 1E). These results suggested that
CHBP pretreatment enhances HK-2 cell viability and reduces
oxidative stress in HK-2 cells.
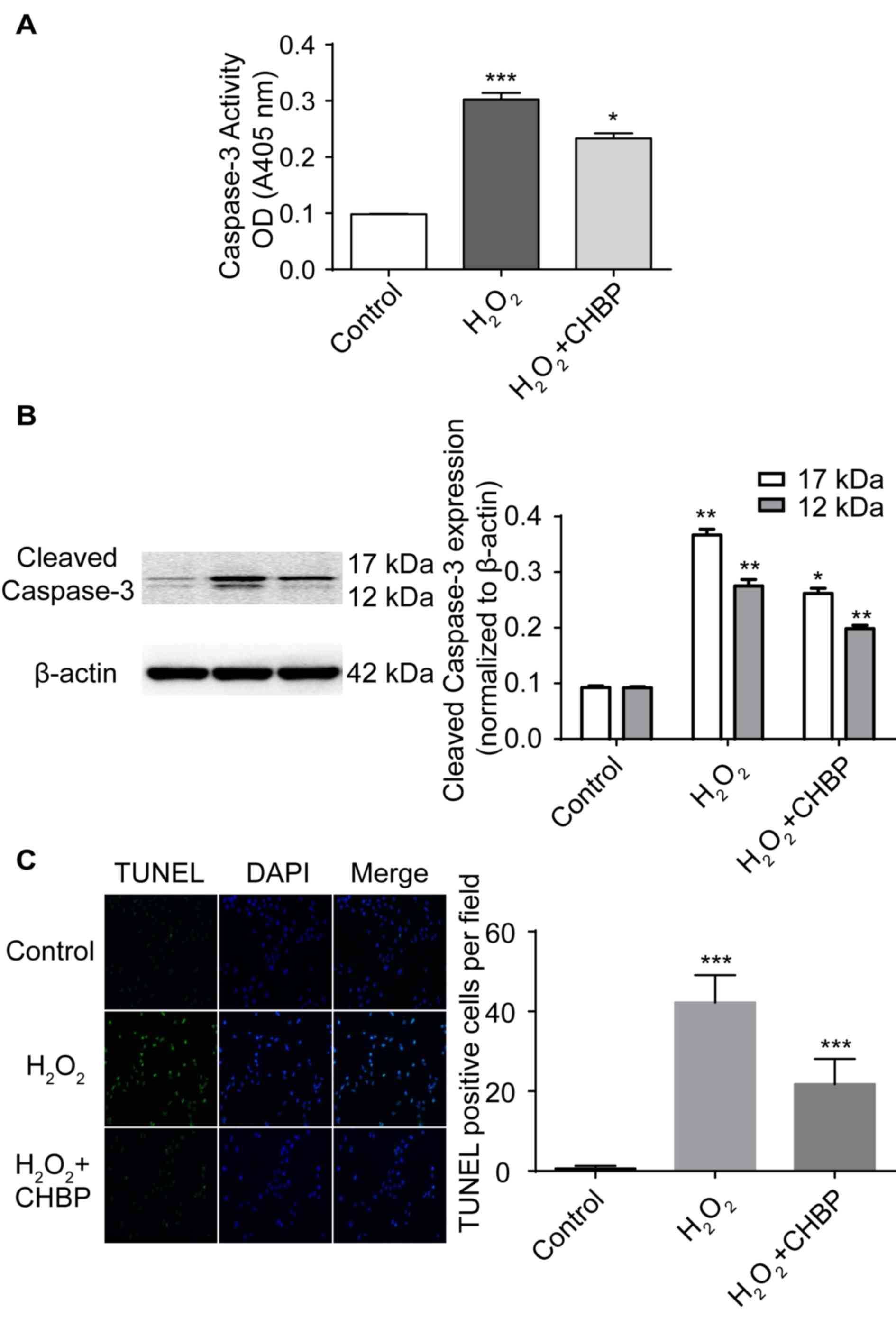
CHBP pretreatment inhibits HK-2
apoptosis induced by H2O2
To determine whether CHBP inhibits apoptosis induced
by H2O2, apoptosis in HK-2 cells was measured
following treatment. In addition, as activation of caspase-3 is a
marker for apoptosis, caspase-3 activity was measured in HK-2 cells
following treatment. The percentage of apoptotic cells was enhanced
following H2O2 treatment alone, whereas
pretreatment with 20 nmol/l CHBP in the presence of
H2O2 significantly reduced the activity
levels of caspase-3 (P<0.01; Fig.
2A). Caspase-3 and cleaved caspase-3 expression levels were
enhanced in HK-2 cells following H2O2
treatment alone, and were significantly reduced by pretreatment
with CHBP (Fig. 2B). Additionally,
Pretreatment with CHBP significantly reduced the number of TUNEL
positive cells in the presence of H2O2
(P<0.001; Fig. 2C). These
results suggested that CHBP inhibits apoptosis induced by
H2O2.
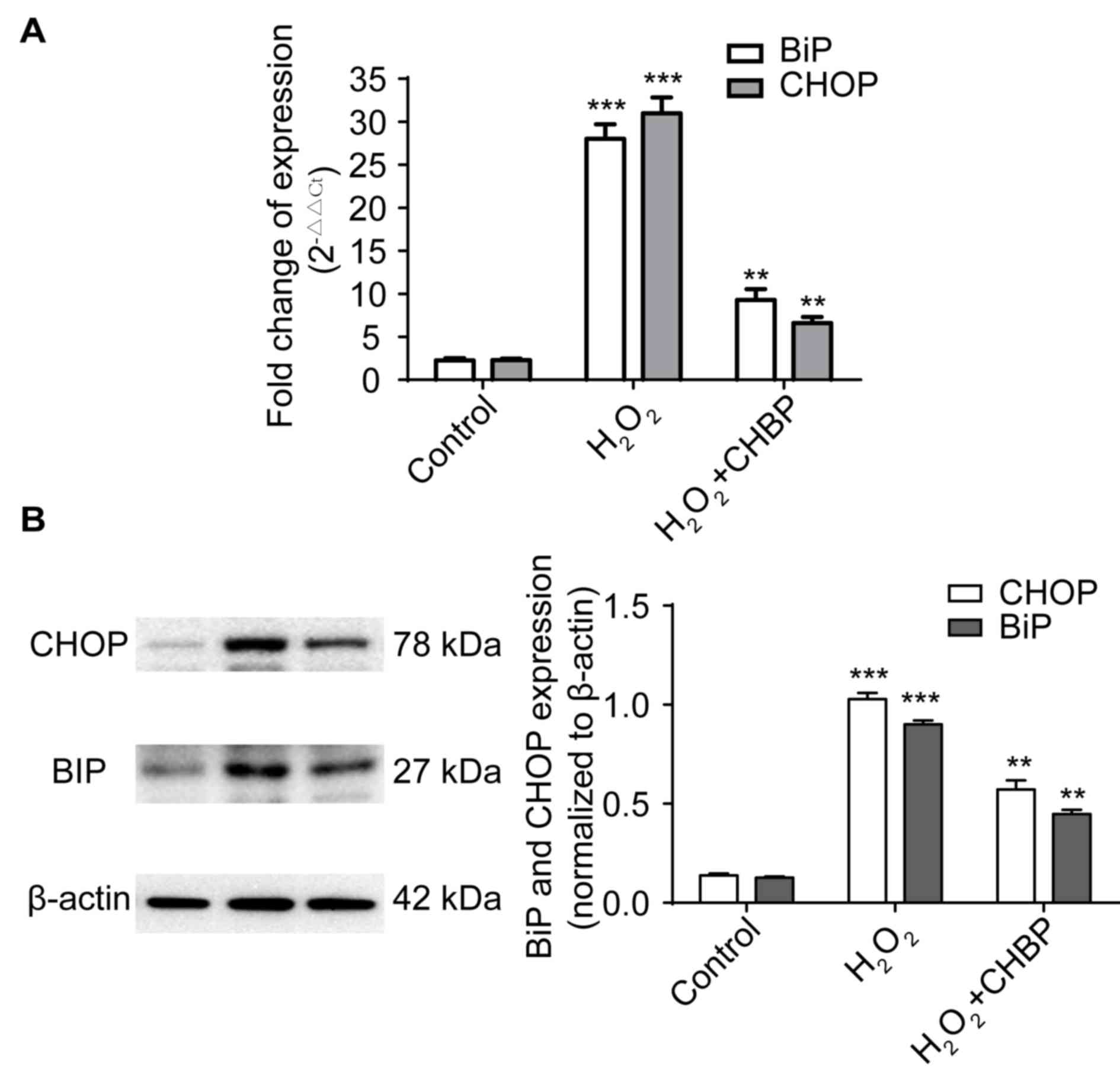
CHBP pretreatment reduces ER stress in
HK-2 cells exposed to H2O2
Elevated expression levels of BiP and CHOP are
features of ER stress (2). To
determine whether CHBP decreased ER stress in HK-2 cells, mRNA and
protein expression levels of BiP and CHOP were measured in HK-2
cells following H2O2 treatment. The mRNA
expression levels of the genes encoding BiP and CHOP were enhanced
in cells treated with H2O2 alone, and these
levels were significantly reduced by pretreatment with CHBP
(P<0.01; Fig. 3A). This was
supported by the protein expression levels of BiP and CHOP
(P<0.01; Fig. 3B). These
results suggested that CHBP pretreatment reduces ER stress in HK-2
cells exposed to H2O2.
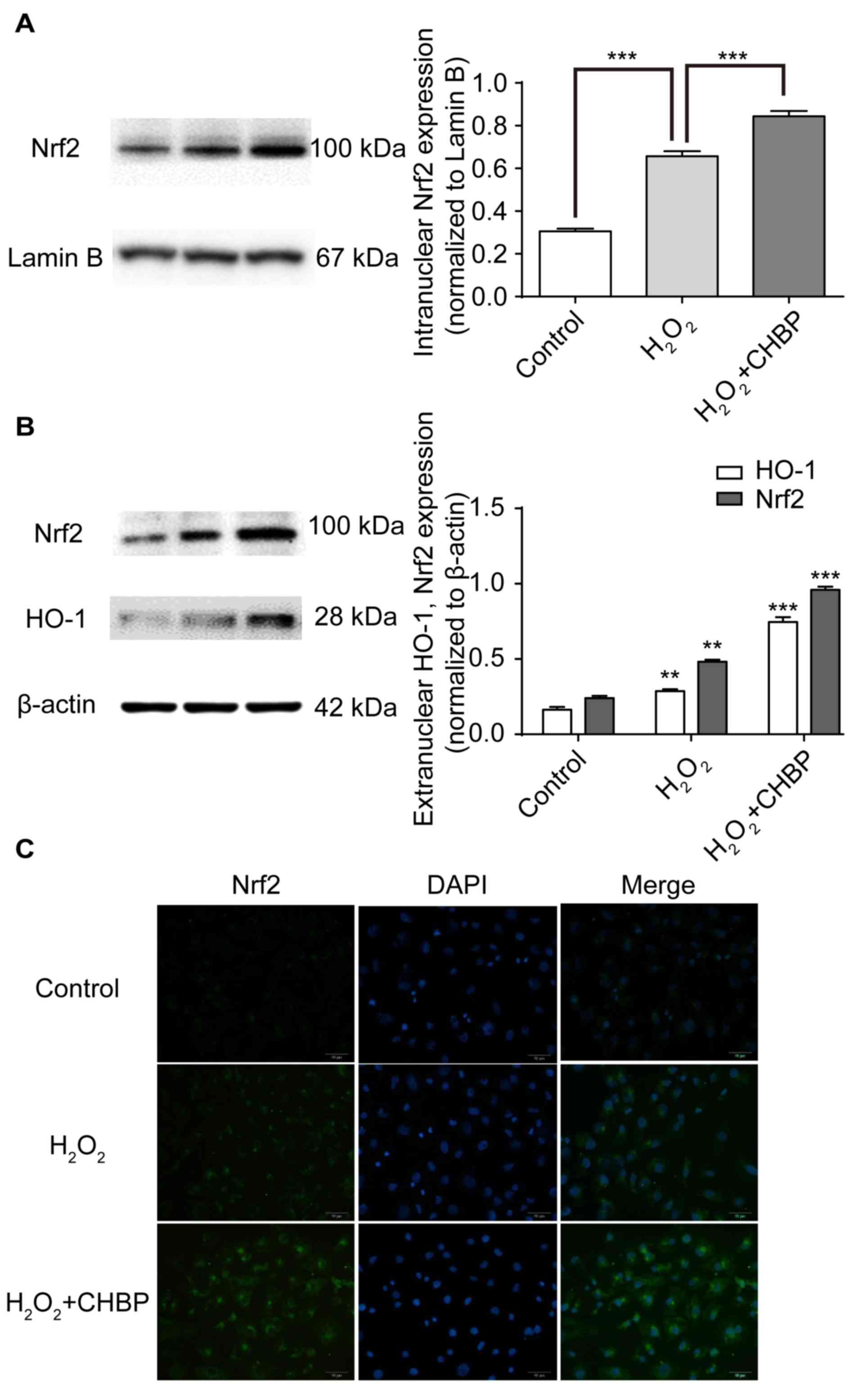
CHBP pretreatment enhances activation
of the Nrf2 signalling pathway in HK-2 cells treated with
H2O2
Nrf2 is a transcriptional regulator of antioxidant
proteins, including HO-1 (12). To
determine whether CHBP activates the Nrf2 signalling pathway in
HK-2 cells treated with H2O2, expression
levels of intranuclear and extranuclear Nrf2 and HO-1 were measured
following pretreatment with CHBP. Nrf2 expression levels were
enhanced in the nuclei of HK-2 cells after
H2O2 treatment alone, (P<0.001; Fig. 4A), and were further enhanced by
pretreatment with CHBP (P<0.001; Fig. 4A). Similar results were observed in
the extranuclear portion of HK-2 cells (P<0.001; Fig. 4B) via western blotting. In
addition, these results were supported by the immunocytochemistry
data for the Nrf2 protein (Fig.
4C). These results suggested that CHBP pretreatment enhances
activation of the Nrf2 signalling pathway in HK-2 cells exposed to
H2O2.
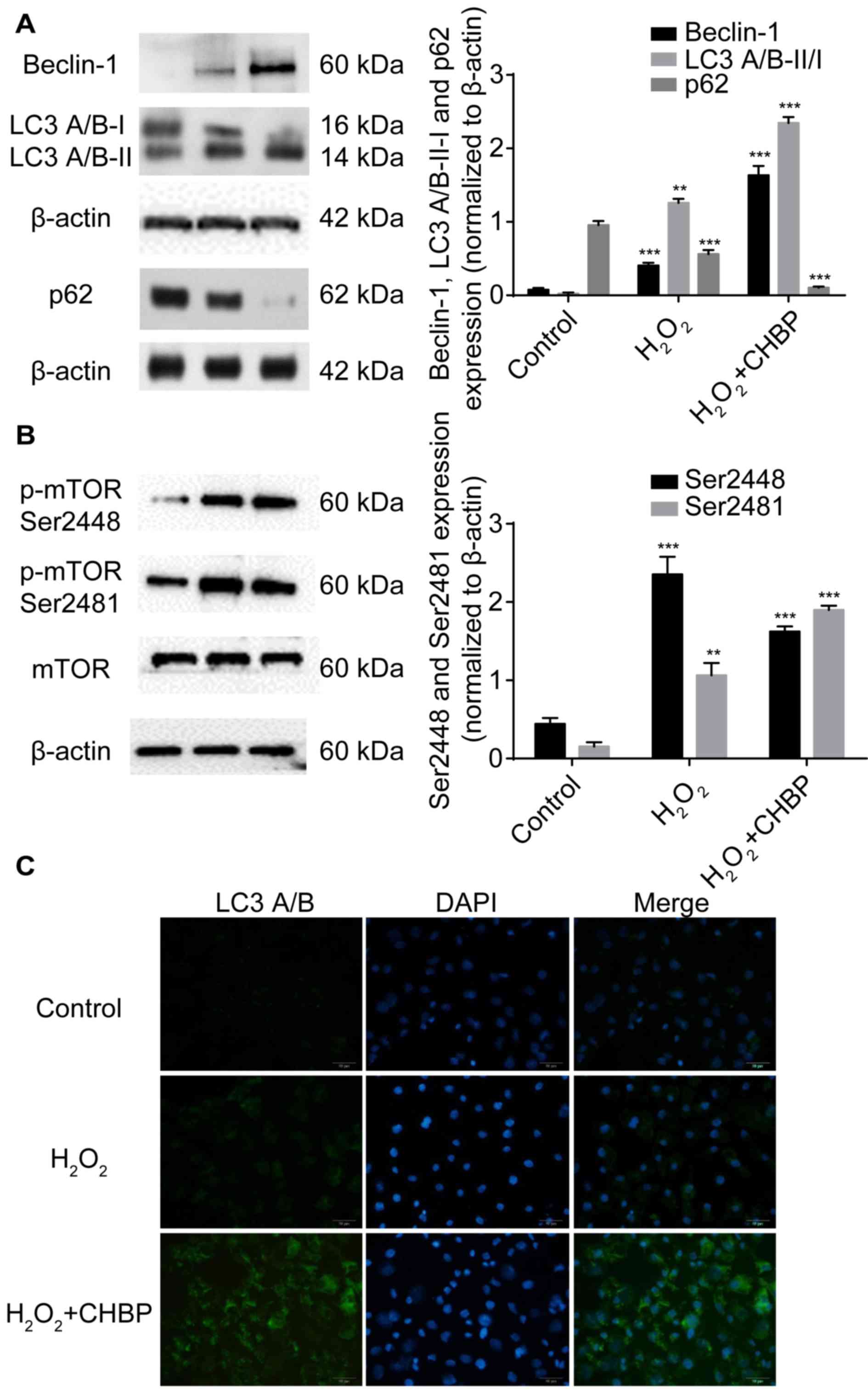
CHBP pretreatment enhances autophagy
in HK-2 cells treated with H2O2
Our previous in vivo study demonstrated that
the renoprotective effect of CHBP against IRI is mediated by
induction of autophagy via inhibition of mTOR complex (C) 1 and
activation of mTORC2 (9). As
oxidative stress may crosstalk with autophagic machinery, the
present study determined whether the renoprotective function of
CHBP is mediated by autophagy in HK-2 cells after
H2O2 treatment. Results demonstrated that
beclin-1 and LC3 A/B-II/I expression levels were significantly
enhanced, and the expression of p62 following CHBP pretreatment was
reduced much more compared with H2O2 alone
(Fig. 5A). However, the expression
of p-mTOR Ser2481 following CHBP pretreatment was increased more
than that of H2O2 alone. By contrast, p-mTOR
Ser2448 levels were enhanced in HK-2 cells after
H2O2 stimulation, and were significantly
reduced by CHBP pretreatment (Fig.
5B). These results suggested that CHBP pretreatment enhances
autophagy in HK-2 cells under oxidative stress.
Discussion
In the present study, to further evaluate the
protective role of CHBP in renal IRI, the effect and underlying
mechanism of CHBP on the HK-2 human renal proximal tubular cell
line was investigated under oxidative stress induced by
H2O2. Under
H2O2-induced oxidative stress, CHBP
pretreatment enhanced HK-2 cell viability, the GSH/GSSG ratio,
activation of the Nrf2 signalling pathway and proteins involved in
autophagy. However, CHBP pretreatment reduced the activity levels
of ROS, apoptosis and ER stress. This suggested that CHBP protects
cells from renal IRI, possibly via the inhibition of ER stress and
pro-apoptotic pathways, and via activation of the Nrf2 signalling
pathway.
In the present study, HK-2 cells were treated with
H2O2 in an in vitro model to mimic
oxidative stress-induced injury during IRI, and to evaluate the
protective effect of CHBP. IRI is characterized by TEC injury. It
is present in numerous diseases and following certain treatments,
including shock, renal transplantation and cardiac surgery
(1). TECs contribute to key renal
function (13). It has been
demonstrated that following renal transplantation, apoptosis of TEC
during IRI results in loss of kidney function and delays graft
function, which greatly affects clinical outcome (13). Therefore, HK-2 cells are a useful
in assessing the effect of CHBP on IRI in vitro.
In the present study, ROS activity levels, the
GSH/GSSG ratio and Nrf2 signalling proteins were measured to
investigate the underlying mechanism of the protective effect of
CHBP. The GSH/GSSG ratio is an indicator of prevention of cell
damage caused by oxygen species, including free radicles and
peroxides. A high GSH/GSSG ratio indicates less oxidative damage
(14). Nrf2 mediates the cellular
antioxidant response. This is a critical cellular defence in the
adaptive UPR response (11). ER
stress activates extracellular regulated kinases via
phosphorylation, including protein kinase RNA-like endoplasmic
reticulum kinase and the IRE1α-JNK-Nrf2 axis, which are initiators
of Nrf2 signalling (6). Nrf2
serves a key role in renal IRI. A previous study has demonstrated
that Nrf2 knockout mice are more susceptible to renal IRI (15). The gene that encodes HO-1 is a
target of Nrf2 (16). Our results
suggest that CHBP protects HK-2 cells from
H2O2-induced injury by reducing oxidative
stress and activating the antioxidant Nrf2 signalling pathway. The
present in vitro study supports our previous study using a
murine model (9).
In the present study, caspase-3, CHOP and BiP
expression levels were measured to investigate the underlying
mechanism of the protective effect of CHBP. Caspases serve a key
role in the initiation and effector phases of apoptosis. Caspase-3
is a downstream effector of the caspase activation cascade, and
directly mediates apoptosis when activated by various upstream
signals (17). Elevated caspase
activity is associated with enhanced apoptosis. The ER is an
important organelle in eukaryotes. ER stress contributes to
apoptosis of TECs in renal IRI. Moderate ER stress is associated
with the adaptive response of the UPR, leading to re-equilibration
of the ER (18). During IRI,
intensive ER stress occurs and the UPR shifts into the apoptotic
phase. CHOP and BiP are involved in this process. BiP is an
important chaperone in the ER lumen, and mediates polypeptide
folding and the structural maturation of nascent glycoproteins
(19). Accumulation of CHOP
initiates intensive ER stress (2).
The results of the present study suggested that CHBP protects HK-2
cells from H2O2-induced injury by inhibiting
ER stress and pro-apoptotic pathways, and is consistent with our
previous study using a murine model (9).
Our previous in vivo study demonstrated that
the renoprotective effect of CHBP against IRI is mediated by
induction of autophagy via inhibition of mTORC1 and activation of
mTORC2 (9). As oxidative stress
may crosstalk with autophagic machinery, it was determined whether
the renoprotective function of CHBP is mediated by autophagy in
vitro under conditions of oxidative stress. Consistently,
expression levels of beclin-1 and LC3 A/B-II/I in HK-2 cells
exposed to H2O2 were enhanced following CHBP
pretreatment. p62, is additionally known as SQSTM1 or sequestome 1,
and interacts with polyubiquitinated protein aggregates via a
ubiquitin-binding domain, and with LC3 via its LC3-binding domain,
therefore targeting these aggregates for degradation by the
autolysosome (20). In the present
study, pretreatment with CHBP further reduced the expression levels
of p62 in HK-2 cells treated with H2O2. In
addition, pretreatment with CHBP reduced p-mTOR Ser2448 and
enhanced p-mTOR Ser2481 expression levels. These results support
the theory that CHBP induces autophagy to protect against oxidative
stress.
CHBP is derived from HBSP, which has a very short
plasma half-life, and therefore requires frequent administration at
high doses to achieve tissue-protective effects (21). However, our previous study
demonstrated that CHBP is metabolically stable and protects mice
from renal IRI via inhibition of apoptosis and inflammation
(9).
In conclusion, the present study revealed that CHBP
protects HK-2 cells from H2O2-induced injury
by inhibiting ER stress and pro-apoptotic pathways, and via
activation of the Nrf2 signalling pathway and autophagy. Therefore,
CHBP may be a promising pharmacological agent for the prevention of
renal IRI.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (grant nos. 81270833, 81300621
and 81500568), National Health and Family Planning Commission
Foundation of Shanghai (grant no. 2014JQ008A).
References
|
1
|
Snoeijs MG, Vink H, Voesten N, Christiaans
MH, Daemen JW, Peppelenbosch AG, Tordoir JH, Peutz-Kootstra CJ,
Buurman WA, Schurink GW and van Heurn LW: Acute ischemic injury to
the renal microvasculature in human kidney transplantation. Am J
Physiol Renal Physiol. 299:F1134–F1140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong B, Zhou H, Han C, Yao J, Xu L, Zhang
M, Fu Y and Xia Q: Ischemia/reperfusion-induced CHOP expression
promotes apoptosis and impairs renal function recovery: The role of
acidosis and GPR4. PLoS One. 9:e1109442014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL,
Zhan J, Tong YN, Lin LR and He YN: Ischemia-reperfusion induces
renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J
Physiol Renal Physiol. 306:F75–F84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Ren F, Cheng Q, Bai L, Shen X, Gao
F, Busuttil RW, Kupiec-Weglinski JW and Zhai Y: Endoplasmic
reticulum stress modulates liver inflammatory immune response in
the pathogenesis of liver ischemia and reperfusion injury.
Transplantation. 94:211–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierre N, Barbe C, Gilson H, Deldicque L,
Raymackers JM and Francaux M: Activation of ER stress by hydrogen
peroxide in C2C12 myotubes. Biochem Biophys Res Commun.
450:459–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Digaleh H, Kiaei M and Khodagholi F: Nrf2
and Nrf1 signaling and ER stress crosstalk: Implication for
proteasomal degradation and autophagy. Cell Mol Life Sci.
70:4681–4694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueba H, Brines M, Yamin M, Umemoto T, Ako
J, Momomura S, Cerami A and Kawakami M: Cardioprotection by a
nonerythropoietic, tissue-protective peptide mimicking the 3D
structure of erythropoietin. Proc Natl Acad Sci USA. 107:pp.
14357–14362. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brines M, Patel NS, Villa P, Brines C,
Mennini T, De Paola M, Erbayraktar Z, Erbayraktar S, Sepodes B,
Thiemermann C, et al: Nonerythropoietic, tissue-protective peptides
derived from the tertiary structure of erythropoietin. Proc Natl
Acad Sci USA. 105:pp. 10925–10930. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang C, Xu Z, Zhao Z, Li L, Zhao T, Peng
D, Xu M, Rong R, Long YQ and Zhu T: A novel proteolysis-resistant
cyclic helix B peptide ameliorates kidney ischemia reperfusion
injury. Biochim Biophys Acta. 1842:2306–2317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Altern Med. 14:192014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
González-Guerrero C, Ocaña-Salceda C,
Berzal S, Carrasco S, Fernández-Fernández B, Cannata-Ortiz P, Egido
J, Ortiz A and Ramos AM: Calcineurin inhibitors recruit protein
kinases JAK2 and JNK TLR signaling and the UPR to activate
NF-kappaB-mediated inflammatory responses in kidney tubular cells.
Toxicol Appl Pharmacol. 272:825–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gobé G, Willgoss D, Hogg N, Schoch E and
Endre Z: Cell survival or death in renal tubular epithelium after
ischemia-reperfusion injury. Kidney Int. 56:1299–1304. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manikonda PK, Rajendra P, Devendranath D,
Gunasekaran B, Channakeshava, Aradhya SR, Sashidhar RB and
Subramanyam C: Extremely low frequency magnetic fields induce
oxidative stress in rat brain. Gen Physiol Biophys. 33:81–90. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue
S, Kamo N, Zhai Y, Yamamoto M, Busuttil RW and Kupiec-Weglinski JW:
KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of
mouse liver transplants. J Hepatol. 59:1200–1207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang JS, Han MH, Kim GY, Kim CM, Kim BW,
Hwang HJ and Hyun Y: Nrf2-mediated HO-1 induction contributes to
antioxidant capacity of a Schisandrae Fructus ethanol extract in
C2C12 myoblasts. Nutrients. 6:5667–5678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo HS, Ku JM, Choi HS, Woo JK, Jang BH,
Shin YC and Ko SG: Induction of caspase-dependent apoptosis by
apigenin by inhibiting STAT3 signaling in HER2-overexpressing
MDA-MB-453 breast cancer cells. Anticancer Res. 34:2869–2882.
2014.PubMed/NCBI
|
|
18
|
Lindenmeyer MT, Rastaldi MP, Ikehata M,
Neusser MA, Kretzler M, Cohen CD and Schlöndorff D: Proteinuria and
hyperglycemia induce endoplasmic reticulum stress. J Am Soc
Nephrol. 19:2225–2236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimazawa M, Tanaka H, Ito Y, Morimoto N,
Tsuruma K, Kadokura M, Tamura S, Inoue T, Yamada M, Takahashi H, et
al: An inducer of VGF protects cells against ER stress-induced cell
death and prolongs survival in the mutant SOD1 animal models of
familial ALS. PLoS One. 5:e153072010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/lC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McVicar CM, Hamilton R, Colhoun LM,
Gardiner TA, Brines M, Cerami A and Stitt AW: Intervention with an
erythropoietin-derived peptide protects against neuroglial and
vascular degeneration during diabetic retinopathy. Diabetes.
60:2995–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|