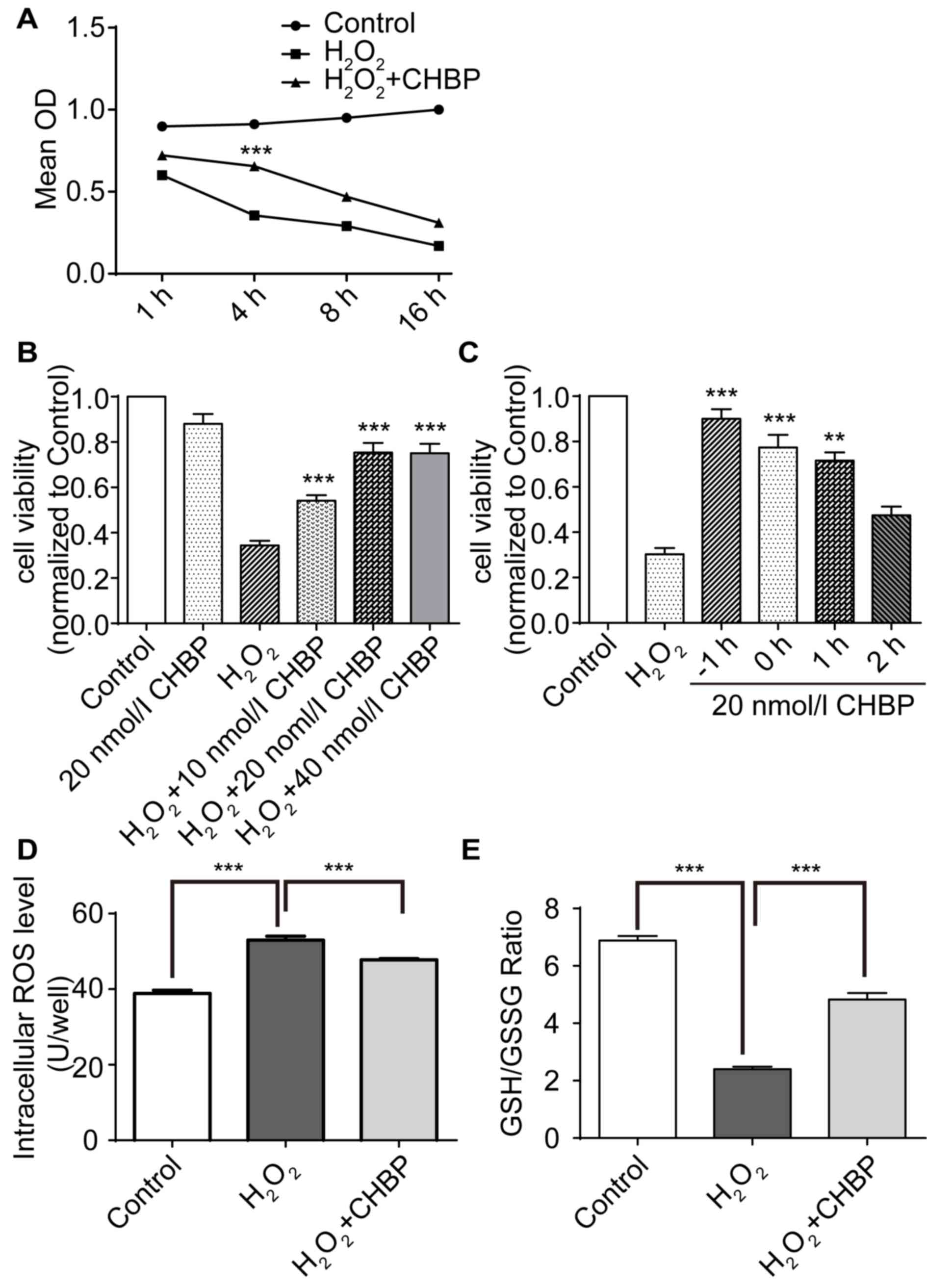
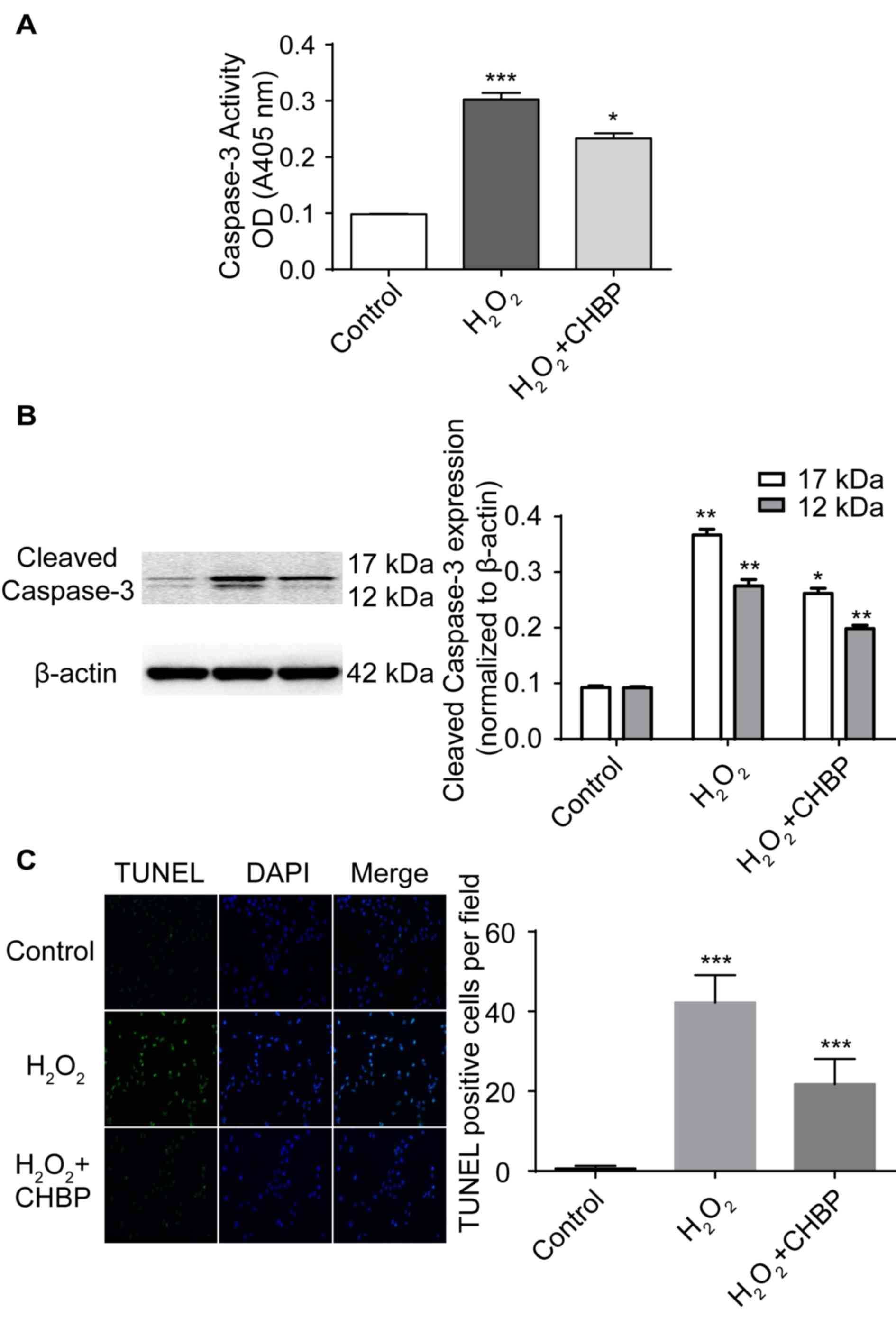
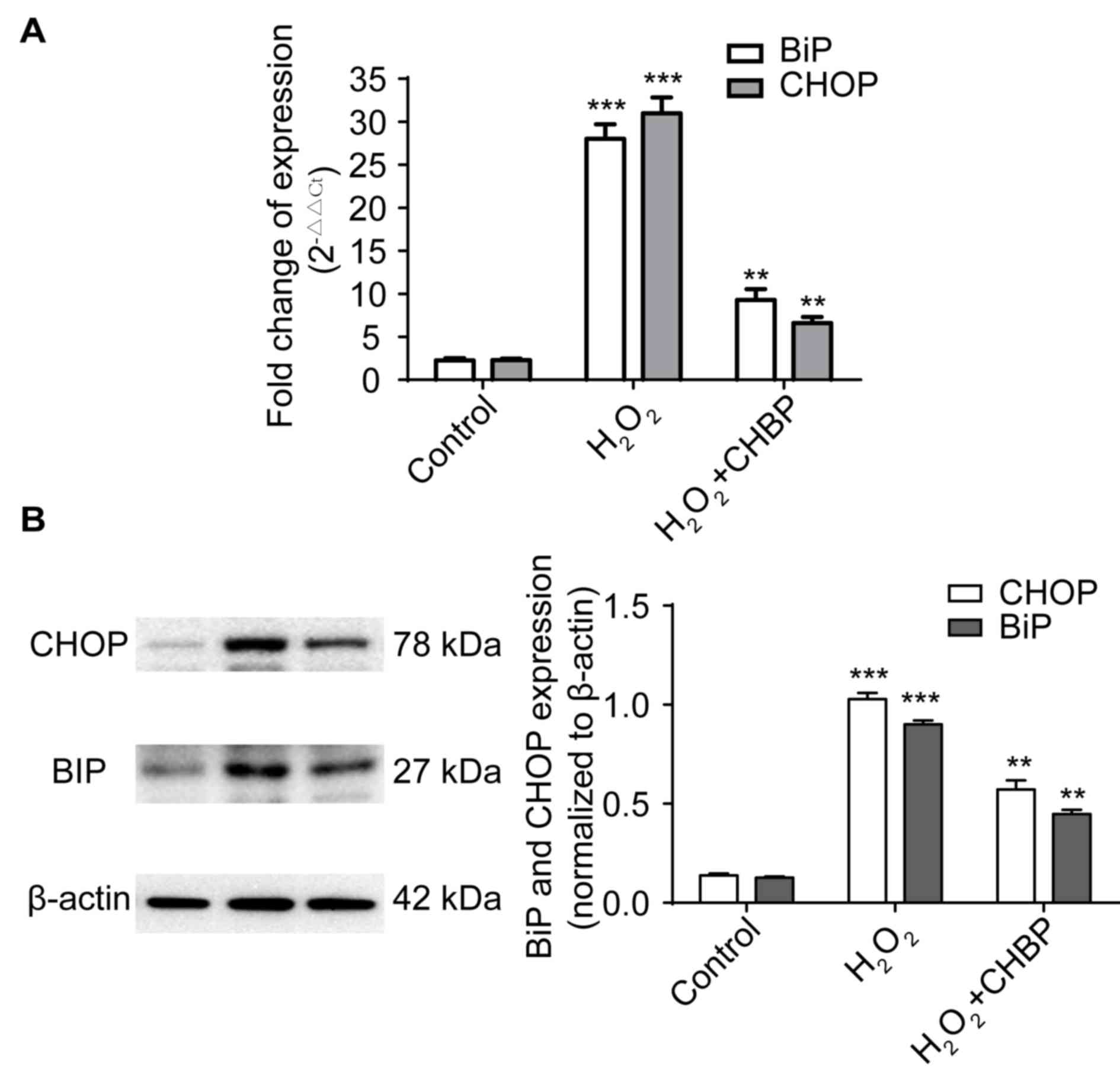
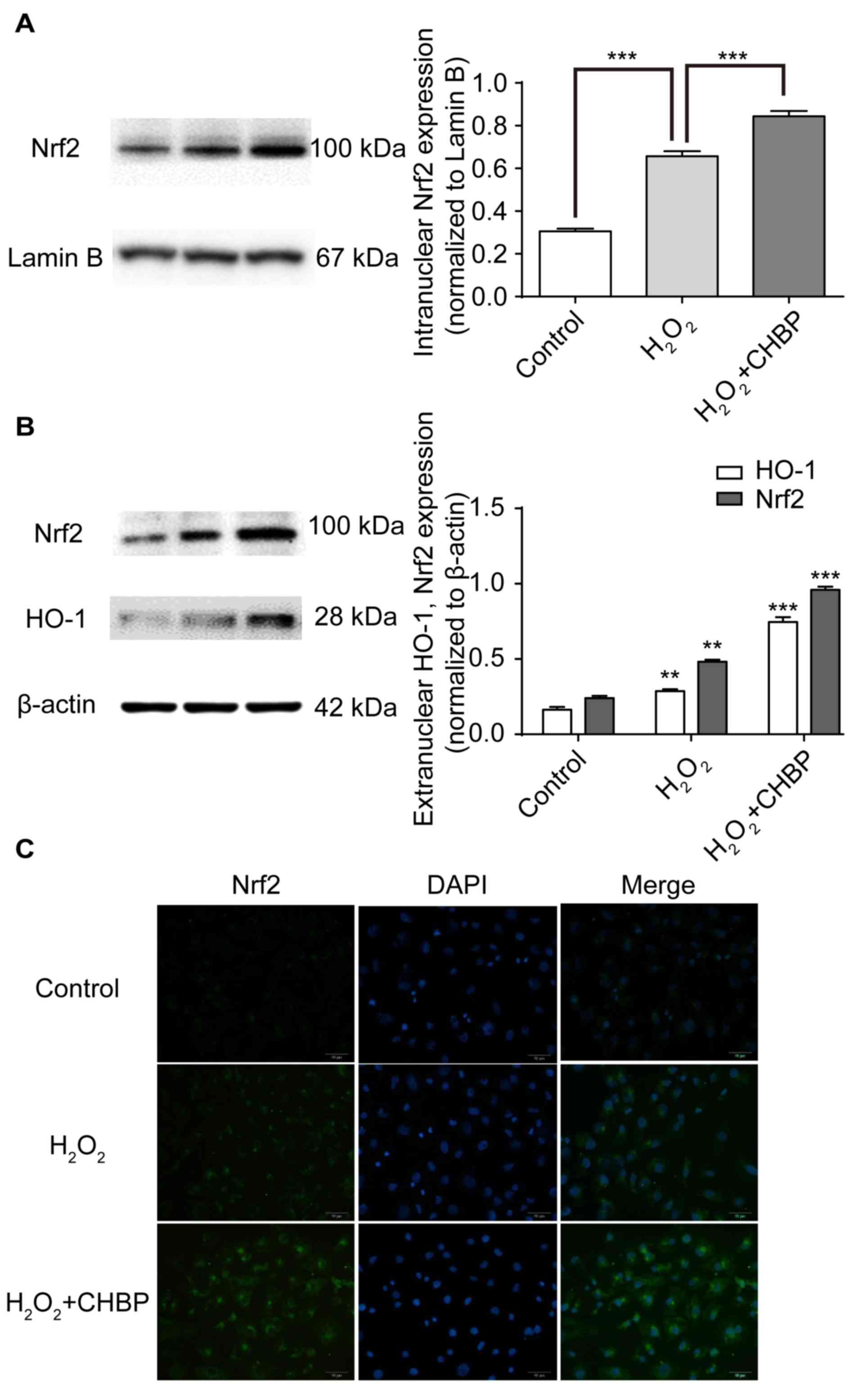
|
1
|
Snoeijs MG, Vink H, Voesten N, Christiaans
MH, Daemen JW, Peppelenbosch AG, Tordoir JH, Peutz-Kootstra CJ,
Buurman WA, Schurink GW and van Heurn LW: Acute ischemic injury to
the renal microvasculature in human kidney transplantation. Am J
Physiol Renal Physiol. 299:F1134–F1140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong B, Zhou H, Han C, Yao J, Xu L, Zhang
M, Fu Y and Xia Q: Ischemia/reperfusion-induced CHOP expression
promotes apoptosis and impairs renal function recovery: The role of
acidosis and GPR4. PLoS One. 9:e1109442014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL,
Zhan J, Tong YN, Lin LR and He YN: Ischemia-reperfusion induces
renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J
Physiol Renal Physiol. 306:F75–F84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Ren F, Cheng Q, Bai L, Shen X, Gao
F, Busuttil RW, Kupiec-Weglinski JW and Zhai Y: Endoplasmic
reticulum stress modulates liver inflammatory immune response in
the pathogenesis of liver ischemia and reperfusion injury.
Transplantation. 94:211–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierre N, Barbe C, Gilson H, Deldicque L,
Raymackers JM and Francaux M: Activation of ER stress by hydrogen
peroxide in C2C12 myotubes. Biochem Biophys Res Commun.
450:459–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Digaleh H, Kiaei M and Khodagholi F: Nrf2
and Nrf1 signaling and ER stress crosstalk: Implication for
proteasomal degradation and autophagy. Cell Mol Life Sci.
70:4681–4694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueba H, Brines M, Yamin M, Umemoto T, Ako
J, Momomura S, Cerami A and Kawakami M: Cardioprotection by a
nonerythropoietic, tissue-protective peptide mimicking the 3D
structure of erythropoietin. Proc Natl Acad Sci USA. 107:pp.
14357–14362. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brines M, Patel NS, Villa P, Brines C,
Mennini T, De Paola M, Erbayraktar Z, Erbayraktar S, Sepodes B,
Thiemermann C, et al: Nonerythropoietic, tissue-protective peptides
derived from the tertiary structure of erythropoietin. Proc Natl
Acad Sci USA. 105:pp. 10925–10930. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang C, Xu Z, Zhao Z, Li L, Zhao T, Peng
D, Xu M, Rong R, Long YQ and Zhu T: A novel proteolysis-resistant
cyclic helix B peptide ameliorates kidney ischemia reperfusion
injury. Biochim Biophys Acta. 1842:2306–2317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Altern Med. 14:192014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
González-Guerrero C, Ocaña-Salceda C,
Berzal S, Carrasco S, Fernández-Fernández B, Cannata-Ortiz P, Egido
J, Ortiz A and Ramos AM: Calcineurin inhibitors recruit protein
kinases JAK2 and JNK TLR signaling and the UPR to activate
NF-kappaB-mediated inflammatory responses in kidney tubular cells.
Toxicol Appl Pharmacol. 272:825–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gobé G, Willgoss D, Hogg N, Schoch E and
Endre Z: Cell survival or death in renal tubular epithelium after
ischemia-reperfusion injury. Kidney Int. 56:1299–1304. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manikonda PK, Rajendra P, Devendranath D,
Gunasekaran B, Channakeshava, Aradhya SR, Sashidhar RB and
Subramanyam C: Extremely low frequency magnetic fields induce
oxidative stress in rat brain. Gen Physiol Biophys. 33:81–90. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue
S, Kamo N, Zhai Y, Yamamoto M, Busuttil RW and Kupiec-Weglinski JW:
KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of
mouse liver transplants. J Hepatol. 59:1200–1207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang JS, Han MH, Kim GY, Kim CM, Kim BW,
Hwang HJ and Hyun Y: Nrf2-mediated HO-1 induction contributes to
antioxidant capacity of a Schisandrae Fructus ethanol extract in
C2C12 myoblasts. Nutrients. 6:5667–5678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo HS, Ku JM, Choi HS, Woo JK, Jang BH,
Shin YC and Ko SG: Induction of caspase-dependent apoptosis by
apigenin by inhibiting STAT3 signaling in HER2-overexpressing
MDA-MB-453 breast cancer cells. Anticancer Res. 34:2869–2882.
2014.PubMed/NCBI
|
|
18
|
Lindenmeyer MT, Rastaldi MP, Ikehata M,
Neusser MA, Kretzler M, Cohen CD and Schlöndorff D: Proteinuria and
hyperglycemia induce endoplasmic reticulum stress. J Am Soc
Nephrol. 19:2225–2236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimazawa M, Tanaka H, Ito Y, Morimoto N,
Tsuruma K, Kadokura M, Tamura S, Inoue T, Yamada M, Takahashi H, et
al: An inducer of VGF protects cells against ER stress-induced cell
death and prolongs survival in the mutant SOD1 animal models of
familial ALS. PLoS One. 5:e153072010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/lC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McVicar CM, Hamilton R, Colhoun LM,
Gardiner TA, Brines M, Cerami A and Stitt AW: Intervention with an
erythropoietin-derived peptide protects against neuroglial and
vascular degeneration during diabetic retinopathy. Diabetes.
60:2995–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|