Introduction
Hypertension is a global public health issue, which
contributes to the burden of heart disease, stroke and kidney
failure as well as premature mortality and disability (1). Increasing experimental and clinical
evidence supports a key role for the renin-angiotensin system (RAS)
in the pathogenesis of hypertension (2). In the RAS, the angiotensin-converting
enzyme (ACE2)-Ang (1–7)-Mas axis acts as a counter-regulation
system against the ACE-Ang II-AT 1 pathway (3), and changes in the expression and
activity of various components of the RAS are implicated in
hypertension (4).
In previous years, the focus on the role of the RAS
in the pathophysiology of hypertension has changed towards the role
of local RAS in specific tissues (5). A previous study reported that in
addition to systemic RAS, there is also a local RAS in many
tissues, including the kidneys, heart, brain, lungs, liver and
blood vessels. Genes of all the components of RAS are expressed in
important organs including the heart, brain, kidneys and aorta
(6). Research has indicated that
tissue RAS can operate independently of circulating RAS (7). Each local RAS has a distinct
enzymatic profile resulting in different patterns of angiotensin
fragment generation in different tissues (8). Currently, intrarenal RAS is
considered to be an important tissue RAS, which controls blood
pressure (BP) and is involved in the pathogenesis of hypertension.
Appropriate activation of the intrarenal RAS ensures kidneys
maintain a normal Na+ balance at normal renal perfusion
pressures to prevent hypertension (9). Regarding intrarenal RAS, the kidney
expresses all the major components of the RAS, such as Ang, renin
and ACE, suggesting that intrarenal generation of Ang II plays a
key role in BP regulation (10,11).
Studies indicate that the intrarenal RAS functions separately from
systemic Ang II generation (11).
In addition, the kidney is one of the most important organs in
which Ang (1–7) is generated from the metabolism of Ang
II by ACE2, and the proximal tubule exhibits a high level of ACE2
activity. However, the intrarenal effects of the ACE2-Ang (1–7)-Mas
receptor pathway are controversial, and further research is
required (9).
Alterations in the circadian gene expression of the
heart RAS may participate in the development of hypertension
(12). Much research was committed
to the cardiac RAS (13,14). Fedoseeva et al (15) measured the mRNA expression of
kidney and myocardium RAS in hypertensive inbred and normotensive
Wistar Albino Glaxo rats respectively, without making analysis and
comparison to the mRNA levels between the two tissues local RAS.
Further studies should be dedicated to exploring whether the
synthesis of components of the myocardial and renal local RAS are
differentially regulated after treatment. In a previous study of
the authors, they demonstrated the 6-week antihypertensive effects
of Ile-Gln-Pro (IQP), Val-Glu-Pro (VEP) and Spirulina
platensis hydrolysates (SH) on the local RAS in the myocardium
of spontaneously hypertensive rats (SHR). It was identified that
ACE, Ang II and AT 1 were downregulated in the myocardium, while AT
2, ACE2, Ang (1–7) and Mas were upregulated in the
myocardium (16).
However, how kidney local RAS expression is
regulated during the development of antihypertension by IQP, VEP
and SH has not been elucidated. Few studies have explored the
differences between the regulation of the kidney and myocardium
local RAS to reduce BP although some components of the local RSA of
different tissues were determined and analyzed in hypertensive rats
(17,18). The current study investigated the
effects of intrarenal RAS by examining how IQP, VEP and SH
regulated the mRNA levels and the protein concentrations of major
components of the RAS [ACE, ACE2, Ang (1–7), Ang
II, AT 1, AT 2 and Mas] in the kidney of SHR, and investigated
whether SH had different effects on the BP-reducing mechanisms of
the local kidney RAS and local myocardium RAS, using reverse
transcription-quantitative polymerase chain reaction, ELISA or
western blotting.
Materials and methods
Reagents
Spirulina platensis powder was purchased from
Zaihuishou Bio-engineering (Ererduosi, China, http://nmzhs.com/). IQP and VEP were provided by
Beijing SciLight Biotechnology LLC (Beijing, China) with a purity
of >98%. SH was produced by a papain enzymatic method according
to the authors’ previous studies, and the percentage of IQP and VEP
in SH were ~1.81 and 2.14%, respectively (16,19,20).
These three reagents were stored at −20°C. Captopril and Nembutal
were purchased from YTHX Biotechnology Co., Ltd. (Beijing, China).
Coomassie Brilliant Blue Total Protein Quantification kit was
purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). ACE ELISA kit (catalog no. ab155452) and ACE2 ELISA kit
(catalog no. ab213843) were purchased from Abcam (Cambridge, UK).
Ang-II ELISA kit (catalog no. CSB-E07304r) and AT 1 ELISA kit
(catalog no. CSB-E13746r) were purchased from Cusabio Biotech.,
Ltd. (College Park, MD, USA). AT 2 ELISA kit (catalog no. SEA973Ra)
and Ang (1–7) ELISA kit (catalog no. CES085Ra) was
purchased from Cloud-Clone Corp. (Katy, TX, USA). Unless otherwise
stated, all reagents and kits were of analytical grade and were
purchased from Tiangen Biotech Co., Ltd. (Beijing, China).
Animal model
A total of 80 male spontaneously hypertensive rats
(SHR) purchased from Experimental Animal Center of Weitonglihua
(.Beijing,, China, http://www.vitalriver.com/), aged 6 weeks and weighing
235.0±6.6 g, whose weighted systolic BP was 181±1 mmHg and weighted
diastolic BP was 145±1 mmHg, were used in the present study.
Animals were housed in 12-h light/dark cycles under controlled
conditions of 25±2°C, relative humidity of 60±5%, and had free
access to food and water. These SHR were divided into 5 groups
(IQP, VEP, SH, saline and captopril; each 10 mg/kg/day) and
received continuous monitoring of BP during six-week treatment
period and 2-week observation period. The administration was
conducted by ‘passive swallow’ gavage instead of usual gavage as to
protect the rats' esophagus at 9:00 a.m. every day.
At the end of the first three-week treatment period,
rats (n=5, each group) were anesthetized with 3% pentobarbital (30
mg/kg, intraperitoneal injection). Kidney tissues were removed, cut
into small pieces and stored in RNAstore Reagent or in saline for
24 h at 4°C, and immediately transferred to an
ultra-low-temperature freezer at −80°C. Other rats were raised as
for the first 3 weeks. At the end of the six-week treatment period,
rats (n=5, each group) were euthanized with 3% pentobarbital (30
mg/kg, intraperitoneal injection). Other rats were raised for
another 2 weeks, treated daily with saline, and euthanized as
before.
All the cares of the rats were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals, Committee of Beijing Experimental Animal Care and Use. The
experiment on the rats was authorized by the Ethics Committee of
the Beijing Experimental Animal Association (Beijing, China).
Blood pressure measurement
The systolic BP and diastolic BP were measured twice
a week (on Wednesday and Sunday, respectively) at 10:00 a.m. by the
tail-cuff method (21) for 8
weeks. Each measurement was repeated five times, and the highest
and lowest values were discarded. The mean value was determined for
the remaining three values. All measurements were performed by the
same person in a quiet environment.
Isolation of RNA from rat kidney
Total RNA from SHR kidneys was isolated using the
RNAprep Pure Tissue Kit. RNA purities and concentrations were
determined (NanoDrop ND-1000; NanoDrop; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) by the ratio of
A260/A280 and
A260/A230.
Single-strand cDNA synthesis
Transcriptor first-strand cDNA was synthesized from
1 µg RNA using the First-Strand Synthesis kit. An RNA template was
placed on ice to defrost, and 5X gDNA Buffer, FQ-RT Primer Mix, 10X
Fast RT Buffer and RNase-Free ddH2O were placed on ice
following thawing at room temperature. Each solution was mixed by
vortex oscillation before use and the remaining liquid on the tube
wall was collected through brief centrifugation. Then, 2 µl 5X gDNA
Buffer was added to a thin-walled PCR tube on ice, and 1,000
ng/concentration of RNA (ng/µl) total RNA was added. RNA-free water
was added to a total volume of 10 µl. The contents were completely
mixed and incubated at 42°C for 3 min, and then the reaction tube
was placed on ice. The reverse transcription reaction system
consisting of 1 µl RT Enzyme Mix, 2 µl FQ-RT Primer Mix, 2 µl 10X
Fast RT Buffer, 5 µl RNase-Free ddH2O were added to the
thin-walled reaction tube, and the contents were incubated at 42°C
for 15 min followed by 95°C for another 3 min. The reaction tube
was placed on ice and then stored at −20°C for RT-qPCR
analysis.
RT-qPCR
RT-qPCR was performed for ACE, ACE2, AT 1, AT 2,
Mas, and the housekeeping gene GAPDH using the SuperReal
PreMix Plus kit. The primers for RT-qPCR analysis were previously
reported (16), and were ordered
from Tiangen Biotech Co., Ltd. The following experimental
operations were performed under dark conditions. All samples were
run in triplicate in 96-well plates. PCR reactions were carried out
in a 20 µl solution consisting of 10 µl 2X SuperReal PreMix Plus,
0.6 µl forward primer (10 µM), 0.6 µl reverse primer (10 µM), 4 µl
cDNA template and 4.8 µl RNase-Free ddH2O. The reaction
tube was centrifuged to ensure that all components were at the
bottom of the tube. The PCR reaction was initiated with initial
denaturation at 95°C for 15 min and then 40 cycles of 10 sec at
95°C for denaturation, 20 sec at 60°C for annealing, and 32 sec at
72°C for extension.
Quantification of a target gene was expressed as the
relative expression ratio of the target gene in a sample vs. that
of a control (housekeeping gene GAPDH). The relative
quantitative method (2−ΔΔCt method) was used for the
analysis of relative expression ratios of the target genes
(22). The value of
2−ΔΔCq was the relative expression ratio of the target
gene.
Western blot analysis
The kidney tissues' total protein was extracted by a
Coomassie Brilliant Blue Total Protein Quantification kit following
homogeneity (5 min, 0°C) and centrifugation (3,000 × g, 10 min,
4°C). The protein samples were separated by 10% SDS-PAGE, and then
transferred to a nitrocellulose membrane. The membrane was blocked
with 5% skimmed milk powder in TBS containing 0.5% Tween 20 for 2 h
at 37°C and then incubated overnight for 2 h with MAS1L primary
antibody (catalog no. ab200685; Abcam) for the Mas receptor at a
1:500 dilution or GAPDH primary antibody (catalog no.
sc-47724; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a
1:800 dilution for GAPDH. After washing with TBS-T, the
blots were then incubated with goat anti-rabbit IgG/HRP secondary
antibody (catalog no. ab97040; Abcam) at a 1:3,000 dilution for the
Mas receptor or a goat anti-mouse IgG/HRP secondary antibody
(catalog no. sc-2005; Santa Cruz Biotechnology, Inc.) at a 1:8,000
dilution for GAPDH, then rinsed thoroughly with TBS-T.
Proteins were detected by electrochemiluminescence (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The protein hands were
analyzed by Gel Pro 4.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
ELISA
The kidney tissues' total protein content was
measured by a Coomassie Brilliant Blue Total Protein Quantification
kit following homogeneity (5 min, 0°C) and centrifugation (3,000 ×
g, 10 min, 4°C). Kidney proteins ACE, ACE2, Ang (1–7), Ang
II, AT 1 and AT 2 were measured by rat ELISA kits (R&D Systems,
Inc., Minneapolis, MN, USA) according to the manufacturer's
instructions. Samples in 96-well microplates were read by a
Benchmark plus microplate spectrophotometer (Bio-Rad Laboratories,
Inc.) within 20 min after the reaction was stopped. Test samples
and standards were measured in duplicate. Microplate manager
version 5.2.1 software (Bio-Rad Laboratories, Inc.), which was
equipped with the spectrophotometer, automatically calculated
sample concentrations according to corresponding standard curves
with four-parameter logistics and log-logit function.
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. Statistical comparisons between groups were
analyzed with one-factor analysis of variance using SPSS
statistical software (version, 17.0; SPSS Inc., Chicago, IL, USA),
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of IQP, VEP and SH on growth
parameters and blood pressure changes in SHR
During the eight-week experiment, body weight,
consumption of drinking fluid and food of SHR were not
significantly different between the groups. As reported previously
(16), IQP, VEP and SH had
significant anti-hypertensive effects on SHR over an eight-week
experimental course.
Effects of IQP, VEP and SH on the
ACE-Ang II-AT 1/AT 2 axis in local kidney RAS
The qRT-PCR analysis of renal ACE, AT 1 and AT 2 is
demonstrated in Fig. 1. In each
experimental period (at weeks 3, 6 and 8) there was a significant
difference in ACE mRNA levels between the every two groups. The SH
group reported the lowest ACE mRNA levels compared with the blank
control group (SHR administered saline), except the positive
control group (SHR administered captopril). At week 6, when all the
experimental groups reached the lowest BP, the ACE mRNA levels of
the IQP, VEP and SH group were significantly decreased by 76.80,
68.60 and 81.70%, respectively, compared with the blank control
group (Fig. 1A). AT 1 mRNA
expression in renal tissues had a similar trend to ACE. However, at
week 6, the AT 1 mRNA level of the VEP group was significantly
lower than that of the SH group, and there was no significant
difference between the VEP and IQP group. Significant differences
of AT 1 mRNA levels were identified at week 6, when compared with
week 3 in the same experimental group. At week 6, the AT 1 mRNA
levels of the IQP, VEP and SH group were significantly decreased,
by 73.00, 74.20 and 70.10%, respectively, compared with the blank
control group (Fig. 1B). For AT 2
mRNA levels, there was a significant difference between the every
two groups at week 6, when all the experimental groups reached the
highest expression of AT 2 mRNA (IQP, VEP and SH group AT 2 mRNA
expression was significantly increased by 36.01-, 34.03- and
38.23-fold, respectively, compared with the negative group;
Fig. 1C).
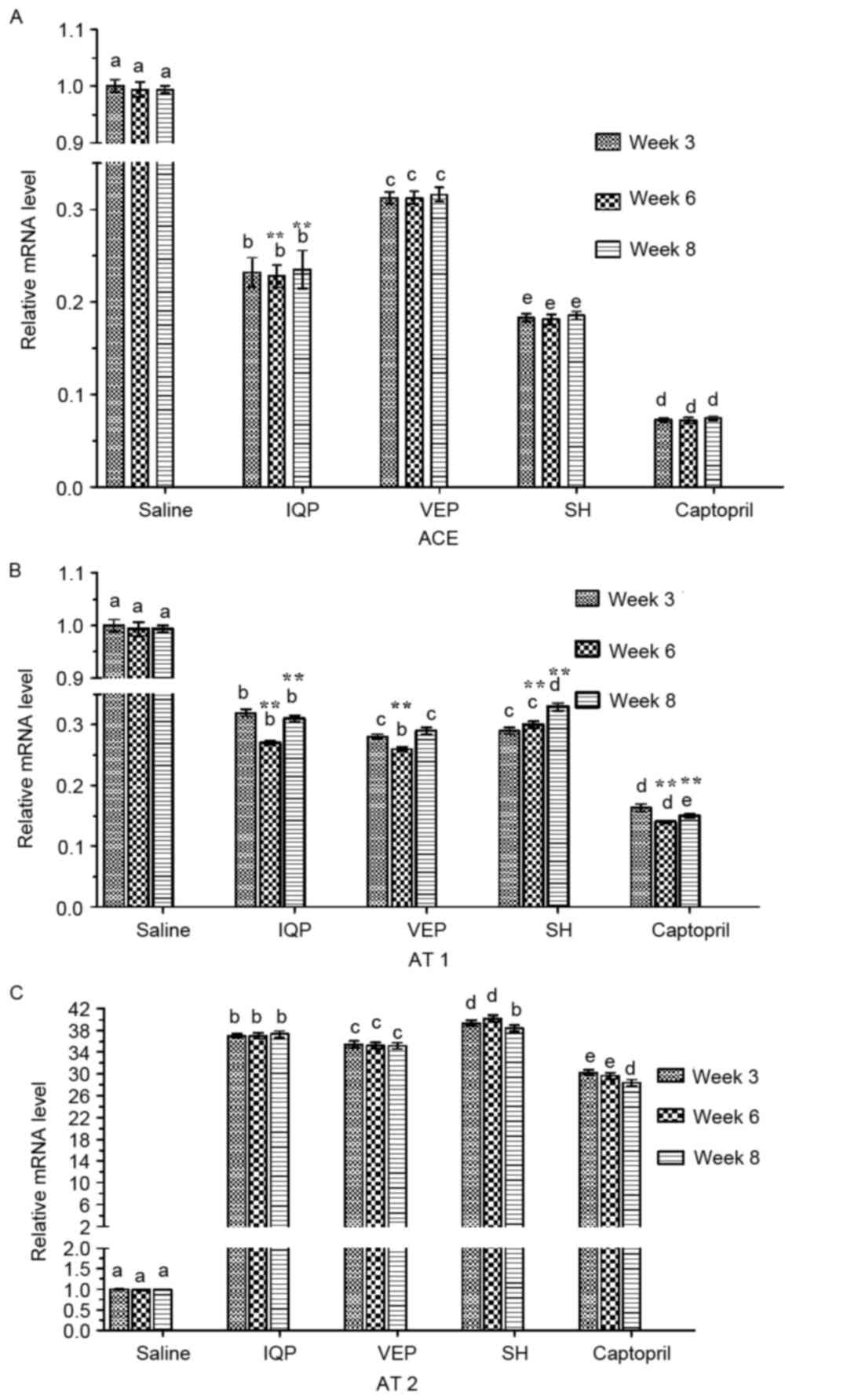 | Figure 1.Effects of IQP, VEP and SH on the
mRNA levels of the ACE-Ang II-AT 1/AT 2 axis in SHR kidney during
different experimental periods (weeks 3, 6, and 8): (A) ACE, (B) AT
1, (C) AT 2. GAPDH was used as a housekeeping gene. Data are
represented as the mean ± standard error of the mean (n=5 animals
per treatment group). In each experimental period, comparisons
between different groups were performed by one-way analysis of
variance. Values with dissimilar lowercase letters (a-e) were
significantly different, P<0.05. Comparisons between different
experimental periods of the same treatment group were performed by
one-way analysis of variance. **P<0.05 vs. week 3. IQP,
Ile-Gln-Pro; VEP, Val-Glu-Pro; SH, Spirulina platensis
hydrolysates; ACE, angiotensin-converting enzyme. |
ACE, Ang II, AT 1 and AT 2 concentrations in the
kidney were measured and quantified by ELISA (Fig. 2). When compared with week 3, the
ACE concentrations of all the experimental groups reached the
lowest values at week 6, most notably the IQP and SH group. At week
6, although the ACE concentration of the captopril group was lower
than that of SH group, there was no significant difference between
the two groups (Fig. 2A). Ang II
presented the same trend. At week 6, the Ang II concentrations of
the IQP, VEP and SH groups were significantly decreased by 32.74,
22.10 and 36.67%, respectively, compared with the blank control
group (Fig. 2B). Similar to ACE
and Ang II, at week 6, the AT 1 concentrations of the IQP, VEP and
SH were significantly decreased by 29.12, 21.27 and 37.37%
respectively, compared with the blank control group. In each
experimental period, there was a significant difference in AT 1
concentration between the every two groups (Fig. 2C). AT 2 concentrations increased to
a maximum in all the experimental groups at week 6: the AT 2
concentrations in the IQP, VEP and SH groups were significantly
increased by 1.08-, 0.74- and 1.61-fold, respectively (Fig. 2D).
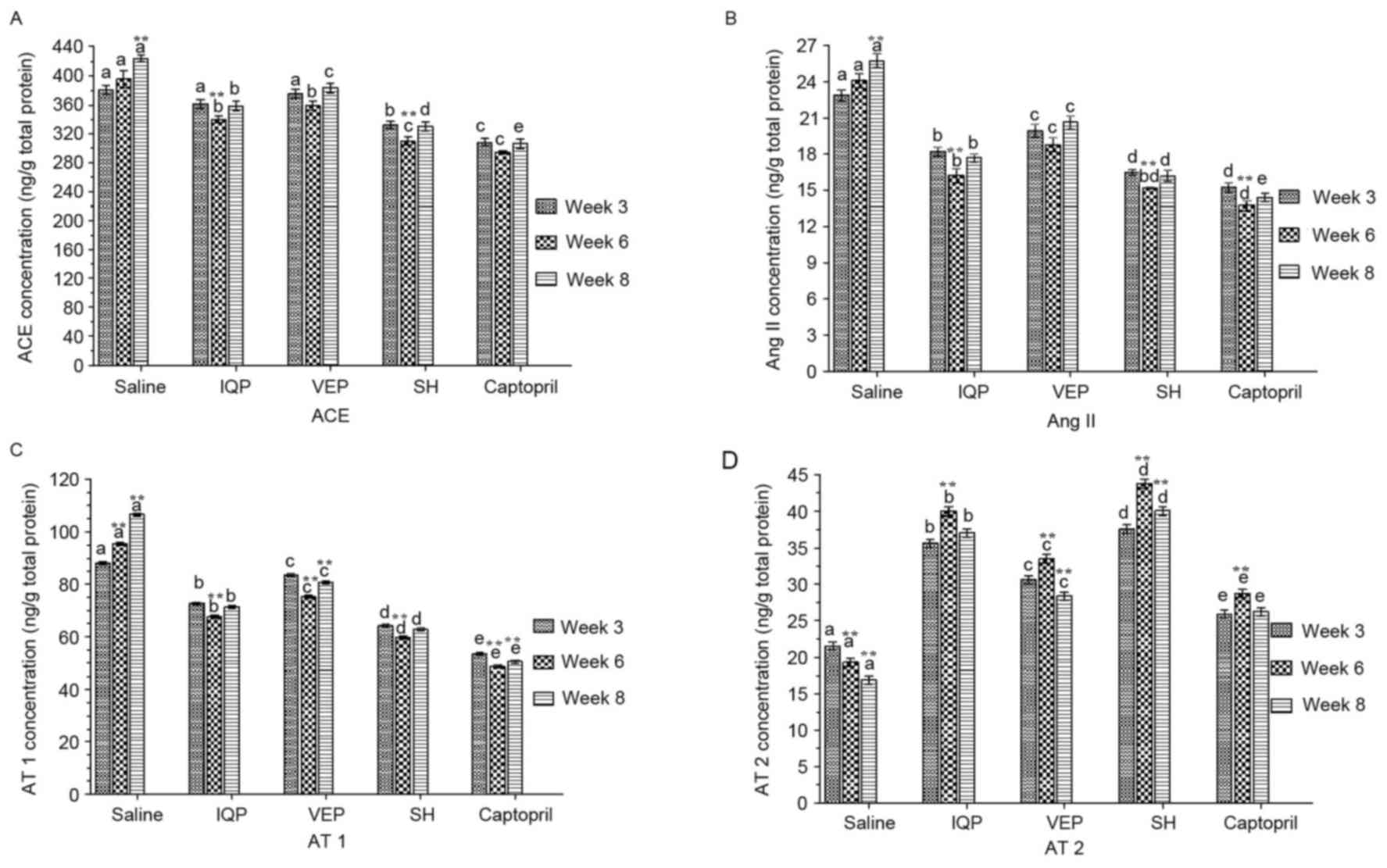 | Figure 2.Effects of IQP, VEP and SH on protein
concentrations of ACE-Ang II-AT 1/AT 2 axis in the SHR kidney
during the different experimental periods (weeks 3, 6, and 8): (A)
ACE, (B) Ang II, (C) AT 1, (D) AT 2. Data are represented as mean ±
standard error of the mean (n=5 animals per treatment group). In
each experimental period, comparisons between different groups were
performed by one-way analysis of variance. Values with dissimilar
lowercase letters (a-e) were significantly different, P<0.05.
Comparisons between different experimental periods of the same
treatment group by one-way analysis of variance. **P<0.05 vs.
week 3. IQP, Ile-Gln-Pro; VEP, Val-Glu-Pro; SH, Spirulina
platensis hydrolysates; ACE, angiotensin-converting enzyme. |
Effects of IQP, VEP and SH on ACE2-Ang
(1–7)-Mas axis in local kidney RAS
Fig. 3 presents the
results of the RT-qPCR analysis of renal ACE2 and Mas mRNA. In each
experimental period, there was a significant difference in ACE2
mRNA levels between the every two groups. At week 6, when all the
experimental groups reached the highest values, the ACE2 mRNA
levels of the IQP, VEP and SH group were significantly increased by
1.31-, 0.91- and 2.01-fold, respectively, compared with the blank
control group. Significant differences of ACE2 mRNA levels were
identified at week 6, when compared with week 3 in the same
experimental group (Fig. 3A).
Similar to ACE2, Mas mRNA levels were increased maximally in all
the experimental groups at week 6: Mas mRNA levels in IQP, VEP and
SH group were significantly increased by 0.76-, 0.51- and
1.68-fold, respectively, compared with the blank control group. At
week 8, although the Mas mRNA of captopril group was slightly
higher than that of SH group, there was no significant difference
between the groups (Fig. 3B).
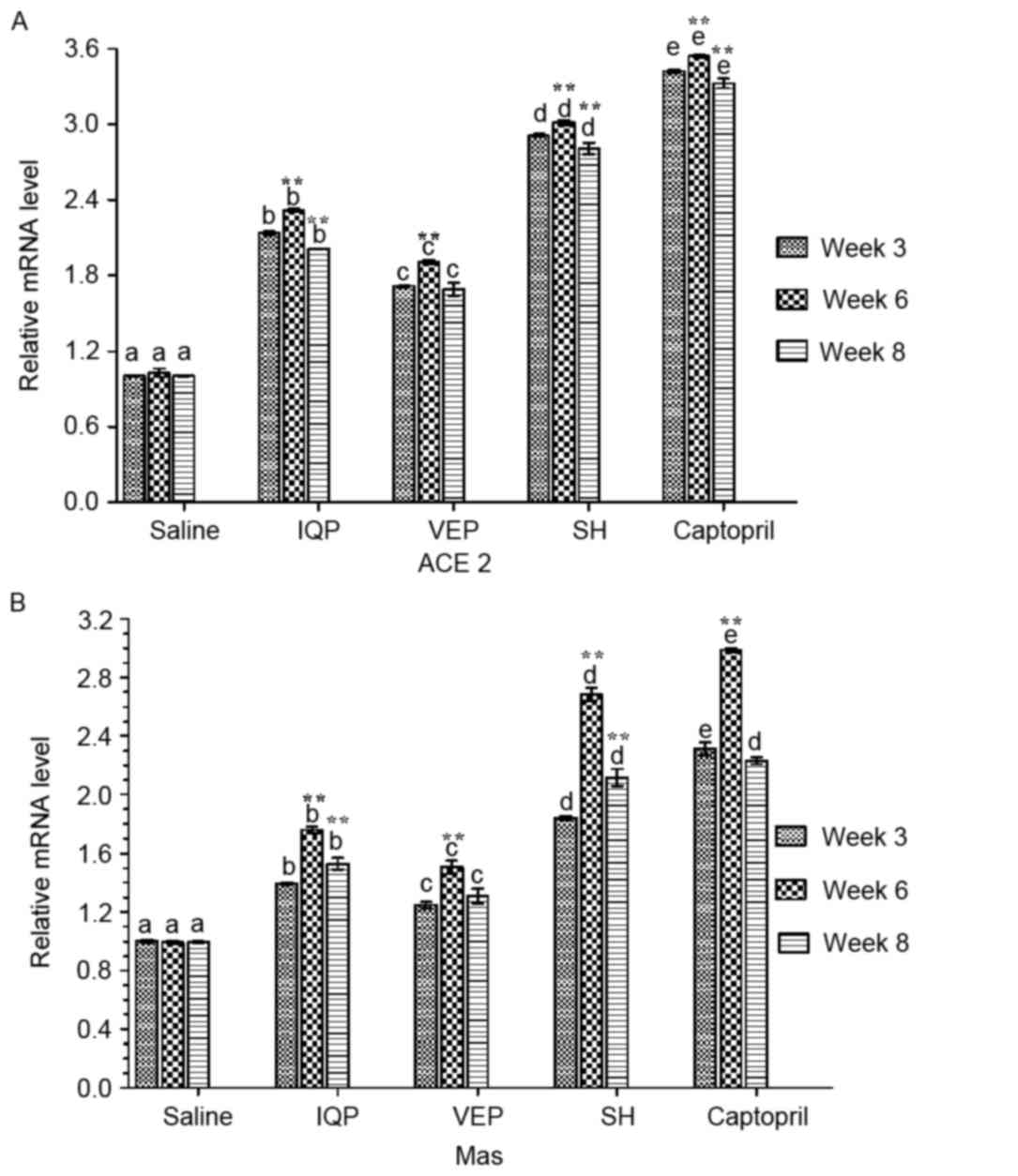 | Figure 3.Effects of IQP, VEP and SH on the
mRNA levels of ACE2-Ang (1–7)-Mas
axis in the SHR kidney during the different during the different
experimental periods (weeks 3, 6, and 8): (A) ACE2, (B) Mas.
GAPDH was the housekeeping gene. Data are represented as
mean ± standard error of the mean (n=5 animals per treatment
group). In each experimental period, comparisons between different
groups were performed by one-way analysis of variance. Values with
dissimilar lowercase letters (a-e) were significantly different,
P<0.05. Comparisons between different experimental periods of
the same treatment group by one-way analysis of variance.
**P<0.05 vs. week 3. IQP, Ile-Gln-Pro; VEP, Val-Glu-Pro; SH,
Spirulina platensis hydrolysates; ACE,
angiotensin-converting enzyme. |
The ACE2, Ang (1–7) and
Mas concentrations in the kidney were measured and quantified by
ELISA and western blotting (Fig.
4). In each experimental period, there was a significant
difference in ACE2 concentrations between the every two groups. At
week 6, the ACE2 concentration levels in the IQP, VEP and SH group
were significantly increased by 1.09-, 0.83-, and 1.43-fold,
respectively, compared with the blank control group. At week 8,
following cessation of administration, the ACE concentrations
increased significantly in IQP, VEP and SH groups, respectively,
compared to week 3 (Fig. 4A). In
each experimental period, the captopril group had the highest level
of Ang (1–7) concentration, followed the SH group.
Only in the SH group was there a significant increase in Ang
(1–7) concentration at week 8 compared with
week 3 (Fig. 4B). Mas
concentrations in the kidney demonstrated the same trend as Ang
(1–7). At week 6, Mas concentrations in the
IQP, VEP and SH group were significantly upregulated by 1.68-,
1.41- and 2.64-fold, respectively, compared with the blank control
group. However, in captopril group, there was a significant
decrease in Mas concentration at week 8 compared with week 3
(Fig. 4C).
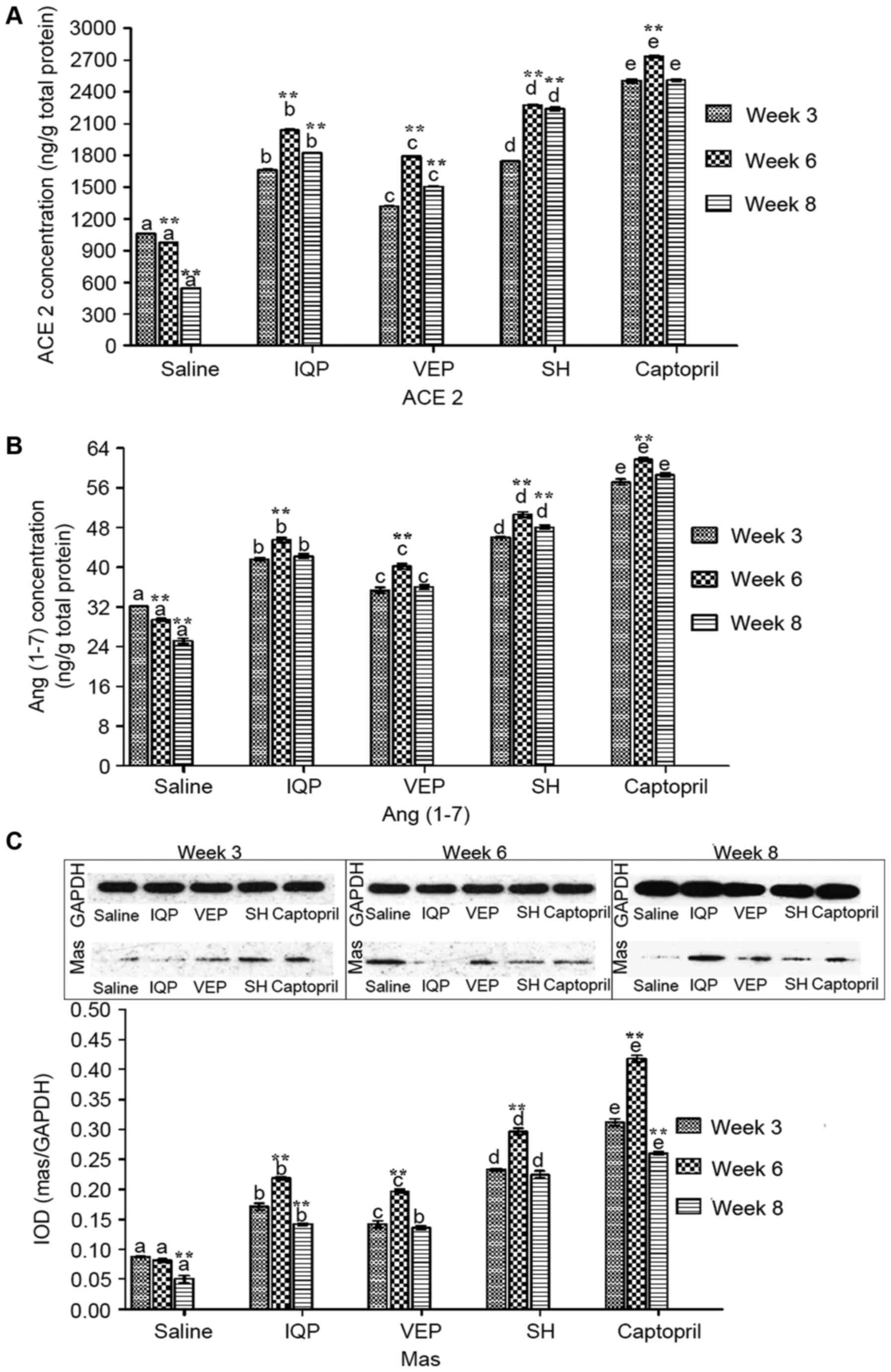 | Figure 4.Effects of IQP, VEP and SH on protein
concentrations of ACE2-Ang (1–7)-Mas
axis in the SHR kidney during the different experimental periods
(weeks 3, 6, and 8): (A) ACE2, (B) Ang (1–7), (C)
Mas. Data are represented as mean ± standard error of the mean (n=5
animals per treatment group). In each experimental period,
comparisons between different groups were performed by one-way
analysis of variance. Values with dissimilar lowercase letters
(a-e) were significantly different, P<0.05. Comparisons between
different experimental periods of the same treatment group by
one-way analysis of variance. **P<0.05 vs. week 3. IQP,
Ile-Gln-Pro; VEP, Val-Glu-Pro; SH, Spirulina platensis
hydrolysates; ACE, angiotensin-converting enzyme; Ang,
angiotensin. |
Effects of SH on the main components
in local kidney and myocardium RAS
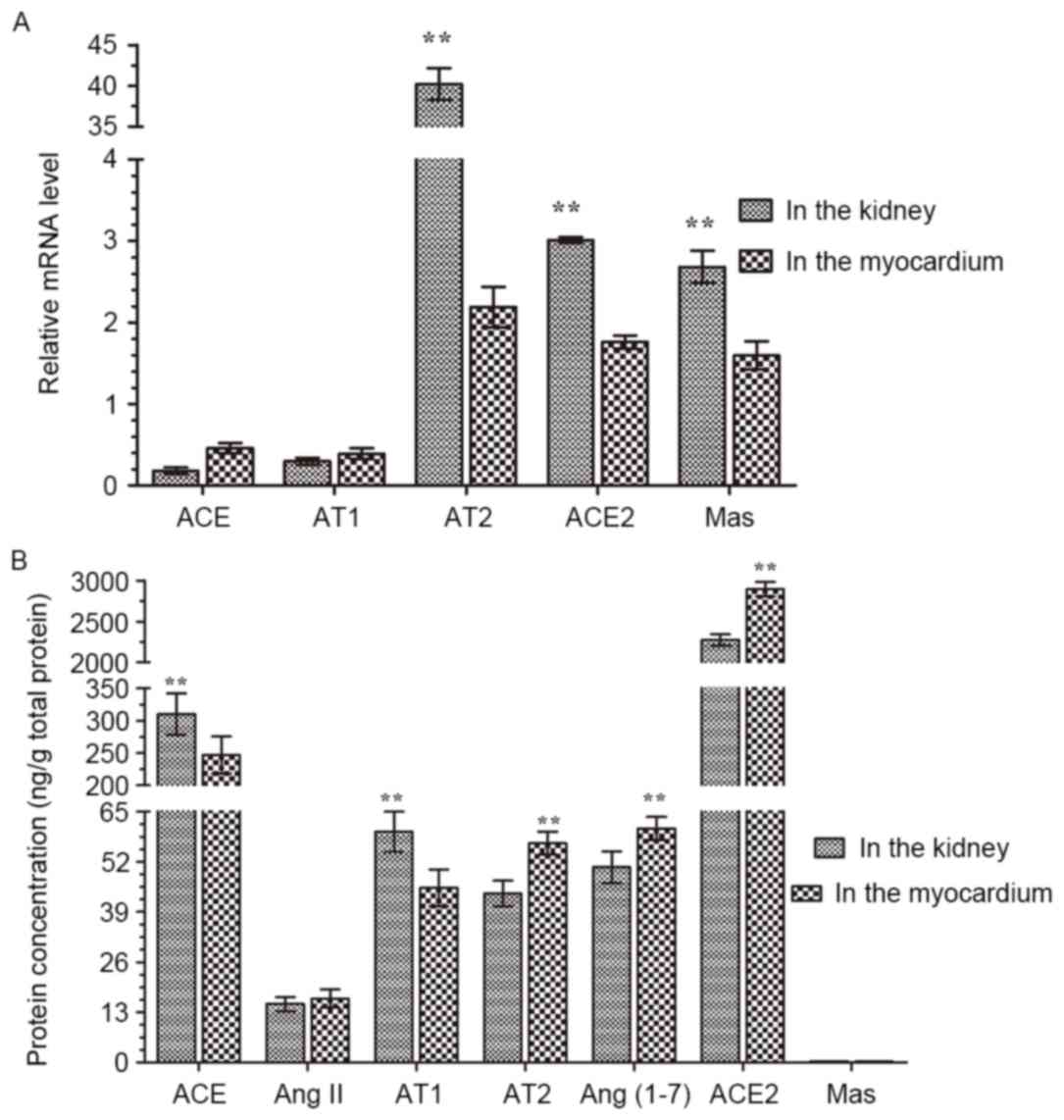
A comparison of the effects of SH on ACE, Ang II, AT
1, AT 2, ACE2, Ang (1–7) and Mas at week 6 between local kidney
and myocardium local RAS is presented in Fig. 5. For local kidney RAS, the mRNA
levels of AT 2, ACE2 and Mas were significantly higher than those
in the local myocardium RAS respectively. Of note, the AT 2 mRNA
level was 17-fold higher in the local kidney RAS than that in the
local myocardium RAS (Fig. 5A).
Protein concentrations of ACE and AT 1 in the local kidney RAS were
significantly higher than those in the local myocardium RAS, and
the concentrations of AT 2, ACE2 and Ang (1–7) were
significantly lower than those in the local myocardium RAS
(Fig. 5B).
Discussion
SHR is a well-established model of human essential
hypertension. Various studies to determine the antihypertensive
effects of food-derived bioactive peptides have used spontaneous
hypertensive animals as a model system (23–25).
Previous studies by Lu et al (26,27)
unraveled the short-term antihypertensive effects of IQP and VEP,
as well as the regulation on renin, Ang and other components of the
renal ACE-Ang II-AT 1 axis in SHR. Research by Pan et al
(16) demonstrated the long-term
antihypertensive effects of IQP, VEP and SH in SHR, and explained
the effects of these antihypertensive peptides on the expression of
local myocardium RAS components. In the current study, the authors
investigated the regulative effects of IQP, VEP and SH on the major
components of both the ACE-Ang II-AT 1 and ACE2-Ang (1–7)-Mas
axes in the local kidney RAS.
In the kidney, all RAS components are present, where
both classic and alternate pathways are operational (28). Studies by Lu et al (26,27)
demonstrated that one-week IQP and VEP treatment significantly
downregulated the mRNA levels of ACE, and AT 1 and upregulated the
mRNA expression of AT 2 in the kidney of SHR. The same trend was
also reported in this study when comparing ACE, AT 1 and AT 2 mRNA
expression at weeks 3 and 6 among the blank control group and the
three experimental groups, suggesting there was a close
relationship between the antihypertensive effects of IQP, VEP and
SH and the downregulation of ACE, and AT 1. Furthermore, the local
kidney RAS may be of high relevance for BP regulation as an
amplifier of circulating Ang II actions. Mice generating increased
renal Ang II, either by transgenic human RAS or by the local
overexpression of rat angiotensinogen, develop high BP (29). Results from the present study
indicated that the expression levels of Ang II in the kidney of SHR
were significantly reduced following treatment with IQP, VEP or SH,
especially at week 6, suggesting that BP reduction was accompanied
by lowered Ang II expression levels in the kidney. Therefore, the
authors conclude that, for kidney local RAS, suppression of the
ACE-Ang II-AT 1 axis in the kidney promotes protection against
hypertension. Furthermore, most of the evidence suggests that Ang
II increases BP by stimulating AT 1, and that AT 2 stimulates a
vasodilator signaling cascade that includes bradykinin, nitric
oxide and cGMP. AT 2 mediates some of the beneficial actions of AT
1 blockade through this pathway (30). In the present study, at week 6,
renal AT 2 mRNA level in the SH group was significantly increased
by 38.23-fold, and the concentration was increased by 1.61-fold
compared with the blank control group. Contrary results were
observed for AT 1. These results were similar to those of Yu et
al (31). Therefore, the
authors propose that, for long-term antihypertensive effects of
IQP, VEP and SH, renal AT 2 has an important role in
counterbalancing the effects of Ang II mediated by AT 1. In
addition, the ACE2-Ang (1–7)-Mas axis is also present in the local
kidney RAS. Červenka et al (32) proposed that the primary mechanism
responsible for the BP-lowering effects on chronic hypoxia in Ren-2
transgenic rats was suppression of the hypertensiogenic ACE-Ang II
axis in the circulation and kidney tissues, combined with
augmentation of the intrarenal vasodilator ACE2-Ang (1–7) axis
(32). In the current study, mRNA
levels and concentrations of ACE2, Ang (1–7) and
Mas in the kidney of the positive control group and experimental
groups were significantly upregulated, especially the SH group, in
the same experimental period, compared with the blank control
group. The results above indicated that IQP, VEP and SH treatments
decrease BP, at least in part, by affecting the expression of major
local RAS components by downregulating ACE, Ang II and AT 1 while
upregulating ACE2, Ang (1–7) and Mas in the kidney of SHR.
The authors investigated the effect of SH on local
tissue RAS (i.e., kidney and myocardium) in SHR following 6 weeks
of SH treatment. ACE and AT 1 mRNA levels in the local kidney were
significantly lower compared with those in the local myocardium
RAS, suggesting that for the local kidney RAS, SH reduces BP by
downregulating the ACE-Ang II-AT 1 pathway. The high AT 2 mRNA
level in the kidney also suggested that, for the local kidney RAS,
AT 2 mRNA level was influenced by SH to reduce the BP. The
catabolism of Ang II to produce Ang (1–7) is
the main function of ACE2 (33).
Although Zhong et al (17)
reported a significant upregulation of ACE2 mRNA and protein
expression in the heart and kidney, a comparison between the two
local RAS was not stated. Other studies reported that ACE2 is
highly expressed in the kidney, and its activity is much higher in
the kidney cortex than that in heart tissue (34,35).
In the current study, the ACE2 mRNA level was significantly higher
in the kidney compared with the myocardium, possibly because the
pathogenesis and progression of kidney diseases suggest that the
renal expression of ACE2 may have a role in controlling local RAS
rather than regulating systemic BP (35). Mas mRNA level in the kidney was
significantly higher than that in the myocardium, suggesting that
kidney local RAS upregulates the ACE2-Ang (1–7)-Mas
axis. Therefore, from the mRNA data, the kidney local RAS regulates
the two pathways to a greater extent than in the myocardium local
RAS. In addition, different components of the RAS are taken up by
different tissues, thereby influencing the local synthesis of Ang
II (36). However, in the current
study, there was no significant difference in Ang II content
between the myocardium and kidney local RAS, while the protein
levels of Ang (1–7) and ACE2 in myocardium were
significantly higher than those in kidney. Accumulating evidence
supports the idea that ACE2 is a key negative regulator of the RAS
where it metabolizes Ang II into Ang (1–7)
(37). Therefore, it was
speculated that a decrease of Ang II in the myocardium may be lower
than in the kidney, but abundant ACE2 in the myocardium might
convert Ang II to Ang (1–7). Moreover, there is good evidence that
the ACE2-Ang (1–7)-Mas axis serves an important role in
counterbalancing Ang II effects on the heart to maintain normal
cardiac function (38). Therefore,
from the perspective of the protein levels, in the myocardium local
RAS, SH reduced BP primarily by upregulating the ACE2-Ang (1–7)-Mas
axis.
Accumulating evidence suggests that local RAS exists
in various tissues, which operates independently of their systemic
counterparts (39). In the present
study, the differences in RAS regulation in the myocardium and
kidney were preliminarily compared. Analysis of the expression of
various RAS components suggests that the local kidney RAS regulates
BP by the ACE-Ang II-AT 1 axis and the ACE2-Ang (1–7)-Mas
axis mainly at the mRNA level, while the myocardium local RAS
regulates BP mainly at the protein level. However, the specific RAS
regulatory mechanism of gene expression in the two organs remains
to be determined, and the relationship between the two tissues and
circulating RAS is worthy of further investigation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31571772,
31671963 and 31201339) and the Major State Research Development
Program of China (grant no. 2016YFD0400604).
Glossary
Abbreviations
Abbreviations:
|
RAS
|
renin-angiotensin system
|
|
BP
|
lood pressure
|
|
IQP
|
Ile-Gln-Pro
|
|
VEP
|
Val-Glu-Pro
|
|
SH
|
Spirulina platensis
hydrolysates
|
|
SHR
|
spontaneously hypertensive rats
|
|
ACE
|
angiotensin-converting enzyme
|
|
Ang II
|
angiotensin II
|
|
AT 1
|
angiotensin type-1 receptor
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
World Health Organization (WHO), . A
global brief on hypertension. http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdfApril
3–2013
|
|
2
|
Varagic J, Ahmad S, Nagata S and Ferrario
CM: ACE2: Angiotensin II/angiotensin-(1–7) balance in cardiac and
renal injury. Curr Hypertens Rep. 16:4202014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwai M and Horiuchi M: Devil and angel in
the renin-angiotensin system: ACE-angiotensin II-AT 1 axis vs.
ACE2-angiotensin-(1–7)-Mas receptor axis. Hypertens Res.
32:533–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samuel P, Ali Q, Sabuhi R, Wu Y and
Hussain T: High Na intake increases renal angiotensin II levels and
reduces expression of the ACE2-AT(2)R-MasR axis in obese Zucker
rats. Am J Physiol Renal Physiol. 303:F412–F419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobori H, Nangaku M, Navar LG and
Nishiyama A: The intrarenal renin-angiotensin system: From
physiology to the pathobiology of hypertension and kidney disease.
Pharmacol Rev. 59:251–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozkayar N, Dede F, Akyel F, Yildirim T,
Ateş I, Turhan T and Altun B: Relationship between blood pressure
variability and renal activity of the renin-angiotensin system. J
Hum Hypertens. 30:297–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen Dinh Cat A and Touyz RM: A new look
at the renin-angiotensin system-focusing on the vascular system.
Peptides. 32:2141–2150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Velez JC: The importance of the intrarenal
renin-angiotensin system. Nat Clin Pract Nephrol. 5:89–100. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carey RM: The intrarenal renin-angiotensin
system in hypertension. Adv Chronic Kidney Dis. 22:204–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrão FM, Lara LS and Lowe J:
Renin-angiotensin system in the kidney: What is new? World J
Nephrol. 3:64–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giani JF, Shah KH, Khan Z, Bernstein EA,
Shen XZ, McDonough AA, Gonzalez-Villalobos RA and Bernstein KE: The
intrarenal generation of angiotensin II is required for
experimental hypertension. Curr Opin Pharmacol. 21:73–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naito Y, Tsujino T, Fujioka Y, Ohyanagi M
and Iwasaki T: Augmented diurnal variations of the cardiac
renin-angiotensin system in hypertensive rats. Hypertension.
40:827–833. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Re RN: The clinical implication of tissue
renin angiotensin systems. Curr Opin Cardiol. 16:317–327. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paul M, Mehr A Poyan and Kreutz R:
Physiology of local renin-angiotensin systems. Physiol Rev.
86:747–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fedoseeva LA, Riazanova MA, Antonov EV,
Dymshits GM and Markel' AL: Renin-angiotensin system gene
expression in the kidney and in the heart in hypertensive ISIAH
rats. Biomed Khim. 57:410–419. 2011.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan H, She X, Wu H, Ma J, Ren D and Lu J:
Long-term regulation of the local renin-angiotensin system in the
myocardium of spontaneously hypertensive rats by feeding bioactive
peptides derived from spirulina platensis. J Agric Food Chem.
63:7765–7774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong JC, Huang DY, Yang YM, Li YF, Liu
GF, Song XH and Du K: Upregulation of angiotensin-converting enzyme
2 by all-trans retinoic acid in spontaneously hypertensive rats.
Hypertension. 44:907–912. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malikova E, Galkova K, Vavrinec P,
Vavrincova-Yaghi D, Kmecova Z, Krenek P and Klimas J: Local and
systemic renin-angiotensin system participates in
cardiopulmonary-renal interactions in monocrotaline-induced
pulmonary hypertension in the rat. Mol Cell Biochem. 418:147–157.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Ren DF, Xue YL, Sawano Y, Miyakawa T
and Tanokura M: Isolation of an antihypertensive peptide from
alcalase digest of spirulina platensis. J Agric Food Chem.
58:7166–7171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu J, Ren DF, Wang JZ and Tanokura M:
Purification and characterization of an angiotensin I-converting
enzyme inhibitory peptide derived from Spirulina platensis. Prog
Biochem Biophys. 37:568–574. 2010. View Article : Google Scholar
|
|
21
|
Buñag RD: Validation in awake rats of a
tail-cuff method for measuring systolic pressure. J Appl Physiol.
34:279–282. 1973.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siragy HM: AT(1) and AT(2) receptors in
the kidney: Role in disease and treatment. Am J Kidney Dis. 36(3
Suppl 1): S4–S9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marczak ED, Usui H, Fujita H, Yang Y,
Yokoo M, Lipkowski AW and Yoshikawa M: New antihypertensive
peptides isolated from rapeseed. Peptides. 24:791–798. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Igarashi K, Yoshioka K, Mizutani K,
Miyakoshi M, Murakami T and Akizawa T: Blood pressure-depressing
activity of a peptide derived from silkworm fibroin in
spontaneously hypertensive rats. Biosci Biotechnol Biochem.
70:517–520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu J, Sawano Y, Miyakawa T, Xue YL, Cai
MY, Egashira Y, Ren DF and Tanokura M: One-week antihypertensive
effect of Ile-Gln-Pro in spontaneously hypertensive rats. J Agric
Food Chem. 59:559–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu J, Yang Y, Chen L, Ren DF, Cai MY, Wang
JZ, Egashira Y and Tanokura M: In vivo antihypertensive effect of
Val-Glu-Pro in spontaneously hypertensive rats. Prog Biochem
Biophys. 38:353–360. 2011. View Article : Google Scholar
|
|
28
|
Majumder K, Chakrabarti S, Morton JS,
Panahi S, Kaufman S, Davidge ST and Wu J: Egg-derived tri-peptide
IRW exerts antihypert effects in spontaneously hypertensive rats.
PLoS One. 8:e828292013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bader M and Ganten D: Update on tissue
renin-angiotensin systems. J Mol Med (Berl). 86:615–621. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carey RM, Jin XH and Siragy HM: Role of
the angiotensin AT2 receptor in blood pressure regulation and
therapeutic implications. Am J Hypertens. 14:98S–102S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Z, Yin Y, Zhao W, Chen F and Liu J:
Antihypertensive effect of angiotensin-converting enzyme inhibitory
peptide RVPSL on spontaneously hypertensive rats by regulating gene
expression of the renin-angiotensin system. J Agric Food Chem.
62:912–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Červenka L, Bíbová J, Husková Z,
Vaňourková Z, Kramer HJ, Herget J, Jíchová Š, Sadowski J and Hampl
V: Combined suppression of the intrarenal and circulating
vasoconstrictor renin-ACE-ANG II axis and augmentation of the
vasodilator ACE2-ANG 1–7-Mas axis attenuates the systemic
hypertension in Ren-2 transgenic rats exposed to chronic hypoxia.
Physiol Res. 64:11–24. 2015.PubMed/NCBI
|
|
33
|
Mizuiri S and Ohashi Y: ACE and ACE2 in
kidney disease. World J Nephrol. 4:74–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soler MJ, Wysocki J and Batlle D: ACE2
alterations in kidney disease. Nephrol Dial Transpl. 28:2687–2697.
2013. View Article : Google Scholar
|
|
35
|
Kuba K, Imai Y and Penninger JM: Multiple
functions of angiotensin-converting enzyme 2 and its relevance in
cardiovascular diseases. Circ J. 77:301–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Mello WC and Frohlich ED: On the local
cardiac renin angiotensin system. Basic and clinical implications.
Peptides. 32:1774–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Patel VB, Clarke N, Wang Z, Fan D,
Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ and Oudit GY:
Angiotensin II induced proteolytic cleavage of myocardial ACE2 is
mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS.
J Mol Cell Cardiol. 66:167–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patel KP and Schultz HD: Angiotensin
peptides and nitric oxide in cardiovascular disease. Antioxid Redox
Sign. 19:1121–1132. 2013. View Article : Google Scholar
|
|
39
|
Wolf G and Ritz E: Combination therapy
with ACE inhibitors and angiotensin II receptor blockers to halt
progression of chronic renal disease: Pathophysiology and
indications. Kidney Int. 67:799–812. 2005. View Article : Google Scholar : PubMed/NCBI
|