Introduction
Age-related macular degeneration (AMD) is a leading
cause of irreversible blindness worldwide, and affects 6.5% of the
US population aged ≥40 years (1).
AMD is classified into wet and dry AMD. The latter is the most
common phenotype, which is characterized by an increase in the
number and diameter of drusen, progressive atrophy of the retinal
pigment epithelium (RPE), pigmentary irregularities and a graded
loss in visual acuity (2–4). Previous studies have indicated that
environmental and genetic factors influence the disease, including
smoking, obesity and dietary fat intake (5–8).
It has been observed that oxidative damage and
inflammation are involved in the pathogenesis of AMD (9,10).
Furthermore, the complement system, a vital component of innate
immunity and a complex network that contributes to inflammation,
has been identified to be associated with the pathogenesis and
progression of AMD (11).
Clusterin, also known as apolipoprotein J because it is present in
high-density lipoprotein complexes, has been indicated to be a
negative regulator of the complement cascade and exhibits increased
mRNA expression levels in the RPE cells of AMD patients (12). Clusterin is involved in cholesterol
transportation and protein aggregation in human plasma, and a
deficiency of this complement regulatory protein may contribute to
inflammation and neovascularization (13–15).
Epigenetic mechanisms, including DNA methylation,
chromatin remodeling, histone modification and non-coding
RNA-mediated regulation, have been demonstrated to influence gene
expression and interactions between the genetics and the
environment during development (16). Non-coding RNA consists of long
non-coding RNAs (lncRNAs) and microRNAs (miRNAs). Recent epigenetic
and genetic evidence has indicated that miRNAs, such as miR-146A,
miRNA-9, miRNA-155 and miRNA-125b are upregulated to modulate a
family of potentially pathogenic genes involved in Alzheimer's
disease and cancer (17–20). In 2011, Salmena et al
(21) proposed a competing
endogenous RNA (ceRNA) hypothesis, which is now supported by
further evidence (22–24). The hypothesis suggests that a
regulatory network exists whereby protein-coding and non-coding
RNAs compete for binding to miRNAs by sharing miRNA-binding sites.
Nevertheless, there is a lack of data on expression patterns of
specific lncRNAs in AMD patients. In addition, it is unknown
whether lncRNAs participate in the regulation of clusterin-related
inflammation, or whether some lncRNAs are induced to aberrantly
express or to participate in an altered ceRNA network after RPE
cells are exposed to clusterin.
The aim of the present study was to elucidate the
effects of clusterin on the expression of coding and non-coding RNA
in RPE cells. The expressed mRNAs, miRNAs and lncRNAs were profiled
in RPE cells treated with or without clusterin, and the ceRNA
network was characterized. To the best of our knowledge, this was
the first study to investigate the disease-specific lncRNA
expression patterns and the associated ceRNA network in RPE
cells.
Materials and methods
Cell culture
The adult human RPE cell line D407 (Procell Life
Science and Technology Co., Ltd., Wuhan, China) was cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 µg/ml streptomycin, 100 U/ml penicillin and 2.5 µg/ml
amphotericin B at 37°C and 95% humidity with a 5% CO2
atmosphere.
MTT viability assay
D407 cells were seeded in 96-well plates at a
density of 1×104 cells/well and allowed to reach 90%
confluence. The growth medium was subsequently replaced with medium
supplemented with clusterin at 0 (control), 0.1, 1.0 or 10 µg/µl.
Cells were incubated for 48 h and analyzed using an MTT assay.
Briefly, the medium was removed and replaced with DMEM supplemented
with 5 mg/ml MTT reagent. Following incubation for 4 h, cells were
solubilized with 95% dimethyl sulfoxide and the absorbance of the
solution was measured at 590 nm using an ELISA plate reader. Each
sample well was assessed in triplicate, and all assays were run in
triplicate. Statistical analysis was performed using one-way
analysis of variance and Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference. Data
were presented as the mean ± standard error of the mean.
RNA extraction
Total RNA was isolated from D407 cells treated
without clusterin (0 µg/µl; control) or 1.0 µg/µl clusterin using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The extracted RNA
sample was treated with DNase I (Invitrogen; Thermo Fisher
Scientific, Inc.) to remove the DNA. The RNA quality was analyzed
using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Inc.)
and 10% gel electrophoresis. RNA samples were stored at −80°C for
sequencing library construction.
Construction of sequencing library and
RNA-sequencing (Seq)
An mRNA library was constructed according to the
manufacturer's instructions using TruSeq Stranded mRNA Library Prep
kit (Illumina, Inc., San Diego, CA, USA). Briefly, mRNA was
enriched using oligo (dT) magnetic beads and then broken to ~200 bp
fragments. The first-strand cDNA was synthesized using random
hexamer primers, and then the second strand was synthesized. The
purified DNA fragments were then sequenced using an Illumina Hiseq
2000 (Illumina, Inc.) by Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). The small RNA library was constructed according to
Illumina's recommended protocols and sequenced on a Hiseq 2000
platform at RiboBio Co., Ltd. All sequences were deposited onto the
GEO database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE87167).
Data analysis
Sequence reads were aligned to the hg19 human genome
using Bowtie (25) with Tophat
(26). Only reads that had ≤2
mismatches and 2 gaps to the hg19 genome were accepted. To assess
the transcript level in the sample (slug-ml-3) and control (NC-3),
the reads per kilobase of transcript per million mapped reads value
was calculated. The number of mapped reads was calculated for each
miRNA and the read count of each unique sequence was normalized
using the reads per million (RPM) normalization, according to the
formula: RPM = (number of reads mapping to miRNA reference / total
number of mapped reads) × 106. Differential expression
analysis was performed using Cuffdiff (26). Protein-coding transcripts and
lncRNAs with fold change (FC)>1.5 or FC<0.67 and P<0.05
were considered to be differentially expressed in D407 cells
treated with clusterin, whereas miRNAs with FC>2 or FC<0.5
(P<0.05) were considered aberrantly expressed.
Each sequence was annotated by aligning to known RNA
sequences downloaded from Rfam11.0 (http://rfam.xfam.org/; rRNA, snRNA and snoRNA),
miRBase (http://www.mirbase.org/release version 21.0; miRNA),
UCSC (http://genome.ucsc.edu; tRNA) and
pirnabank (http://pirnabank.ibab.ac.in/; piRNA). Novel miRNAs
were predicted using the miREAP algorithm (27) according to the manual. RNA
secondary structures were predicted using RNAfold (28).
Functional annotation of
differentially expressed mRNAs
Gene Ontology (GO) (29) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) (30) pathway
analysis of differentially expressed mRNAs was performed using the
database for annotation, visualization and integrated discovery
(DAVID, version 6.7) (31). The
obtained GO terms and KEGG categories were filtered at a threshold
significance value of P<0.05.
Construction of ceRNA network
The ceRNA network was constructed and visualized
using Cytoscape software as preciously described (32). The process included prediction of
lncRNA-miRNA interactions by PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html)
(33), RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
(34) and miRanda (http://www.microrna.org) (35), targeting of mRNAs by miRNAs based
on experimental data using miRTarBase (http://www.microrna.gr/tarbase) (36), miRwalk (http://mirwalk.uni-hd.de/) (37) and miRanda, and filtration of
specific miRNA-lncRNA and miRNA-mRNA pairs, where the interaction
pairs containing differentially expressed miRNA and lncRNA or mRNA
were retained. A network map was constructed and visualized using
Cytoscape software v3.5.1 (32).
Results
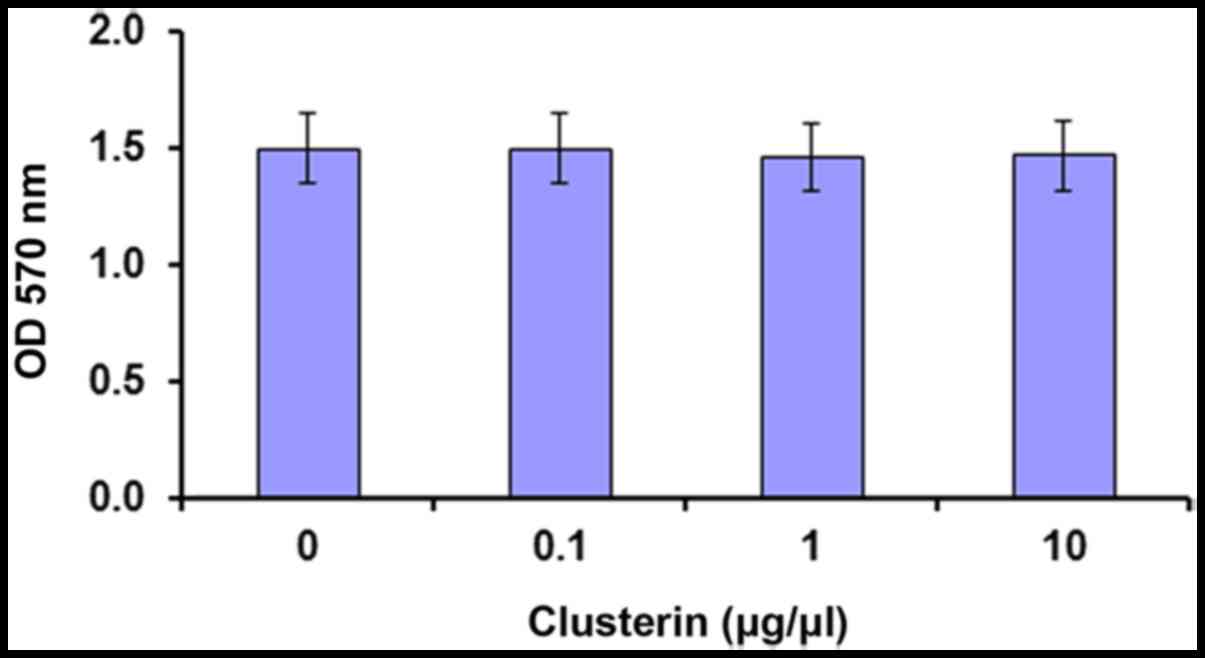
Effect of clusterin on cell
viability
In consideration of the abnormal secretion of
clusterin in RPE cells from AMD donors (38), clusterin activity on the viability
of D407 cell viability was investigated using MTT assay. The
viability of D407 cells was not affected by clusterin at tested
concentrations (Fig. 1).
RNA profiling in RPE cells
Total RNA isolated from D407 cells treated or
untreated with clusterin was sequenced. After filtering out reads
without a 3′, 5′ adaptor sequence and poly-A reads, the slug-ml-s
and NC-3 mRNA libraries had 12,378,042 and 12,385,191 mapped reads,
respectively (Table I). Notably,
the distribution of the mapped reads in the experimental and
control groups were similar between control and clusterin-treated
RPE cells. The small RNA sequences were annotated according to
their overlap with the sequences of known RNAs. The annotated
results suggested that small RNA libraries from slug-ml-3 and NC-3
contained the same classes of RNAs (Table II). Furthermore, the abundance of
each class was similar between the two libraries.
 | Table I.The distribution of mapped reads in
experimental and control groups. |
Table I.
The distribution of mapped reads in
experimental and control groups.
| Region | Slug-ml-3 mapped
reads | NC-3 mapped
reads |
|---|
| All | 12,378,042 | 12,385,191 |
|
Upstream/downstream | 171,120 | 160,934 |
| Exonic | 66,229 | 80,603 |
| Intergenic | 3,191,258 | 2,709,256 |
| Intronic | 1,116,005 | 1,116,007 |
| NcRNA_exonic | 7,642,921 | 8,092,812 |
| NcRNA_intronic | 110,292 | 134,888 |
| Splicing | 2,720 | 2,856 |
| UTR | 77,497 | 87,835 |
 | Table II.Annotation of noncoding RNAs. |
Table II.
Annotation of noncoding RNAs.
|
| Slug-ml-3 | NC-3 |
|---|
|
|
|
|
|---|
| Type | Total sRNA | % | Total sRNA | % |
|---|
| All | 13,393,799 | 100.0 | 13,233,217 | 100.0 |
| miRNA | 5,547,804 | 41.4 | 6,436,571 | 48.7 |
| tRNA | 472,065 | 3.5 | 452,319 | 3.4 |
| rRNA | 1,648,269 | 12.3 | 1,417,235 | 10.7 |
| snRNA | 621,981 | 4.6 | 435,469 | 3.3 |
| snoRNA | 423,883 | 3.2 | 440,867 | 3.3 |
| piRNA | 504,767 | 3.8 | 451,597 | 3.4 |
| Y_RNA | 126,656 | 1.0 | 126,592 | 1.0 |
| Others | 4,048,374 | 30.2 | 3,472,567 | 26.2 |
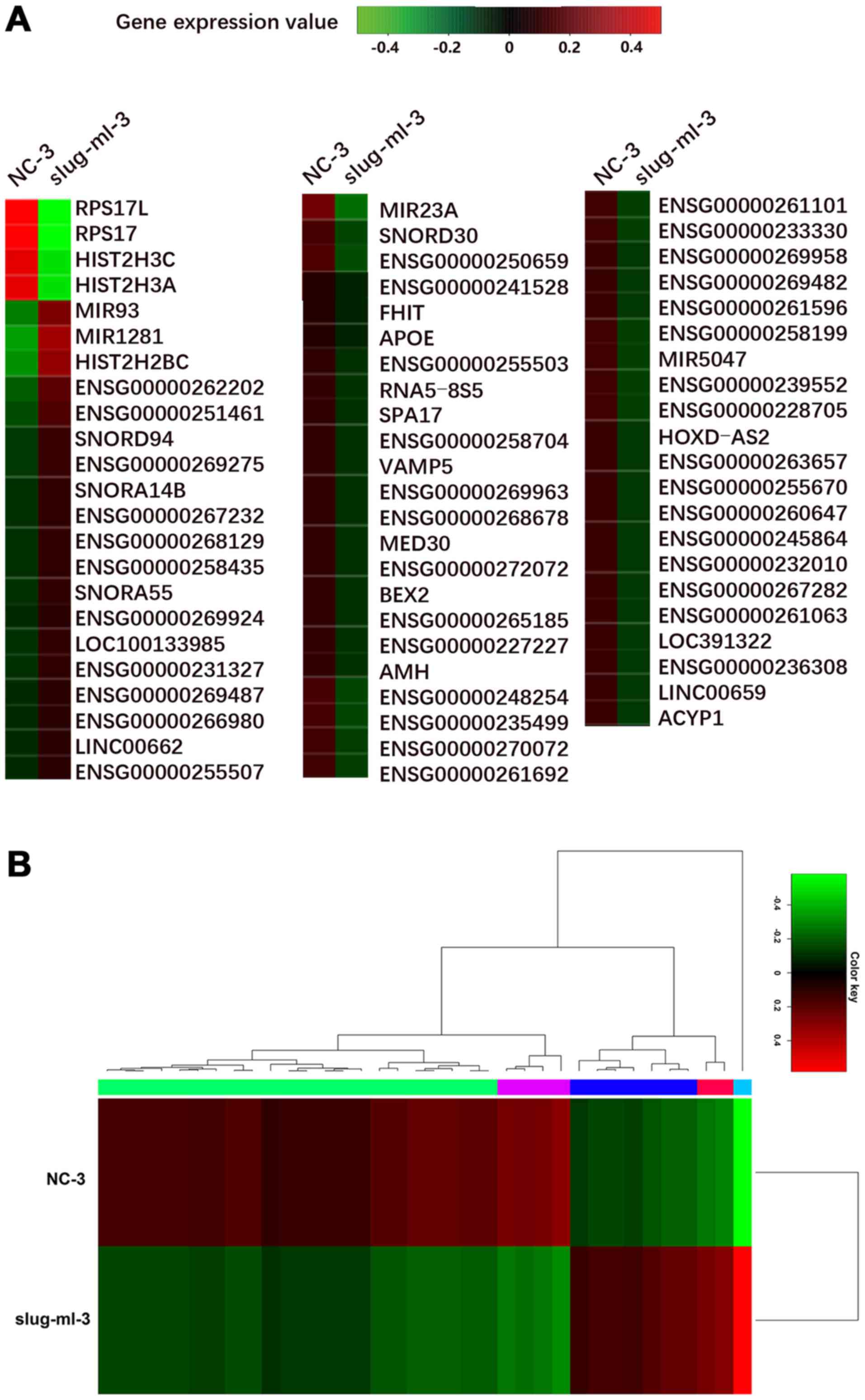
Identification of differentially
expressed coding and non-coding genes
The expression levels of differentially expressed
mRNA, miRNA and lncRNA were further analyzed between slug-ml-3 and
NC-3 cells. A total of 321 genes were significantly differentially
expressed, including 71 up- and 250 downregulated genes. Among
them, 67 important genes are presented in Fig. 2A.
Aberrantly expressed miRNAs were detected based on
the threshold of FC>2 or FC<0.5, and P<0.05. When miRNA
expression was compared between D407 cells treated with and without
clusterin, 36 miRNAs were observed as aberrantly expressed
(Fig. 2B). To identify novel
miRNAs from the reads mapped to unannotated genomic regions, the
described miRNA discovery algorithm, miREAP, which is a tool for
discovering novel miRNAs from small RNA deep sequencing data, was
employed. A total of 71 and 76 candidate miRNAs were identified and
considered to be novel in slug-ml-3 and NC-3, respectively.
Differentially expressed lncRNAs were also detected
between slug-ml-3 and NC-3 cells. In total, 296 previously
annotated lncRNAs and 90 novel lncRNAs were aberrantly regulated in
cells treated with clusterin.
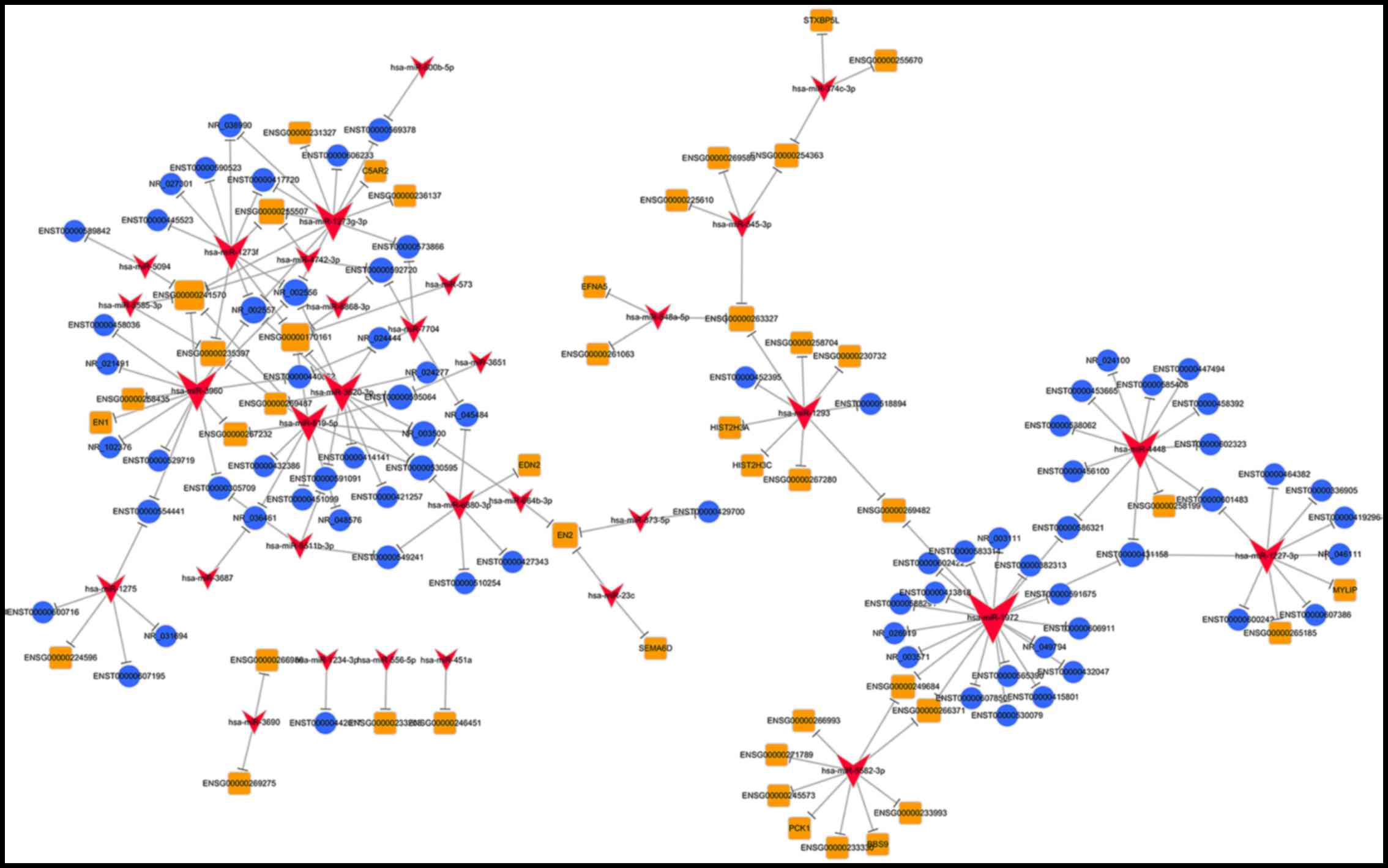
ceRNA network generation
miRNAs interact with lncRNAs via miRNA response
elements within the ceRNA network. Therefore, potential miRNA
response elements in lncRNAs were investigated. The results
indicated that 22 of 36 specific miRNAs may interact with 75 of 386
lncRNAs (data not shown). To establish a lncRNA-miRNA-mRNA network
(ceRNA network), mRNAs that were targeted by miRNAs were
investigated. Using the list of miRNAs that may interact with
lncRNAs, miRNA targeting mRNAs were searched for using miRTarBase,
miRwalk and miRanda. From this analysis, 23 miRNAs were identified
(data not shown). Most of the targets of these miRNAs were
AMD-associated genes such as engrailed homeobox (EN)1, EN2,
endothelin 2, myosin regulatory light chain interacting protein and
complement component 5a receptor 2 (C5AR2). Based on these data, a
ceRNA network (Fig. 3) was
established; 75 lncRNAs and 32 miRNAs were involved in the proposed
ceRNA network.
Functional analysis of differentially
expressed mRNAs
In order to elucidate the molecular model of action
of clusterin in AMD; biological processes and pathways regulated by
clusterin were analyzed using DAVID. Enriched GO terms including
Biological Process, Cellular Component and Molecular Function
annotations were assessed. The results indicated that
differentially expressed mRNAs were predominantly associated with
the regulation of lipid transport across the blood brain barrier,
receptor catabolic process, postsynaptic membrane organization and
phospholipid transport (Fig. 4A and
B). To understand the signaling pathways involved in the ceRNA
network, the mRNAs were analyzed using the DAVID algorithm. The
principally enriched pathways were environmental information
processing, genetic information processing, cellular processing,
organismal systems, drug development, human diseases and metabolism
(Fig. 4C and D). The results
indicated that differentially expressed mRNAs were enriched in the
adherens junction and cell adhesion molecules in addition to
extracellular matrix-receptor interactions. Furthermore, these
mRNAs were largely involved in diseases including small cell lung
cancer, arrhythmogenic right ventricular cardiomyopathy and
hypertrophic cardiomyopathy.
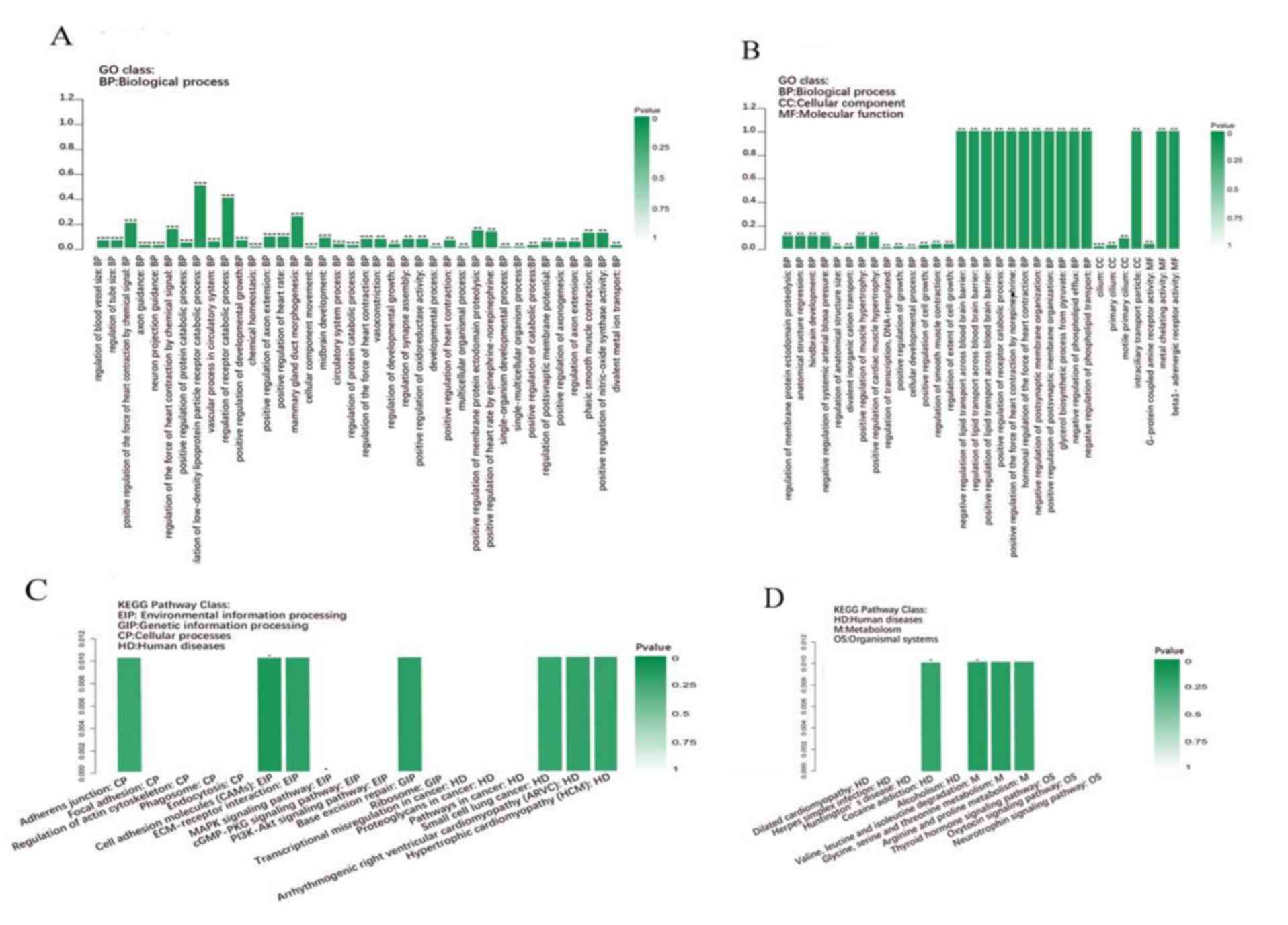 | Figure 4.GO enrichment analysis of
differentially expressed genes. (A and B) GO analysis and (C and D)
KEGG pathway enrichment analysis of differentially expressed genes
identified from human retinal pigment epithelial cell line D407
treated with or without clusterin. GO, gene ontology; BP,
biological process; CC, cellular component; MF, molecular function;
KEGG, Kyoto Encyclopedia of Genes and Genomes; EIP, environmental
information processing; GIP, genetic information processing; CP,
cellular processes; HD, human diseases; M, metabolism; OS,
organismal systems. ***P<0.001; **P<0.01; *P<0.05. |
Discussion
lncRNAs are considered as important regulators of
biological processes in human diseases (39,40).
However, few studies have reported the lncRNAs expression profile
in retinal disorder-associated diseases including AMD, using a
high-throughput sequencing strategy (41). Increasing evidence has demonstrated
that there are interactions between mRNA and lncRNA (42) or lncRNA and miRNA (43,44)
in cancer development, suggesting that lncRNAs may function as a
part of ceRNA network. However, ceRNA networks in AMD are still
poorly explored. The present study served to identify miRNA and
lncRNA expression and distribution in RPE cells following treatment
with clusterin, based on genome-wide expression profiling.
Furthermore, a ceRNA network was constructed among mRNAs, miRNAs
and lncRNAs, to provide a systematic view of lncRNA-miRNA-mRNA
interactions.
Based on next generation RNA sequence data, 386
lncRNAs were observed to be differentially expressed between RPE
cells treated with or without clusterin. Abnormally expressed
miRNAs and mRNAs were also identified, and 75 lncRNAs were
identified to be involved in the ceRNA network, suggesting they may
serve role in AMD development. Among the lncRNAs involved in the
ceRNA network, NR_036461 (HIST2H2BC) was reported to promote cell
proliferation and invasion in triple-negative breast cancer
(45). Furthermore, NR_102376
(small integral membrane protein 27) was identified to be a
potential biomarker for human breast cancer (46). From these results, it was concluded
that there was a differential expression pattern of certain lncRNAs
between RPE cells with and without clusterin treatment. Further
studies are required to elucidate the role of these differentially
expressed lncRNAs.
To improve the predicted network, three databases
including PITA, RNAhybrid and miRanda were applied to filter the
pair-wised relationships based on lncRNA-miRNA expression
correlations. This strategy served to identify additional lncRNAs,
compared with using a single database. To enhance the reliability
of the data, validated miRNA target genes were acquired from the
miRTarBase database that had associated experimental supporting
data.
Several genes involved in inflammation and cell
adhesion were identified to participate in the ceRNA network after
treating RPE cells with clusterin. For instance, engrailed (EN)1,
EN2, endothelin 2, myosin regulatory light chain interacting
protein, complement component 5a receptor 2 (C5AR2), lncRNA
NR_036461 (HIST2H2BC) and NR_102376 (TOPORS-AS1) miRNA
hsa-miR-1273g-3p, hsa-miR-5582-3p and hsa-miR-1293 were identified
to be associated with AMD. Moreover, we found that hsa-miR-1273g-3p
was closely associated with inflammation. C5AR2, which is mediated
by hsa-miR-1273g-3p, has been indicated to be involved in
C5a-mediated human mast cell adhesion and proinflammatory mediator
production (47). Inflammation is
well recognized as a molecular mechanism of AMD (48). Therefore, differentially expressed
lncRNAs may serve important roles in AMD development.
In conclusion, the present study identified lncRNAs
specifically induced by clusterin in RPE cells and identified an
abnormal expression pattern of these lncRNAs. A ceRNA network was
constructed that may provide a novel method to identify AMD-related
lncRNA alterations in AMD. These results suggest that lncRNAs
specifically identified in RPE cells may participate in a complex
ceRNA network and in unknown regulatory pathways in the network,
and may contribute to our understanding of the biological mechanism
underlying AMD.
Acknowledgements
The current study was supported by the China Social
Assistance Fund (grant no. BJ-LM2015001 J).
References
|
1
|
Klein R, Chou CF, Klein BE, Zhang X, Meuer
SM and Saaddine JB: Prevalence of age-related macular degeneration
in the US population. Arch Ophthalmol. 129:75–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gehrs KM, Anderson DH, Johnson LV and
Hageman GS: Age-related macular degeneration-emerging pathogenetic
and therapeutic concepts. Ann Med. 38:450–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gehrs KM, Jackson JR, Brown EN, Allikmets
R and Hageman GS: Complement, age-related macular degeneration and
a vision of the future. Arch Ophthalmol. 128:349–358. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson DH, Radeke MJ, Gallo NB, Chapin
EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G,
et al: The pivotal role of the complement system in aging and
age-related macular degeneration: Hypothesis re-visited. Prog Retin
Eye Res. 29:95–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chakravarthy U, Wong TY, Fletcher A,
Piault E, Evans C, Zlateva G, Buggage R, Pleil A and Mitchell P:
Clinical risk factors for age-related macular degeneration: A
systematic review and meta-analysis. BMC Ophthalmol. 10:312010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chong EW, Robman LD, Simpson JA, Hodge AM,
Aung KZ, Dolphin TK, English DR, Giles GG and Guymer RH: Fat
consumption and its association with age-related macular
degeneration. Arch Ophthalmol. 127:674–680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seddon JM, George S and Rosner B:
Cigarette smoking, fish consumption, omega-3 fatty acid intake, and
associations with age-related macular degeneration: The US twin
study of age-related macular degeneration. Arch Ophthalmol.
124:995–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Age-Related Eye Disease Study Research
Group, ; SanGiovanni JP, Chew EY, Clemons TE, Ferris FL III,
Gensler G, Lindblad AS, Milton RC, Seddon JM and Sperduto RD: The
relationship of dietary carotenoid and vitamin AE, and C intake
with age-related macular degeneration in a case-control study:
AREDS Report No. 22. Arch Ophthalmol. 125:1225–1232. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donoso LA, Kim D, Frost A, Callahan A and
Hageman G: The role of inflammation in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 51:137–152.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beatty S, Koh H, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reynolds R, Hartnett ME, Atkinson JP,
Giclas PC, Rosner B and Seddon JM: Plasma complement components and
activation fragments: Associations with age-related macular
degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci.
50:5818–5827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suuronen T, Nuutinen T, Ryhänen T,
Kaarniranta K and Salminen A: Epigenetic regulation of
clusterin/apolipoprotein J expression in retinal pigment epithelial
cells. Biochem Biophys Res Commun. 357:397–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson DH, Mullins RF, Hageman GS and
Johnson LV: A role for local inflammation in the formation of
drusen in the aging eye. Am J Ophthalmol. 134:411–431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jenne DE, Lowin B, Peitsch M, Böttcher A,
Schmitz G and Tschopp J: Clusterin (complement lysis inhibitor)
forms a high density lipoprotein complex with apolipoprotein AI in
human plasma. J Biol Chem. 266:11030–11036. 1991.PubMed/NCBI
|
|
15
|
Gemenetzi M and Lotery AJ: The role of
epigenetics in age-related macular degeneration. Eye (Lond).
28:1407–1417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bell JT and Spector TD: A twin approach to
unraveling epigenetics. Trends Genet. 27:116–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li YY, Cui JG, Hill JM, Bhattacharjee S,
Zhao Y and Lukiw WJ: Increased expression of miRNA-146a in
Alzheimer's disease transgenic mouse models. Neurosci Lett.
487:94–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li YY, Cui JG, Dua P, Pogue AI,
Bhattacharjee S and Lukiw WJ: Differential expression of
miRNA-146a-regulated inflammatory genes in human primary neural,
astroglial and microglial cells. Neurosci Lett. 499:109–113. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pogue AI, Percy ME, Cui JG, Li YY,
Bhattacharjee S, Hill JM, Kruck TP, Zhao Y and Lukiw WJ:
Up-regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal
sulfate-stressed human astroglial (HAG) primary cell cultures. J
Inorg Biochem. 105:1434–1437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kociok N and Joussen AM: Enhanced
expression of the complement factor H mRNA in proliferating human
RPE cells. Graefes Arch Clin Exp Ophthalmol. 248:1145–1153. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ebert MS, Neilson JR and Sharp PA:
MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian
cells. Nat Methods. 4:721–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poliseno L, Salmena L, Zhang J, Carver B,
Haveman WJ and Pandolfi PP: A coding-independent function of gene
and pseudogene mRNAs regulates tumour biology. Nature.
465:1033–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H: CREB up-regulates long non-coding
RNA, HULC expression through interaction with microRNA-372 in liver
cancer. Nucleic Acids Res. 38:5366–5383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Langmead B: Aligning short sequencing
reads with Bowtie. Curr Protoc Bioinformatics Chapter. 11:Unit
11.72010.
|
|
26
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Li Q, Wang J, Guo X, Jiang X, Ren
Z, Weng C, Sun G, Wang X, Liu Y, et al: Identification and
characterization of novel amphioxus microRNAs by Solexa sequencing.
Genome Biol. 10:R782009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hofacker IL, Fontana W, Stadler PF,
Bonhoeffer LS, Tacker M and Schuster P: Fast folding and comparison
of RNA secondary structures. Monatshefte für Chemie/Chemical
Monthly. 125:167–188. 1994. View Article : Google Scholar
|
|
29
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization, and integrated discovery. Genome Biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nat Genet. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rehmsmeier M, Steffen P, Hochsmann M and
Giegerich R: Fast and effective prediction of microRNA/target
duplexes. RNA. 10:1507–1517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vlachos IS, Paraskevopoulou MD, Karagkouni
D, Georgakilas G, Vergoulis T, Kanellos I, Anastasopoulos IL,
Maniou S, Karathanou K, Kalfakakou D, et al: DIANA-TarBase v7.0:
Indexing more than half a million experimentally supported
miRNA:mRNA interactions. Nucleic Acids Res. 43(Database issue):
D153–D159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
An E, Lu X, Flippin J, Devaney JM,
Halligan B, Hoffman EP, Strunnikova N, Csaky K and Hathout Y:
Secreted proteome profiling in human RPE cell cultures derived from
donors with age related macular degeneration and age matched
healthy donors. J Proteome Res. 5:2599–2610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guduric-Fuchs J, O'Connor A, Cullen A,
Harwood L, Medina RJ, O'Neill CL, Stitt AW, Curtis TM and Simpson
DA: Deep sequencing reveals predominant expression of miR-21
amongst the small non-coding RNAs in retinal microvascular
endothelial cells. J Cell Biochem. 113:2098–2111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Augoff K, McCue B, Plow EF and
Sossey-Alaoui K: miR-31 and its host gene lncRNA LOC554202 are
regulated by promoter hypermethylation in triple-negative breast
cancer. Mol Cancer. 11:52012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsang FH, Au SL, Wei L, Fan DN, Lee JM,
Wong CC, Ng IO and Wong CM: Long non-coding RNA HOTTIP is
frequently up-regulated in hepatocellular carcinoma and is targeted
by tumour suppressive miR-125b. Liver Int. 35:1597–1606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu YR, Jiang YZ, Xu XE, Hu X, Yu KD and
Shao ZM: Comprehensive transcriptome profiling reveals multigene
signatures in triple-negative breast cancer. Clin Cancer Res.
22:1653–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Su X, Malouf GG, Chen Y, Zhang J, Yao H,
Valero V, Weinstein JN, Spano JP, Meric-Bernstam F, Khayat D and
Esteva FJ: Comprehensive analysis of long non-coding RNAs in human
breast cancer clinical subtypes. Oncotarget. 5:9864–9876. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pundir P, MacDonald CA and Kulka M: The
novel receptor C5aR2 is required for C5a-mediated human mast cell
adhesion, migration, and proinflammatory mediator production. J
Immunol. 195:2774–2787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Donoso LA, Kim D, Frost A, Callahan A and
Hageman G: The role of inflammation in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 51:137–152.
2006. View Article : Google Scholar : PubMed/NCBI
|