Introduction
Glioma accounts for the majority of central nervous
system (CNS) malignancies. They are difficult to cure and always
present a poor prognosis. Histologically, glioma can be divided
into four classes: Astrocytomas, oligodendrogliomas, ependymomas
and mixed gliomas; diffuse gliomas are the most common. According
to the 2007 World Health Organisation CNS tumor classification
(1), CNS gliomas are diagnosed as
grade II (diffuse astrocytoma, oligodendroglioma and
oligoastrocytic tumors), grade III (anaplastic astrocytoma,
oligodendroglioma and oligoastrocytic tumors) and grade IV
(glioblastoma multiforme; GBM). The evidence is that high grade
gliomas (III and IV) are the most common, and GBM occupies ~30% of
CNS gliomas (2). Although the
therapeutic strategies of glioma involve continuous improvement and
adjustment, the prognosis remains unsatisfactory. The Chinese
Glioma Cooperative Group statistics (3) give a median general survival time
(OS) of GBM at only 14.4 months, along with 5-year OS rates at 9%.
Therefore, identification of the pathogenic mechanism of glioma is
important, and novel therapeutic strategies to reduce the high
mortality rates of CNS malignancies are required.
In the past, researchers have concentrated on
exploring the molecular mechanism of glioma, which led to the
discovery of isocitrate dehydrogenase 1, telomerase reverse
transcriptase and various other molecules. A CNS glioma molecular
classification has been suggested (3–5). The
present review aimed to focus on a widely-studied molecule,
connexin (Cx)43, which is largely expressed in astrocytes and which
participates in the construction of gap junctions (GJs) of
astrocytes or astrocytes and neurons (6). Cx43 is a multifunctional protein
which not only constructs gap junction channels and hemi-channels
(7), but also contains numerous
binding domains which can interrelate with various Cx43 linked
proteins, thus serving an elemental role in several physiological
and pathological functions (8).
Cx43 has been reported to be involved in the inception of certain
neurodegenerative diseases, including Alzheimer's and Parkinson's
disease (9), epilepsy secondary to
focal cortical dysplasia (FCD) (10) and amyotrophic lateral sclerosis
(11), among others. The role of
Cx43 in glioma has also been widely and consistently explored.
The present review aimed at introducing the role of
Cx43 in glioma from the following aspects: i) Expression of Cx43
and glioma grade; ii) inhibition of glioma proliferation, but
improvement in invasion and migration; iii) consideration of Cx43
and the possibility of its promoting glioma-associated epileptic
discharge; and iv) disease diagnosis and therapy.
Structure and function
Cx43 is encoded by the GJA1 gene and is strongly
expressed in astrocytes. Cx43 is an elemental membrane protein,
which contains three intracellular regions, two extracellular loops
together with multiple trans-membrane domains. The intracellular
region is composed of N- and C-terminal (CT) domains along with a
loop that links the trans-membrane domains. Cx43CT comprises of
amino acids 232–381, a plurality of binding-domains and
phosphorylation sites (12). The
present aimed to review the functions of Cx43 and specifically to
its functions in constructing GJs. Astrocyte Cx43 gathers adjacent
to the central pore and forms connexons. Subsequently, it is
coupled with neighbouring astrocytes or neurons through apposing
connexons to form GJ channels, which may directly exchange the
cytoplasm between coupled astrocytes and permit swapping of ions
together with certain small molecules. Astrocyte Cx43 may also form
membrane hemi-channels, which are directly involved in material
exchange between the extracellular milieu and astrocytes or neurons
(13–15).
From its special structure and character (forming GJ
channels and hemi-channels), Cx43 can therefore serve important
physiological and pathological functions in CNS through these two
routes.
GJ channels and hemi-channels
Cx43 is highly expressed in astrocytes, lasting
until adulthood. In relation to neurons, the typical feature of
astrocyte GJs is to support the astrocytes in couple formation.
This involves ions, amino acids, metabolites and certain small
molecules to exchange through the cytomembrane between astrocytes
in addition to extracellular milieu (16). The principal roles of astrocyte GJs
are described below.
Potassium spatial buffering
When neurons are in an energized state, a large
number of K+ ions efflux into the intercellular space.
Aggregation of extracellular milieu K+ activates the
inwardly rectifying K+ channel, and there is an
excessive intake of K+, rapidly dispersed to adjacent
astrocytes or neurons through GJs. Ultimately, the K+
homeostasis of this coupling is maintained and is considered to be
beneficial in maintaining the normal microenvironment, in addition
to the electrical activity of neurons (17).
Signal transduction
Mediated by Cx43, astrocytes form functional group
coupling astrocytes networks which may contribute to long-range
signal transduction. External stimulation can spread through
astrocytes via calcium waves to participate in neuromodulation
(18,19). Astrocytes also contain an adenylate
cycle and phosphoinositide courier delivery system, which can
transmit signals through second messengers, including cyclic
adenosine monophosphate (20).
Nutritional support
Astrocytes take in glucose which can be delivered to
neurons through the GJ to contribute to metabolic regulation of
neurons (21). Additionally,
through the GJs formed by Cx43 between astrocytes and neurons,
these two cell types may directly achieve material exchange along
with signal transduction (22).
Specific binding domains and
phosphorylation sites
Cx43 is a structurally complex protein in the
C-terminal domains. There are certain binding domains which can
interrelate with paired molecules to contribute to the building and
regulation of cell architecture, polarity, mobility, invasion and
growth (8,12,23,24).
According to the reviews put forward by Giepmans (8) and Tabernero et al (12), the interactions of Cx43 when
closely associated with proteins are summarized in Table I.
 | Table I.Cx43-interacting proteins. |
Table I.
Cx43-interacting proteins.
| Protein and
phosphorylation sites | Amino acids | Function | Cx43
interaction |
|---|
|
ZO-1 | 379–382 | Tight junctions,
adherens junctions, cytoskeleton build, signal transduction | Inversely regulates
gap Junctional communication and Hemichannel activity, prevents
cytoplasmic localization and malignization |
|
Src | 247,265,
274–283 | Phosphorylates Cx43
oncogenic activity | Inhibits Cx43-based
GJC, Tumor suppression, but excludes the C-terminal tail for ZO-1
binding |
|
Tubulin | 234–262 | Combines into
dimers, assembles microtubules | Modulates cell
polarity, Motility and directional cell migration |
|
Cadherins, catenin and
actin |
| Adherens junctions,
β-catenin modulates Wnt-mediated gene transcription | Modulate cell
motility |
| CK1,
PKA MAPK, PKG, PKC |
| Phosphorylates
Cx43 | Upregulate Cx43
assembly Inhibit Cx43-based GJC |
Expression of Cx43 and glioma grade
In standard physiological states, Cx43 is
prominently expressed in astrocytes. However, when the cell becomes
malignant, the expression of Cx43 is downregulated. Thus, Sin et
al (25) suggested that
decreased Cx43 expression is accompanied by greater proliferation
and malignancy of tumors. By studying the expression of Cx43 in
human glioma and normal tissue microarray slides, mainly by western
blot analysis and immunohistochemical staining, Sin et al
(26) and Ye et al
(27) noted a reduced expression
of Cx43 in the tumor center as the glioma malignancy increased.
Grade I and II primary astrocyte gliomas may express an enhanced
immunoreactivity compared with normal brain tissue. However, it
lacks the distinct disrupting staining of normal astrocytes. In
high grade glioma, the expression of Cx43 is commonly reduced
compared with normal tissues. It is also decreased in the majority
of GBM, where the expression of Cx43 protein is insignificant
(12,26,27).
However, Crespin et al (28) did not share this point of view.
First, in spite of the modest inverse association between tumor
grade and Cx43 expression, over half of glioblastomas still express
Cx43. Secondly, the expression of Cx43 between grade II and III
astrocytes gliomas is not significantly different. Additionally,
the various expression levels of Cx43 between grade III astrocytoma
and oligodendroglioma suggest that Cx43 can act as a marker in
discriminating against grade III oligodendroglioma in addition to
astrocytoma. In reality, expression of Cx43 differs within the same
tumor. For instance, Cx43 is rarely labelled at the membranes and
in the cytoplasm of GBM cells. Nevertheless, it is abundant at the
plasma membrane of reactive astrocytes in the surrounding tumor
mass (26,28). The notable features of these areas
are tumor cell infiltration and reactive astrocytes (26,29).
The peritumor cortex not infiltrated by glioma cells may increase
Cx43 immunoreactivity and reactive astrocytes. However, this
appearance is perhaps associated with the existence of epileptic
seizures (30). Besides, this
conclusion may not be valid; the origin of glioma associated
seizure stemming from the peritumor area and infiltration by glioma
cells has been widely accepted (31). Therefore, the above conclusion may
require further elucidation. In addition, due to the driving factor
of glioma pathogenesis partly being ascribed to cancer stem cells
(CSCs), Hitomi et al (32)
explored the expression levels of Cx43 in GBM glioma stem cells
(GSCs). The results indicated that Cx43 is predominantly expressed
in non-GSCs while Cx46 is expressed in CSCs. Yu et al
(33) further identified lower
expression of Cxs and the loss of GJ-like structures together with
dysfunction of GJ intracellular function in GSCs.
Cx43 and glioma proliferation, invasion and
migration
Glial tumors, as the most common supratentorial
neoplasms, are particularly difficult to cure. This is made more
difficult with a poor prognosis, largely due to tumor cell
migration, invasion and proliferation. This section briefly
introduces the role of Cx43 in glioma migration, invasion and
proliferation in addition to its possible mechanism. Previous
studies (12,25,34,35)
focused more on the association between Cx43 and cancer, including
astrocytic glioma. However, more recent studies have proposed novel
insights. The present review aimed to examine the association of
Cx43 and astrocytic glioma in light of previous reviews and new
findings.
Inhibition of glioma growth and
proliferation
Thus far, the majority of studies have indicated
that Cx43, as a tumor suppressor factor, inhibits astrocytoma
growth and proliferation in a variety of ways. Treatments that
regulate Cx43 expression, including tolbutamide (36,37),
selective β2-AR agonist (38),
17-β estradiol (E2) (39), ciliary
neurotrophic factor (40) and low
doses of γ-radiation (41) have
been verified. Cx43 may therefore inhibit glioma growth and
proliferation (Table II).
 | Table II.Cx43 and the regulation of glioma
proliferation and growth. |
Table II.
Cx43 and the regulation of glioma
proliferation and growth.
| Author (date) | Model | Regulated
proteins | Regulatory
mechanism Regulation of Cx43 | Effect on cell
growth and proliferation | (Refs.) |
|---|
| Moinfar et
al (2016) | 17-β estradiol (E2)
treated-C6 cell | Estradiol
Receptors | Cx43↓ | Proliferation↑ | (39) |
| Ye et al
(2015) | PKC, MAPK, and PTK
inhibitors treated-U251 cell | PKC, MAPK, and
PTK | Cx43↓ and
p-Cx43↓ | Proliferation↓ | (27) |
| Mostafavi et
al (2014) | Selective β2-AR
agonist treated-1321N1 astrocytoma cells | β2-AR,
cAMP-Epac | Cx43↑ | Proliferation↓ | (38) |
| Jin et al
(2013) |
miR-125b-transfected U87 and U251 glioma
cells |
| Cx43↓ | Proliferation↑ | (64) |
| Ghosh et al
(2014) | Low doses of
γ-radiation treated-U87 cells | ERK-1/2, p38-MAPK
activation | Cx43↑and GJ↑ | Proliferation↓ | (41) |
| Hao et al
(2012) | AS-miR-221/222
transfect U251 cells |
| Cx43↑ | Proliferation↓ | (65) |
| Zhang et al
(2010) | Ad-bFGF-siRNA
transfect U251 cells |
| Cx43↑ | Proliferation↓ | (67) |
| Ozog et al
(2002) | CNTFR-α treated C6
glioma cells | CNTFRα | Cx43↑and GJ↑ | Proliferation and
growth↓ | (40) |
| Sanchez-Alvarez
et al (2001) | Tolbutamide-treated
C6 | p21, p27, Rbp, | Cx43↑and GJ↑ | Proliferation and
growth↓ | (36) |
| Sanchez-Alvarez
(2006) | glioma cells |
|
|
| (37) |
| P.A. Robe et
al (2000) | TGF-β1-treated C6
glioma cells |
| p-Cx43↓and GJ↓ | Proliferation↑ | (66) |
| Effect on the GSCs
phenotype |
| Shi-Cang Yu et
al (2012) | Reconstitution of
Cx43 GSCs | E-Cadherin | Wnt/b-Catenin
Pathway | Proliferation↓ | (33) |
| Gangoso et
al (2014) | Reconstitution of
Cx43 GSCs | c-Src, Sox2, Id1,
cadherin | c-Src
activity↓→Id1↓→ Sox2↑→ GSC self-renewal↓ | Proliferation↓ | (42) |
| Intervention in
cell metabolism |
| Herrero-Gonzalez
et al (2009) | Transfection of
Cx43-siRNA astrocytes | Endothelin-1 | glucose uptake
↑ | Proliferation↑ | (43) |
| Gang Li et
al (2015) | Sprague-Dawley
rats | Cx43 and AQP4 | Cx43↑→edema |
| (44) |
| Kolar et al
(2015) | GL261 glioma cells
and mouse | Cx43 and
Podoplanin |
Podoplanin→ischemia |
| (45) |
| Wei Zhang et
al (2003) | T98G-Cx43
cells | Cx43, GJ, VEGF | VEGF high
expression in T98G-Cx43 cells |
| (46) |
| Ruochun Huang
(2002) | Cx43-transfected
cells U251 | Cx43, MCP-1 | Cx43 downregulates
MCP-1, then inhibits angiogenesis | Proliferation↓ | (47) |
| Participation in
cell cycle regulation |
| Sanchez-Alvarez
et al (2006) | Tolbutamide treated
C6 | p21, p27, Rbp, | p21,
p27↑→Rbp↓→ | Proliferation and
growth ↓ | (37) |
| Tabernero et
al (2006) | glioma cells | E2F, cyclin E | E2F↓→cyclin E↓ |
| (51) |
| Geng Y et al
(1996) |
|
|
|
| (52) |
| Regulated growth
factor and proliferation related protein |
| Sin et al
(2008) | Cx43 expression C6
cell | CCN1↑ |
| Proliferation
↑ | (53) |
| Sin et al
(2008) |
| CCN3↑ |
| Proliferation
↓ | (53) |
| Fu et al
(2004) |
|
|
|
| (54) |
| Bradshaw et
al (1993) |
| Osteopontin↑ |
| Proliferation
↓ | (55) |
| Bradshaw et
al (1993) |
| IGFBP4↑,
IGF-1↓, |
|
| (56) |
| Goldberg et
al (2000) |
| IGFP↓, bFGFK,
PDGF↓ |
| Proliferation
↓ | (57) |
| Xia et al
(2003) |
|
|
|
| (58) |
| González-Sánchez
et al (2016) | Cx43 expression C6
cell | PTEN, Csk | c-Scr
activity↓ | Proliferation
↓ | (59) |
| Ghosh et al
(2014) | Knock-down of Cx43
expression U87 cell | p38, ERK-1/2 | p38, ERK-1/2
activity↓ | Proliferation
↑ | (41) |
The specific mechanism of how Cx43 influences glioma
proliferation remains to be elucidated. However, the following
mechanisms may contribute to the regulation of Cx43 in glioma
proliferation (Table II).
Affecting the GSC phenotype
GSCs are cells which possess the capability for
self-renewal and are considered among the driving factors in glioma
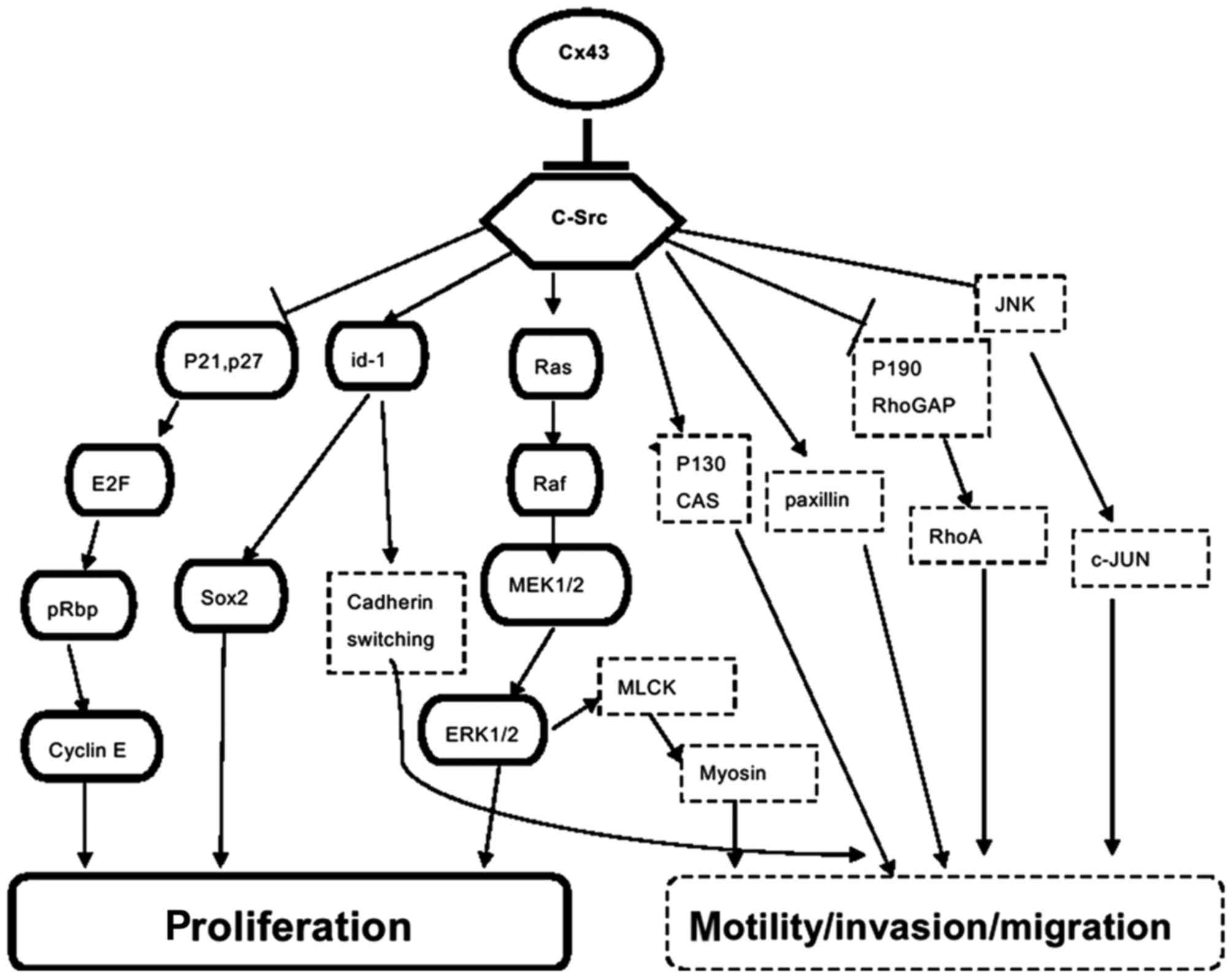
pathogenesis. Notably, Cx43 is mostly expressed in non-GSCs, and
the expression of Cx43 in GSCs is low (32,33).
When reconstituting Cx43 in GSCs, the tumorigenicity of GSCs is
inhibited, while self-renewal and proliferation are delayed. Yu
et al (33) described the
interaction of Cx43 with epithelial cadherin as having an influence
on CSC phenotype through the Wnt/β-Catenin signaling pathway; this
may be a potential mechanism. Additionally, as a proto-oncogene,
Scr and its interaction with Cx43 are also considered to be
involved in glioma proliferation regulation. Tabernero et al
(12) hypothesized c-
proto-oncogene tyrosine-protein kinase (Src) inhibiting Src
activity as the main initiator of Cx43. It has an effect on GSCs:
Gangoso et al (42)
transfected Cx43 to GSCs and identified that Ki-67-positive glioma
cells decreased and expressed Cx43, while downregulating
DNA-binding protein inhibitor (a transcriptional regulator)
expression via inhibition of Src activity. Consequently, there was
reduced (sex determining region Y)-box (Sox2) expression,
downregulation of Sox2 and a reduction in GSC self-renewal
(Fig. 1).
Intervention in cell metabolism
Cancer cells detect rapid proliferation by adapting
to metabolic environmental changes. For glioblastoma, uptake of
enough glucose or the transformation of metabolism strategies to
enable the cells to survive in a hypoxic tumor microenvironment is
a key precondition for the growth and proliferation of GBM cells.
Cx43 increases GJ channel and hemi-channel coupling, enabling the
exchange of ions, amino acids, metabolites and certain small
molecules through the cytomembrane and between astrocytes, together
with the extracellular milieu. Notably, that inhibition of GJs or
downregulation of Cx43 expression leads to an increase in glucose
uptake (43). Accordingly, this is
the main energy substance for glioma cells. Cx43 and its associated
GJs are capable of influencing the peritumoral microenvironment of
edema (44), ischemia (45) and angiogenesis (46,47),
and of interfering with glioma cell metabolism. This may further
influence glioma growth and proliferation. Equally, deletion of
Cx43 in astrocytes has been observed to inhibit oligodendrocyte
precursor cell proliferation by reducing matrix glucose levels
(48).
Participation in cell cycle
regulation
Cx43 may deter the cell cycle from G1 to S-phase or
M-phase (49,50). It is also capable of rebuilding
Cx43 in glioma cells which could delay the progression of cells
from G0/G1 to S-phase (37). Cx43
has been observed to increase the expression of p21 and p27, and
then weaken retinoblastoma phosphorylation (pRb) (37,51).
pRb phosphorylation promotes the release of E2 transcription
factor, which is associated with the expression of cyclin E
(52). Cx43 possibly regulates the
glioma cell cycle by decreasing pRb phosphorylation, subsequently
inhibiting cyclin E expression.
Regulation of growth factor and
proliferation-associated proteins
Several growth factors (GFs) may also affect the
growth and proliferation of cells. Previous research indicates that
Cx43 can regulate certain GF expression levels. For instance,
Cx43-transfected glioblastoma cells (U251) downregulate the
expression of MCP-1, a factor that can further promote
angiogenesis, and then suppress glioma cell proliferation (47). Restored Cx43 expression in C6
glioma cells was also determined as being able to upregulate
secretory proteins cysteine-rich angiogenic inducer (CCN)1 together
with CCN3 expression (53).
Notably, over-expression of CCN3 and its interaction with Cx43 are
conducive to a decrease in glioma growth rate. Overexpression of
CCN1 exhibits an opposite function (53,54).
Similarly, Cx43 transfected to C6 rat glioma cells also regulates
the expression of secreted proteins. For instance, it decreases
insulin-like growth factor protein, basic fibroblast growth factor,
platelet-derived growth factor, insulin-like growth factor 1 and
N-methylpurine DNA glycosylase-E8 protein expression levels while
increasing CCN3, insulin-like growth factor-binding protein 4 and
osteopontin levels (55–58); this is the common outcome of
suppressed glioma cell proliferation. Additionally, Cx43 adjusts
certain kinase activities to affect the growth and proliferation of
cells. For instance, Cx43 recruits phosphatase and tensin homolog
and C-terminal Src kinase to inhibit c-Src activity (59). Additionally, c-Src equally combines
with the C-terminal of Cx43 to reduce the oncogenic activity of
c-Src (12,42,60).
Cx43 can also modify the activity of other proliferation-associated
proteins, including p38, extracellular signal-regulated kinases-1/2
(44) and zonula occludens (ZO)-1
(12), to affect the proliferation
of glioma cells. Suzhi et al (61) noted that Cx43 could transfer
microRNA (miR)-124-3p between coupling cells and improve the
antiproliferative ability of miR-124-3p.
Regulation of gene expression
Cx43 regulates gene expression, perhaps as a
potential mechanism that influences glioma cell proliferation.
However, there are not enough relevant studies to support this
hypothesis. Dang et al (62) identified that the carboxyl-tail of
Cx43 localizes to the nucleus and inhibits cell growth. Mennecier
et al (63) also noted that
Cx43 may enter into the nucleus of glioma cell lines. Thus, it may
be hypothesized that Cx43 regulates gene expression directly or
indirectly to affect the proliferation and growth of glioma
cells.
Controversy of the effect of Cx43 on
glioma cell invasion and migration
From the above discussion, it can be deduced that
Cx43 is a tumor-suppression factor. However, this valuable role can
be weakened by its effects on migration and invasiveness. The
majority of the literature reports that Cx43 enhances glioma
invasion (24,26,68,69),
while certain studies report the inhibitory action of Cx43 in
glioma invasion and migration (65,70,71).
Although Cx43 is present in a lower expression state in a malignant
glioma mass, a high expression of Cx43 is detected at the plasma
membrane of the reactive astrocytes around the peritumor area
(26,28), and in tumor cell infiltration and
reactive astrocytes. In this area, malignant glioma cells form
functional GJ communication between themselves and astrocytes
(28,72), establishing a tight cell network
(71). This may be the structural
basis of the effect of Cx43 effect the invasion and migration of
malignant glioma cells by GJ-dependent and independent
mechanisms.
GJ-dependent mechanisms
Reduction of GJ activity has been reported to
improve cell migration (71,73).
However, more studies report that the overexpression of Cx43
encourages glioma cell migration and invasion in a GJ
channel-dependent manner (69,72,74).
Aftab et al (71)
demonstrated that downregulation of Cx43 expression in the U118
human glioma cell line is a way to increase migration by reducing
cell-extracellular matrix adhesion, and change the migration
pattern from collective to single cell. It was also demonstrated
that Cx43-GJ serves more prominent roles in mediating migration and
invasion behaviors compared with the C-terminal tail interaction.
Functional GJ coupling also contributes to long-range signal
transduction, and adjusts the formation of calcium waves (18,19,41,75).
Additionally, it transmits signals through second messengers
(20). Through these ways, Cx43-GJ
may promote the transformation of malignant astrocytes by
regulating a glioma-associated signaling pathway. Furthermore, Cx43
located in lipid raft microdomains can also regulate homocellular
and heterocellular GJ communications between cancer and stroma
cells, and can control the tumor phenotype (68,69).
Consequently, such actions may influence glioma invasion behaviors.
Additionally, a Cx43-constructed glioma-astrocyte GJ can modulate
glioma invasive behavior by direct transfer of miRs (72). Cx43-GJ is involved in tumor
microtube-mediated cell-to-cell communication and influences the
motility of glioma cells (75).
GJ-independent mechanisms
Cx43 promotes glioma cell invasion through
GJ-reliant mechanisms, which are not always recognized. Sin et
al (26) suggested that
astrocytic Cx43 may aid glioma cells to detach from the glioma
core. However, Cx43 may mediate glioma invasion solely in a
GJ-independent manner since the expression of Cx43-T154A has
demonstrated no effect on glioma invasion (26). This conclusion contradicts the
previously discussed findings in the present review. Certain
Cx43-associated proteins merge with Cx43 extracellular loops or
C-terminal regions to improve adhesive connections or to regulate
cytoskeletal dynamics, which alter the structure of Cx43 to
facilitate malignant glioma cell invasion and migration by
independent mechanisms. A wound healing motility assay indicated
that the C-terminal of Cx43 is required for Cx43-mediated C6 glioma
cell motility (24). For instance,
Cx43 interacts with ZO-1 protein, which could prevent the
cytoplasmic localization and lead to glioma cell invasion (12,76).
Cx43 interacts with other cytoskeleton proteins and tight junctions
or adherens junctions associated with proteins, including tubulin,
cadherins, catenin and actin, to modulate polarity, motility and
directional migration of cells (12,24,77,78).
In addition, Cx43 interacts with c-Src and inhibits Src activity,
sequentially modulating cell polarity, motility and invasion
through several signaling pathways (Fig. 1) (12,79,80).
In brief, there is no consensus on the effect of Cx43 on glioma
invasion and migration, and the detailed mechanism remains
unclear.
Cx43 may promote glioma-associated epileptic
discharge
The association between Cx43/-GJ and epilepsy has
widely been studied in the last 20 years. Earlier studies failed to
consider that Cx43 is associated with epilepsy, since expression of
Cx43 had not identified significant differences between
epileptogenic and nonepileptogenic tissues, in living tissue assays
(81) and animal models (82). Nevertheless, the majority of
studies have identified Cx43 as capable of participating in the
genesis and development of certain types of epilepsy. For instance,
Cx43 is increasingly expressed in the hippocampus tissue of
patients with refractory temporal lobe epilepsy (83,84)
and in FCD type IIB (10).
Cx43/-GJ were also altered in either lithium pilocarpine-induced
epilepsy (84) or in
kainic-acid-induced status epilepticus models (85). Notably, the inhibition of the Cx43
GJ with carbenoxolone can shorten the duration of seizures and
reduce the amplitude of the seizure discharges (86). In view of the above, Cx43 may be to
be associated with the genesis and development of certain types of
epilepsy, in addition to glioma-associated epilepsy.
Glioma-associated epilepsy may be defined as seizure
which directly arises from the existence of supratentorial glioma.
It is the presenting feature in ≤87% of low-grade gliomas and ≤50%
of gliomas overall (87). Epilepsy
is usually the initial symptom of glioma patients and a significant
factor affecting their post-operative quality of life (88). However, the detailed mechanism of
glioma-associated epilepsy remains to be elucidated. It may be a
combination of direct mass effects and the change of the tumor
microenvironment.
Overall, Cx43 is highly expressed in peritumoral
astrocytes (29) which facilitate
glioma cells detachment from the tumor core (26). Glioma cells invade the neocortex
structure, a special peritumoral region where single neurons are
bounded by very few or a single tumor cell (89). This peritumoral region has recently
been considered as the basic focus of glioma-associated seizure
(31,90,91).
GJ changes (89) and the increase
of Cx43 expression (30) have been
identified in the perilesional tissue of seizures associated with
brain tumors. GJ or Cx43-glial coupling may explain glioma-induced
epileptogenesis (92). Cx43 and
its associated GJ are capable of influencing the peritumoral
microenvironment, including edema (44), ischemia (45) and angiogenesis (46,47)
which may induce epileptic discharge through direct effects of
mass. Cx43-GJ is involved in the generation of sharp wave-ripple
(34). It propagates neuronal
activity through long-range signal transduction and Ca+
waves, then promotes a synchronized discharge of neurons (93). Cx43-GJ can also influence seizure
discharge by regulating K+ redistribution and neuronal
energy supply (94). Peritumoral
reactive astrocytes can highly express Cx43. Cx43, in this context,
may serve a predominant role in the regulation of
neurotransmitters, including glutamate. First, Cx43-hem channels/GJ
in astrocytes can control glutamate (95,96),
release ATP (96) and sustain
glutamatergic synaptic efficacy (97). Second, Cx43 knockdown may raise
cortical glutamate transporter (GLT)-1 in addition to glutamate
aspartate transporter (GLAST) protein expression levels, and
control transcription and translation of glial glutamate
transporters excitatory amino acid transporter (EAAT)-1 and EAAT-2
(98). Similarly, blocking the gap
junction has been reported to suppress transcriptional activity of
GLT-1 promoter, but increase GLAST gene transcription (99). The spinal astrocytic Cx43 has also
been reported to be capable of activating N-methyl D-aspartate
receptors (100) and elemental
ionotropic glutamate receptors in the postsynaptic membrane
(101). In essence, peritumoral
Cx43 high immunoreactivity is mainly on the reactive astrocytes
(29), and demonstrates Cx43 to be
potentially associated with astrocyte reactivity. A recent study
observed that reactive astrocytes not only limit glutamate uptake,
but also inhibit the production of gamma-aminobutyric acid.
Furthermore, this leads to a loss of inhibition and an increase in
neuronal excitability (102).
Even so, it can be hypothesized that Cx43 can possibly promote
glioma-associated epileptic discharge through these aforementioned
ways. The relevant studies remain scarce, and further studies are
required to identify the exact mechanism of Cx43 in
glioma-associated epilepsy.
In summary, the special microenvironment of glioma
(tumor cell infiltration and high expression of Cx43 in reactive
astrocytes) may identify why glioma patients present with epilepsy
and why they possess a favorable prognosis but are prone to
relapses (101). Cx43 also
presents in temozolomide resistance and resistance to radiotherapy
in glioblastoma cells. The peritumoral region has been considered
the basic focus of glioma-associated seizure (31). It is hypothesized that early stage
glioma cells highly express Cx43, and migrate and invade the host
parenchyma with a low proliferation index (26).
Facilitating disease diagnosis and
therapy
Investigating biomolecules is useful facilitate
disease diagnosis and therapy. The value of Cx43 in glioma
diagnosis and therapy is beginning to be recognized. Abakumova
(103) demonstrated that the
Cx43-targeted T1 contrast agent may efficiently visualize glioma C6
and its borders in vitro and in vivo. MAbE2Cx43 s was
covalently associated with the Phthalosens derivative
photosensitizer delivery of fluorescent agents to the glioma
tissue. This may be valuable in demonstrating the optimal border
and increase the extent of resection due to improved visualization
of the glioma (104). Similarly,
Cx43/-GJ may help brain tumor cells to interconnect a functional
and resistant network (75), which
confer temozolomide resistance (105–108) and radiotherapy resistance
(75) in glioblastoma cells. The
Cx43-antibody MAbE2Cx43 has been demonstrated to be potentially
part of a combined therapy for poorly differentiated gliomas
(108).
Conclusion
In regular physiological conditions, Cx43 is highly
expressed in astrocytes. However, this expression is restrained
when the malignant transformation of astrocytes and the levels of
Cx43 are reduced, along with an increase in the degrees of
malignancy in astrocytomas. The association between the expression
of Cx43 and degrees of glioma implicates Cx43 as a tumor
suppressor, inhibiting glioma cell proliferation. However, the
majority of data have indicated that Cx43 may enhance the motor
ability and invasiveness of astrocytic glioma cells, and to
facilitate glioma cell separation from the tumor core to the
surroundings. This can be interpreted as Cx43 in the early stages
of glioma progression, with a relatively low proliferative index of
glioma cells, predisposing glioma cells to migrate and integrate
with the host parenchyma (26).
Simultaneously, reactive astrocytes and the tumor cell invade into
peritumoral tissue comprising the significant surrounding
microenvironment, making an ideal environment for epileptic
discharge. It is undoubtable that Cx43 or GJ /hemi-channels have a
contribution in promoting glioma-associated epileptic discharge
through direct mass effects and the change of tumor
microenvironment, particularly the effect in excitatory
neurotransmitter-glutamate regulation. Notably, Cx43 expression is
considerably upregulated in astrocytes reactive due to tissue
damage during surgery. This could promote tumor proliferation, in
addition to migration (109), and
then facilitate glioma recurrence following resection (110). This can partially explain
post-operative epileptic seizures in glioma patients and those with
no epilepsy prior to surgery.
Previously published reviews (12,25)
have presented the roles of Cx43 in glioma proliferation in two
mechanisms: GJ-dependent and GJ-independent. To the best of our
knowledge, the present review was the first to introduce the exact
mechanism of these functions and the roles of Cx43 in
glioma-associated epilepsy. Certainly, there are still a number of
challenges that require further exploration. If Cx43 is associated
with the prognosis of glioma patients, then its potential as a
treatment target requires further study. Peritoneal tissue which
highly express Cx43 is involved in the incidence of glioma and is
associated with epilepsy; thus, should be explored further.
In conclusion, the roles of Cx43 in glioma
proliferation, in the present review, can be directed to the
association between glioma and epilepsy. A number of identifiable
challenges in this current review can be the subject of further
studies. More importantly, future studies could also aid
understanding of any other ways through which Cx43 and other
expressions are associated with incidences of glioma and with
epilepsy.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen F, Wu CX, Yao Y, Peng P, Qin ZY, Wang
Y, Zheng Y and Zhou LF: Transition over 35 years in the incidence
rates of primary central nervous system tumors in Shanghai, China
and histological subtyping based on a single center experience
spanning 60 years. Asian Pac J Cancer Prev. 14:7385–7393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang T, Mao Y, Ma W, Mao Q, You Y, Yang
X, Jiang C, Kang C, Li X, Chen L, et al: CGCG clinical practice
guidelines for the management of adult diffuse gliomas. Cancer
Lett. 375:263–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ceccarelli M, Barthel FP, Malta TM,
Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh
A, Pagnotta SM, et al: Molecular profiling reveals biologically
discrete subsets and pathways of progression in diffuse glioma.
Cell. 164:550–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Foote MB, Papadopoulos N and Diaz LA Jr:
Genetic Classification of Gliomas: Refining Histopathology. Cancer
Cell. 28:9–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Almad AA, Doreswamy A, Gross SK, Richard
JP, Huo Y, Haughey N and Maragakis NJ: Connexin 43 in astrocytes
contributes to motor neuron toxicity in amyotrophic lateral
sclerosis. GLIA. 64:1154–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharrow AC, Li Y, Micsenyi A, Griswold RD,
Wells A, Monga SS and Blair HC: Modulation of osteoblast gap
junction connectivity by serum, TNFalpha, and TRAIL. Exp Cell Res.
314:297–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giepmans BN: Gap junctions and
connexin-interacting proteins. Cardiovasc Res. 62:233–245. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freitas-Andrade M and Naus CC: Astrocytes
in neuroprotection and neurodegeneration: The role of connexin43
and pannexin1. Neuroscience. 323:207–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garbelli R, Frassoni C, Condorelli DF,
Salinaro A Trovato, Musso N, Medici V, Tassi L, Bentivoglio M and
Spreafico R: Expression of connexin 43 in the human epileptic and
drug-resistant cerebral cortex. Neurology. 76:895–902. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Almad AA, Doreswamy A, Gross SK, Richard
JP, Huo Y, Haughey N and Maragakis NJ: Connexin 43 in Astrocytes
Contributes to Motor Neuron Toxicity in Amyotrophic Lateral
Sclerosis. Glia. 64:1154–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tabernero A, Gangoso E, Jaraíz-Rodríguez M
and Medina JM: The role of connexin43-Src interaction in
astrocytomas: A molecular puzzle. Neuroscience. 323:183–194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giaume C, Fromaget C, Aoumari A, Cordier
J, Glowinski J and Gros D: Gap junctions in cultured astrocytes:
Single-channel currents and characterization of channel-forming
protein. Neuron. 6:133–143. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giaume C, Koulakoff A, Roux L, Holcman D
and Rouach N: Astroglial networks: A step further in neuroglial and
gliovascular interactions. Nat Rev Neurosci. 11:87–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giaume C, Leybaert L, Naus CC and Sáez JC:
Connexin and pannexin hemichannels in brain glial cells:
Properties, pharmacology, and roles. Front Pharmacol. 4:882013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bennett MV, Contreras JE, Bukauskas FF and
Sáez JC: New roles for astrocytes: Gap junction hemichannels have
something to communicate. Trends Neurosci. 26:610–617. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagy JI and Rash JE: Connexins and gap
junctions of astrocytes and oligodendrocytes in the CNS. Brain Res
Brain Res Rev. 32:29–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scemes E: Components of astrocytic
intercellular calcium signaling. Mol Neurobiol. 22:167–179. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van den pol AN, Finkberiner SM and
Cornell-Bell AH: Calcium excitability and oscillations in
suprachiasmatic nucleus neurons and glia in vitro. J Neurosci.
12:2648–2664. 1992.PubMed/NCBI
|
|
20
|
Mehta PP, Yamamoto M and Rose B:
Transcription of the gene for the gap junctional protein connexin43
and expression of functional cell-to-cell channels are regulated by
c AMP. Mol Biol Cell. 3:839–850. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giaume C, Tabernero A and Medina JM:
Metabolic trafficking through astrocytic gap junctions. Glia.
21:114–123. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nedergaard M: Direct signaling from
astrocytes to neurons in cultures of mammalian brain cells.
Science. 263:1768–1771. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Nwagwu C, Le DM, Yong VW, Song H
and Couldwell WT: Increased invasive capacity of
connexin43-overexpressing malignant glioma cells. J Neurosurg.
99:1039–1046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bates DC, Sin WC, Aftab Q and Naus CC:
Connexin43 Enhances Glioma Invasion by a Mechanism Involving the
Carboxy Terminus. GLIA. 55:1554–1564. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sin WC, Crespin S and Mesnil M: Opposing
roles of connexin43 in glioma progression. Biochim Biophys Acta.
1818:2058–2067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sin WC, Aftab Q, Bechberger JF, Leung JH,
Chen H and Naus CC: Astrocytes promote glioma invasion via the gap
junction protein connexin43. Oncogene. 35:1504–1516. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye XY, Jiang QH, Hong T, Zhang ZY, Yang
RJ, Huang JQ, Hu K and Peng YP: Altered expression of connexin43
and phosphorylation connexin43 in glioma tumors. Int J Clin Exp
Pathol. 8:4296–4306. 2015.PubMed/NCBI
|
|
28
|
Crespin S, Fromont G, Wager M, Levillain
P, Cronier L, Monvoisin A, Defamie N and Mesnil M: Expression of a
gap junction protein, connexin43, in a large panel of human
gliomas: New insights. Cancer Med. 5:1742–1752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kolar K, Freitas-Andrade M, Bechberger JF,
Krishnan H, Goldberg GS, Naus CC and Sin WC: Podoplanin: A marker
for reactive gliosis in gliomas and brain injury. J Neuropathol Exp
Neurol. 74:64–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aronica E, Gorter JA, Jansen GH, Leenstra
S, Yankaya B and Troost D: Expression of connexin 43 and connexin
32 gap-junction proteins in epilepsy-associated brain tumors and in
the perilesional epileptic cortex. Acta Neuropathol. 101:449–459.
2001.PubMed/NCBI
|
|
31
|
Pallud J, Le van Quyen M, Bielle F,
Pellegrino C, Varlet P, Cresto N, Baulac M, Duyckaerts C,
Kourdougli N, Chazal G, et al: Cortical GABAergic excitation
contributes to epileptic activities around human glioma. Sci Transl
Med. 6:244ra892014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hitomi M, Deleyrolle LP, Mulkearns-Hubert
EE, Jarrar A, Li M, Sinyuk M, Otvos B, Brunet S, Flavahan WA,
Hubert CG, et al: Differential connexin function enhances
self-renewal in glioblastoma. Cell Rep. 11:1031–1042. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu SC, Xiao HL, Jiang XF, Wang QL, Li Y,
Yang XJ, Ping YF, Duan JJ, Jiang JY, Ye XZ, et al: Connexin 43
reverses malignant phenotypes of glioma stem cells by modulating
E-cadherin. Stem Cell. 30:108–120. 2012. View Article : Google Scholar
|
|
34
|
Moinfar Z, Dambach H and Faustmann PM:
Influence of drugs on gap junctions in glioma cell lines and
primary astrocytes in vitro. Front Physiol. 5:1862014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Naus CC and Laird DW: Implications and
challenges of connexin connections to cancer. Nat Rev Cancer.
10:435–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sánchez-Alvarez R, Tabernero A,
Sánchez-Abarca LI, Orfao A, Giaume C and Medina JM: Proliferation
of C6 glioma cells is blunted by the increase in gap junction
communication caused by tolbutamide. FEBS Lett. 509:1–206. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sánchez-Alvarez R, Paíno T,
Herrero-González S, Medina JM and Tabernero A: Tolbutamide reduces
glioma cell proliferation by increasing connexin43, which promotes
the up-regulation of p21 and p27 and subsequent changes in
retinoblastoma phosphorylation. Glia. 54:125–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mostafavi H, Khaksarian M, Joghataei MT,
Soleimani M, Hassanzadeh G, Eftekhari S, Soleimani M, Mousavizadeh
K, Estiri H, Ahmadi S and Hadjighassem MR: Selective β2 adrenergic
agonist increases Cx43 and miR-451 expression via cAMP-Epac. Mol
Med Rep. 9:2405–2410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moinfar Z, Dambach H, Schoenebeck B,
Förster E, Prochnow N and Faustmann PM: Estradiol receptors
regulate differential connexin 43 expression in F98 and C6 glioma
cell lines. PLoS One. 11:e01500072016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ozog MA, Bechberger JF and Naus CC:
Ciliary neurotrophic factor (CNTF) in combination with its soluble
receptor (CNTFRalpha) increases connexin43 expression and
suppresses growth of C6 glioma cells. Cancer Res. 62:3544–3548.
2002.PubMed/NCBI
|
|
41
|
Ghosh S, Kumar A, Tripathi RP and Chandna
S: Connexin-43 regulates p38-mediated cell migration and invasion
induced selectively in tumour cells by low doses of γ-radiation in
an ERK-1/2-independent manner. Carcinogenesis. 35:383–395. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gangoso E, Thirant C, Chneiweiss H, Medina
JM and Tabernero A: A cell-penetrating peptide based on the
interaction between c-Src and connexin43 reverses glioma stem cell
phenotype. Cell Death Dis. 5:e10232014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Herrero-González S, Valle-Casuso JC,
Sánchez-Alvarez R, Giaume C, Medina JM and Tabernero A: Connexin43
is involved in the effect of endothelin-1 on astrocyte
proliferation and glucose uptake. Glia. 57:222–233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li G, Liu X, Liu Z and Su Z: Interactions
of connexin 43 and aquaporin-4 in the formation of glioma-induced
brain edema. Mol Med Rep. 11:1188–1194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kolar K, Freitas-Andrade M, Bechberger JF,
Krishnan H, Goldberg GS, Naus CC and Sin WC: Podoplanin: A marker
for reactive gliosis in gliomas and brain injury. J Neuropathol Exp
Neurol. 74:64–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang W, DeMattia JA, Song H and Couldwell
WT: Communication between malignant glioma cells and vascular
endothelial cells through gap junctions. J Neurosurg. 98:846–853.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang R, Lin Y, Wang CC, Gano J, Lin B,
Shi Q, Boynton A, Burke J and Huang RP: Connexin 43 suppresses
human glioblastoma cell growth by down-regulation of monocyte
chemotactic protein 1, as discovered using protein array
technology. Cancer Res. 62:2806–2812. 2002.PubMed/NCBI
|
|
48
|
Niu J, Li T, Yi C, Huang N, Koulakoff A,
Weng C, Li C, Zhao CJ, Giaume C and Xiao L: Connexin-based channels
contribute to metabolic pathways in the oligodendroglial lineage. J
Cell Sci. 129:1902–1914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang YW, Nakayama K, Nakayama K and
Morita I: A novel route for connexin 43 to inhibit cell
proliferation: Negative regulation of S-phase kinase-associated
protein (Skp 2). Cancer Res. 63:1623–1630. 2003.PubMed/NCBI
|
|
50
|
Kamei J, Toyofuku T and Hori M: Negative
regulation of p21 by beta-catenin/TCF signaling: A novel mechanism
by which cell adhesion molecules regulate cell proliferation.
Biochem Biophys Res Commun. 312:380–387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tabernero A, Sánchez-Alvarez R and Medina
JM: Increased levels of cyclins D1 and D3 after inhibition of gap
junctional communication in astrocytes. J Neurochem. 96:973–982.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Geng Y, Eaton EN, Picón M, Roberts JM,
Lundberg AS, Gifford A, Sardet C and Weinberg RA: Regulation of
cyclin E transcription by E2Fs and retinoblastoma protein.
Oncogene. 12:1173–1180. 1996.PubMed/NCBI
|
|
53
|
Sin WC, Bechberger JF, Rushlow WJ and Naus
CC: Dose-dependent differential upregulation of CCN1/Cyr61 and
CCN3/NOV by the gap junction protein connexin43 in glioma cells. J
Cell Biochem. 103:1772–1782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fu CT, Bechberger JF, Ozog MA, Perbal B
and Naus CC: CCN3 (NOV) interacts with connexin43 in C6 glioma
cells: Possible mechanism of connexin-mediated growth suppression.
J Biol Chem. 279:36943–36950. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bradshaw SL, Naus CC, Zhu D, Kidder GM,
D'Ercole AJ and Han VK: Alterations in the synthesis of
insulin-like growth factor binding proteins and insulin-like growth
factors in rat C6 glioma cells transfected with a gap junction
connexin43 cDNA. Regul Pept. 48:99–112. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bradshaw SL, Naus CC, Zhu D, Kidder GM and
Han VK: Insulin-like growth factor binding protein-4 gene
expression is induced by transfection of gap junction connexin43
gene in a C6 glioma cell line. Growth Regul. 3:26–29.
1993.PubMed/NCBI
|
|
57
|
Goldberg GS, Bechberger JF, Tajima Y,
Merritt M, Omori Y, Gawinowicz MA, Narayanan R, Tan Y, Sanai Y,
Yamasaki H, et al: Connexin43 suppresses MFG-E8 while inducing
contact growth inhibition of glioma cells. Cancer Res.
60:6018–6026. 2000.PubMed/NCBI
|
|
58
|
Xia ZB, Pu PY, Huang Q, You YP, Wang GX
and Wang CY: Preliminary study on the mechanism of connexin 43 gene
transfection in the control of glioma cell proliferation. Zhonghua
Zhong Liu Za Zhi. 25:4–8. 2003.(In Chinese). PubMed/NCBI
|
|
59
|
González-Sánchez A, Jaraíz-Rodríguez M,
Domínguez-Prieto M, Herrero-González S, Medina JM and Tabernero A:
Connexin43 recruits PTEN and Csk to inhibit c-Src activity in
glioma cells and astrocytes. Oncotarget. 7:49819–49833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Herrero-González S, Gangoso E, Giaume C,
Naus CC, Medina JM and Tabernero A: Connexin43 inhibits the
oncogenic activity of c-Src in C6 glioma cells. Oncogene.
29:5712–5723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Suzhi Z, Liang T, Yuexia P, Lucy L,
Xiaoting H, Yuan Z and Qin W: Gap junctions enhance the
antiproliferative effect of microRNA-124-3p in glioblastoma cells.
J Cell Physiol. 230:2476–2488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dang X, Doble BW and Kardami E: The
carboxy-tail of connexin-43 localizes to the nucleus and inhibits
cell growth. Mol Cell Biochem. 242:1–2. 2003. View Article : Google Scholar
|
|
63
|
Mennecier G, Derangeon M, Coronas V, Hervé
JC and Mesnil M: Aberrant expression and localization of connexin43
and connexin30 in a rat glioma cell line. Mol Carcinog. 47:391–401.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jin Z, Xu S, Yu H, Yang B, Zhao H and Zhao
G: miR-125b inhibits connexin43 and Promotes glioma growth. Cell
Mol Neurobiol. 33:1143–1148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hao J, Zhang C, Zhang A, Wang K, Jia Z,
Wang G, Han L, Kang C and Pu P: miR-221/222 is the regulator of
Cx43 expression in human glioblastoma cells. Oncol Rep. 27:1–1510.
2012.
|
|
66
|
Robe PA, Rogister B, Merville MP and Bours
V: Growth regulation of astrocytes and C6 cells by TGFbeta1:
Correlation with gap junctions. NeuroReport. 11:2837–2841. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang B, Feng X, Wang J, Xu X, Liu H and
Lin N: Adenovirus-mediated delivery of bFGF small interfering RNA
increases levels of connexin 43 in the glioma cell line, U251. J
Exp Clin Cancer Res. 29:32010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang W, Nwagwu C, Le DM, Yong VW, Song H
and Couldwell WT: Increased invasive capacity of
connexin43-overexpressing malignant glioma cells. J Neurosurg.
99:1039–1046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Strale PO, Clarhaut J, Lamiche C, Cronier
L, Mesnil M and Defamie N: Down-regulation of Connexin43 expression
reveals the involvement of caveolin-1 containing lipid rafts in
human U251 glioblastoma cell invasion. Mol Carcinog. 51:845–860.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Qin LJ, Jia YS, Zhang YB and Wang YH:
Cyclooxygenase inhibitor induces the upregulation of connexin-43
expression in C6 glioma cells. Biomed Rep. 4:444–448. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Aftab Q, Sin WC and Naus C: Reduction in
gap junction intercellular communication promotes glioma migration.
Oncotarget. 6:11447–11464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hong X, Sin WC, Harris AL and Naus CC: Gap
junctions modulate glioma invasion by direct transfer of microRNA.
Oncotarget. 6:15566–15577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
McDonough WS, Johansson A, Joffee H, Giese
A and Berens ME: Gap junction intercellular communication in
gliomas is inversely related to cell motility. Int J Dev Neurosci.
17:601–611. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Osswald M, Jung E, Sahm F, Solecki G,
Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M,
et al: Brain tumour cells interconnect to a functional and
resistant network. Nature. 528:93–98. 2015.PubMed/NCBI
|
|
75
|
Reichert M, Müller T and Hunziker W: The
PDZ domains of zonula occludens-1 induce an epithelial to
mesenchymal transition of Madin-Darby canine kidney I cells.
Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem.
275:9492–9500. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lin JH, Takano T, Cotrina ML, Arcuino G,
Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H, et al:
Connexin 43 enhances the adhesivity and mediates the invasion of
malignant glioma cells. J Neurosci. 22:4302–4311. 2002.PubMed/NCBI
|
|
77
|
Reszec J, Szkudlarek M, Hermanowicz A,
Bernaczyk PS, Mariak Z and Chyczewski L: N-cadherin, beta-catenin
and connexin 43 expression in astrocytic tumours of various grades.
Histol Histopathol. 30:361–371. 2015.PubMed/NCBI
|
|
78
|
Kirschstein T and Köhling R: Animal models
of tumour-associated epilepsy. J Neurosci Methods. 260:109–117.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Patel A, Sabbineni H, Clarke A and
Somanath PR: Novel roles of Src in cancer cell
epithelial-to-mesenchymal transition, vascular permeability,
microinvasion and metastasis. Life Sci. 157:52–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Elisevich K, Rempel SA, Smith BJ and
Edvardsen K: Hippocampal connexin 43 expression in human complex
partial seizure disorder. Exp Neurol. 145:154–164. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Senner V, Köhling R, Püttmann-Cyrus S,
Straub H, Paulus W and Speckmann EJ: A new
neurophysiological/neuropathological ex vivo model localizes the
origin of glioma-associated epileptogenesis in the invasion area.
Acta Neuropathol. 107:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Das A, GC IV Wallace, Holmes C, McDowell
ML, Smith JA, Marshall JD, Bonilha L, Edwards JC, Glazier SS, Ray
SK, et al: Hippocampal tissue of patients with refractory temporal
lobe epilepsy is associated with astrocyte activation,
inflammation, and altered expression of channels and receptors.
Neuroscience. 220:237–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fonseca CG, Green CR and Nicholson LF:
Upregulation in astrocytic connexin 43 gap junction levels may
exacerbate generalized seizures in mesial temporal lobe epilepsy.
Brain Res. 929:105–116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Su M and Tong XX: Astrocytic gap junction
in the hippocampus of rats with lithium pilocarpine-induced
epilepsy. Nan Fang Yi Ke Da Xue Xue Bao. 30:2738–2741. 2010.(In
Chinese). PubMed/NCBI
|
|
85
|
Takahashi DK, Vargas JR and Wilcox KS:
Increased coupling and altered glutamate transport currents in
astrocytes following kainic-acid-induced status epilepticus.
Neurobiol Dis. 40:573–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Oliveira R, Christov C, Guillamo JS, de
Boüard S, Palfi S, Venance L, Tardy M and Peschanski M:
Contribution of gap junctional communication between tumor cells
and astroglia to the invasion of the brain parenchyma by human
glioblastomas. BMC Cell Biol. 6:72005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liubinas SV, O'Brien TJ, Moffat BM,
Drummond KJ, Morokoff AP and Kaye AH: Tumour associated epilepsy
and glutamate excitotoxicity in patients with gliomas. J Clin
Neurosci. 21:899–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Armstrong TS, Grant R, Gilbert MR, Lee JW
and Norden AD: Epilepsy in glioma patients: Mechanisms, management,
and impact of anticonvulsant therapy. Neuro Oncol. 18:779–789.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Elisevich K, Rempel SA, Smith B and Allar
N: Connexin 43 mRNA expression in two experimental models of
epilepsy. Mol Chem Neuropathol. 32:75–88. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Köhling R, Senner V, Paulus W and
Speckmann EJ: Epileptiform activity preferentially arises outside
tumor invasion zone in glioma xenotransplants. Neurobiol Dis.
22:64–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Buckingham SC, Campbell SL, Haas BR,
Montana V, Robel S, Ogunrinu T and Sontheimer H: Glutamate release
by primary brain tumors induces epileptic activity. Nat Med.
17:1269–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kim LC, Song L and Haura EB: Src kinases
as therapeutic targets for cancer. Nat Rev Clin Oncol. 6:587–595.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Mylvaganam S, Ramani M, Krawczyk M and
Carlen PL: Roles of gap junctions, connexins, and pannexins in
epilepsy. Front Physiol. 5:1722014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kékesi O, Ioja E, Szabó Z, Kardos J and
Héja L: Recurrent seizure-like events are associated with coupled
astroglial synchronization. Front Cell Neurosci.
9:2152015.PubMed/NCBI
|
|
95
|
Jiang S, Wang YQ, Xu CF, Li YN, Guo R and
Li L: Involvement of connexin43 in the infrasonic noise-induced
glutamate release by cultured astrocytes. Neurochem Res.
39:833–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wei H, Deng F, Chen Y, Qin Y, Hao Y and
Guo X: Ultrafine carbon black induces glutamate and ATP release by
activating connexin and pannexin hemichannels in cultured
astrocytes. Toxicology. 323:32–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chever O, Pannasch U, Ezan P and Rouach N:
Astroglial connexin 43 sustains glutamatergic synaptic efficacy.
Philos Trans R Soc Lond B Biol Sci. 369:201305962014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Unger T, Bette S, Zhang J, Theis M and
Engele J: Connexin-deficiency affects expression levels of glial
glutamate transporters within the cerebrum. Neurosci Lett.
506:12–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Figiel M, Allritz C, Lehmann C and Engele
J: Gap junctional control of glial glutamate transporter
expression. Mol Cell Neurosci. 35:130–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shen N, Mo LQ, Hu F, Chen PX, Guo RX and
Feng JQ: A novel role of spinal astrocytic connexin 43: Mediating
morphine antinociceptive tolerance by activation of NMDA receptors
and inhibition of glutamate transporter-1 in rats. CNS Neurosci
Ther. 20:728–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Huberfeld G and Vecht CJ: Seizures and
gliomas-towards a single therapeutic approach. Nat Rev Neurol.
12:204–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Robel S and Sontheimer H: Glia as drivers
of abnormal neuronal activity. Nat Neurosci. 19:28–33. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Abakumova T, Abakumov M, Shein S,
Chelushkin P, Bychkov D, Mukhin V, Yusubalieva G, Grinenko N,
Kabanov A, Nukolova N and Chekhonin V: Connexin 43-targeted T1
contrast agent for MRI, diagnosis of glioma. Contrast Media Mol
Imaging. 11:15–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Iusubalieva GM, Zorkina IaA, Baklaushev
VP, Gurina OI, Goriaĭnov SA, Aleksandrova EV, Zhukov VIu, Savel'eva
TA, Potapov AA and Chekhonin VP: Connexin-43 antibodies In
Intraoperative diagnosis of experimental poorly differentiated
gliomas. Zh Vopr Neirokhir Im N N Burdenko. 78:3–13. 2014.(In
Russian). PubMed/NCBI
|
|
105
|
Gielen PR, Aftab Q, Ma N, Chen VC, Hong X,
Lozinsky S, Naus CC and Sin WC: Connexin43 confers Temozolomide
resistance in human glioma cells by modulating the mitochondrial
apoptosis pathway. Neuropharmacology. 75:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Murphy SF, Varghese RT, Lamouille S, Guo
S, Pridham KJ, Kanabur P, Osimani AM, Sharma S, Jourdan J, Rodgers
CM, et al: Connexin 43 inhibition sensitizes chemoresistant
glioblastoma cells to temozolomide. Cancer Res. 76:139–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Munoz JL, Rodriguez-Cruz V, Greco SJ,
Ramkissoon SH, Ligon KL and Rameshwar P: Temozolomide resistance in
glioblastoma cells occurs partly through epidermal growth factor
receptor-mediated induction of connexin 43. Cell Death Dis.
5:e11452014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yusubalieva GM, Baklaushev VP, Gurina OI,
Zorkina YA, Gubskii IL, Kobyakov GL, Golanov AV, Goryainov SA,
Gorlachev GE, Konovalov AN, et al: Treatment of poorly
differentiated glioma using a combination of monoclonal antibodies
to extracellular connexin-43 fragment, temozolomide, and
radiotherapy. Bull Exp Biol Med. 157:510–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Okolie O, Bago JR, Schmid RS, Irvin DM,
Bash RE, Miller CR and Hingtgen SD: Reactive astrocytes potentiate
tumor aggressiveness in a murine glioma resection and recurrence
model. Neuro Oncol. 18:1622–1633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Theodoric N, Bechberger JF, Naus CC and
Sin WC: Role of gap junction protein Connexin43 in astrogliosis
induced by brain injury. PLoS One. 7:e473112012. View Article : Google Scholar : PubMed/NCBI
|