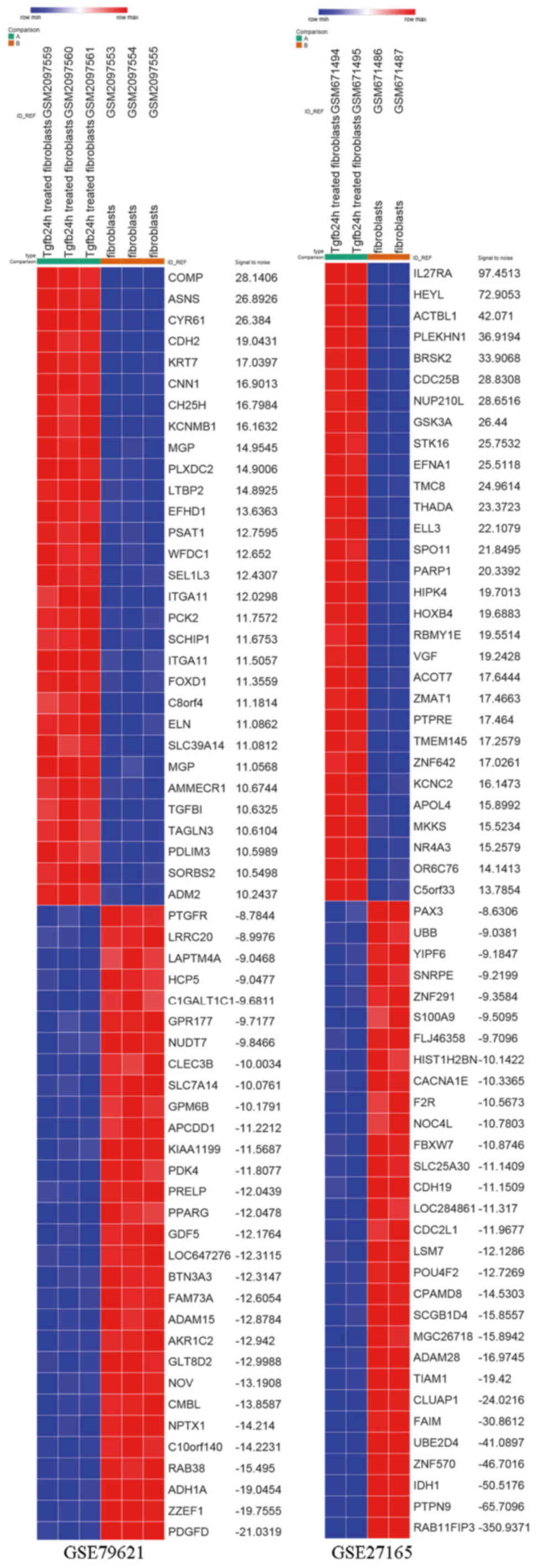
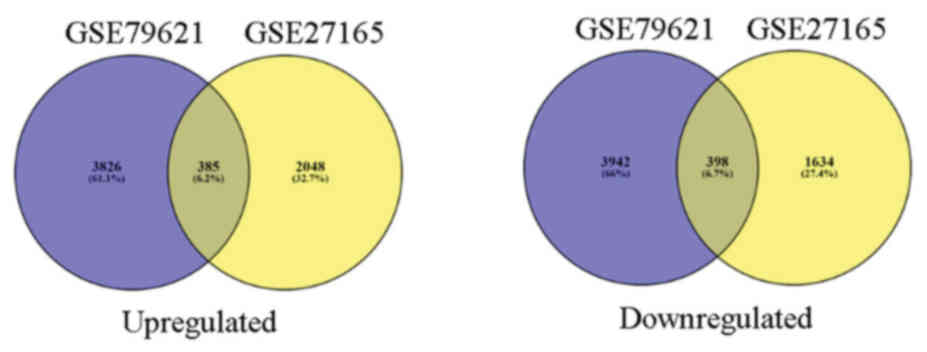
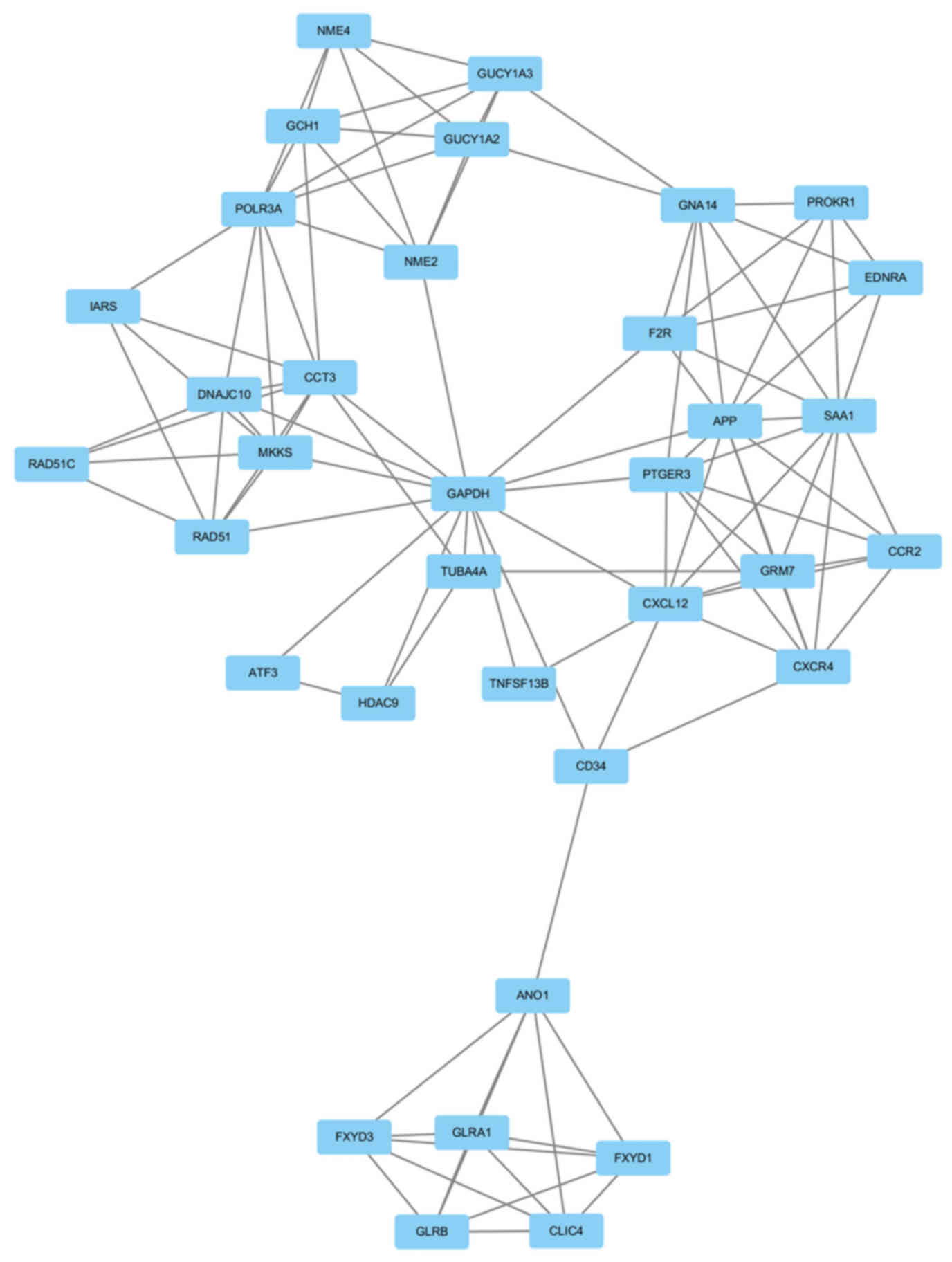
|
1
|
Ueha S, Shand FH and Matsushima K:
Cellular and molecular mechanisms of chronic
inflammation-associated organ fibrosis. Front Immunol. 3:712012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacinto A, Martinez-Arias A and Martin P:
Mechanisms of epithelial fusion and repair. Nat Cell Biol.
3:E117–E123. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baer ML and Colello RJ: Endogenous
bioelectric fields: A putative regulator of wound repair and
regeneration in the central nervous system. Neural Regen Res.
11:861–864. 2016.PubMed/NCBI
|
|
4
|
Xie SY, Peng LH, Shan YH, Niu J, Xiong J
and Gao JQ: Adult stem cells seeded on electrospinning silk fibroin
nanofiberous scaffold enhance wound repair and regeneration. J
Nanosci Nanotechnol. 16:5498–5505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith-Bolton R: Drosophila imaginal discs
as a model of epithelial wound repair and regeneration. Adv Wound
Care (New Rochelle). 5:251–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang S, Ma K, Geng Z, Sun X and Fu X:
Oriented cell division: New roles in guiding skin wound repair and
regeneration. Biosci Rep. 35:pii: e002802015. View Article : Google Scholar
|
|
7
|
Peng LH, Wei W, Shan YH, Zhang TY, Zhang
CZ, Wu JH, Yu L, Lin J, Liang WQ, Khang G and Gao JQ:
β-Cyclodextrin-linked polyethylenimine nanoparticles facilitate
gene transfer and enhance the angiogenic capacity of mesenchymal
stem cells for wound repair and regeneration. J Biomed Nanotechnol.
11:680–690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kotwal GJ, Sarojini H and Chien S: Pivotal
role of ATP in macrophages fast tracking wound repair and
regeneration. Wound Repair Regen. 23:724–727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shingyochi Y, Orbay H and Mizuno H:
Adipose-derived stem cells for wound repair and regeneration.
Expert Opin Biol Ther. 15:1285–1292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eming SA, Martin P and Tomic-Canic M:
Wound repair and regeneration: Mechanisms, signaling, and
translation. Sci Transl Med. 6:265sr62014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu S, Sang L, Zhang Y, Wang X and Li X:
Biological evaluation of human hair keratin scaffolds for skin
wound repair and regeneration. Mater Sci Eng C Mater Biol Appl.
33:648–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sen CK and Roy S: OxymiRs in cutaneous
development, wound repair and regeneration. Semin Cell Dev Biol.
23:971–980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reinke JM and Sorg H: Wound repair and
regeneration. Eur Surg Res. 49:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H and Fu X: Mechanisms of action of
mesenchymal stem cells in cutaneous wound repair and regeneration.
Cell Tissue Res. 348:371–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eldardiri M, Martin Y, Roxburgh J,
Lawrence-Watt DJ and Sharpe JR: Wound contraction is significantly
reduced by the use of microcarriers to deliver keratinocytes and
fibroblasts in an in vivo pig model of wound repair and
regeneration. Tissue Eng Part A. 18:587–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng LH, Tsang SY, Tabata Y and Gao JQ:
Genetically-manipulated adult stem cells as therapeutic agents and
gene delivery vehicle for wound repair and regeneration. J Control
Release. 157:321–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu X and Li H: Mesenchymal stem cells and
skin wound repair and regeneration: Possibilities and questions.
Cell Tissue Res. 335:317–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao F and Eriksson E: Gene therapy in
wound repair and regeneration. Wound Repair Regen. 8:443–451. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Puchelle E: Airway epithelium wound repair
and regeneration after injury. Acta Otorhinolaryngol Belg.
54:263–270. 2000.PubMed/NCBI
|
|
21
|
Clark LD, Clark RK and Heber-Katz E: A new
murine model for mammalian wound repair and regeneration. Clin
Immunol Immunopathol. 88:35–45. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sicard RE and Nguyen LM: An in vivo model
for evaluating wound repair and regeneration microenvironments. In
Vivo. 10:477–481. 1996.PubMed/NCBI
|
|
23
|
Suga H, Sugaya M, Fujita H, Asano Y, Tada
Y, Kadono T and Sato S: TLR4, rather than TLR2, regulates wound
healing through TGF-β and CCL5 expression. J Dermatol Sci.
73:117–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee MJ, Shin JO and Jung HS: Thy-1
knockdown retards wound repair in mouse skin. J Dermatol Sci.
69:95–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han
J, Zhu X, Tang C and Hu D: Wnt/β-catenin pathway forms a negative
feedback loop during TGF-β1 induced human normal skin
fibroblast-to-myofibroblast transition. J Dermatol Sci. 65:38–49.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Zeng B, Yao H and Xu J: The effect
of TLR4/7 on the TGF-β-induced Smad signal transduction pathway in
human keloid. Burns. 39:465–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Diegelmann RF and Evans MC: Wound healing:
An overview of acute, fibrotic and delayed healing. Front Biosci.
9:283–289. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elgaaen BV, Olstad OK, Sandvik L, Odegaard
E, Sauer T, Staff AC and Gautvik KM: ZNF385B and VEGFA are strongly
differentially expressed in serous ovarian carcinomas and correlate
with survival. PLoS One. 7:e463172012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li C, Zhen G, Chai Y, Xie L, Crane JL,
Farber E, Farber CR, Luo X, Gao P, Cao X and Wan M: RhoA determines
lineage fate of mesenchymal stem cells by modulating CTGF-VEGF
complex in extracellular matrix. Nat Commun. 7:114552016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Rekabi Z, Wheeler MM, Leonard A, Fura
AM, Juhlin I, Frazar C, Smith JD, Park SS, Gustafson JA, Clarke CM,
et al: Activation of the IGF1 pathway mediates changes in cellular
contractility and motility in single-suture craniosynostosis. J
Cell Sci. 129:483–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chigurupati S, Mughal MR, Okun E, Das S,
Kumar A, McCaffery M, Seal S and Mattson MP: Effects of cerium
oxide nanoparticles on the growth of keratinocytes, fibroblasts and
vascular endothelias cells in cutaneous wound healing.
Biomaterials. 34:2194–2201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao H, Han T, Hong X and Sun D: Adipose
differentiation-related protein knockdown inhibits vascular smooth
muscle cell proliferation and migration and attenuates neointima
formation. Mol Med Rep. 16:3079–3086. 2017.PubMed/NCBI
|
|
33
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le Clainche C and Carlier MF: Regulation
of actin assembly associated with protrusion and adhesion in cell
migration. Physiol Rev. 88:489–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fotedar R and Margolis RL: WISp39 and
Hsp90: Actin' together in cell migration. Oncotarget.
6:17871–17872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mast BA, Diegelmann RF, Krummel TM and
Cohen IK: Hyaluronic acid modulates proliferation, collagen and
protein synthesis of cultured fetal fibroblasts. Matrix.
13:441–446. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balaji S, King A, Marsh E, LeSaint M,
Bhattacharya SS, Han N, Dhamija Y, Ranjan R, Le LD, Bollyky PL, et
al: The role of interleukin-10 and hyaluronan in murine fetal
fibroblast function in vitro: Implications for recapitulating fetal
regenerative wound healing. PLoS One. 10:e1243022015. View Article : Google Scholar
|
|
38
|
Wu YS and Chen SN: Apoptotic cell: Linkage
of inflammation and wound healing. Front Pharmacol. 5:12014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuki K, Hathaway CK, Lawrence MG,
Smithies O and Kakoki M: The role of transforming growth factor β1
in the regulation of blood pressure. Curr Hypertens Rev.
10:223–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agarwal SK, Wu M, Livingston CK, Parks DH,
Mayes MD, Arnett FC and Tan FK: Toll-like receptor 3 upregulation
by type I interferon in healthy and scleroderma dermal fibroblasts.
Arthritis Res Ther. 13:R32011. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Alexander PB and Wang XF: TGF-β
family signaling in the control of cell proliferation and survival.
Cold Spring Harb Perspect Biol. 9:pii: a0221452017. View Article : Google Scholar
|
|
42
|
DiRenzo DM, Chaudhary MA, Shi X, Franco
SR, Zent J, Wang K, Guo LW and Kent KC: A crosstalk between
TGF-β/Smad3 and Wnt/beta-catenin pathways promotes vascular smooth
muscle cell proliferation. Cell Signal. 28:498–505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ghosh AK, Mori Y, Dowling E and Varga J:
Trichostatin A blocks TGF-beta-induced collagen gene expression in
skin fibroblasts: Involvement of Sp1. Biochem Biophys Res Commun.
354:420–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu J, Shi J, Li M, Gui B, Fu R, Yao G,
Duan Z, Lv Z, Yang Y, Chen Z, et al: Activation of AMPK by
metformin inhibits TGF-β-induced collagen production in mouse renal
fibroblasts. Life Sci. 127:59–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takino K, Ohsawa S and Igaki T: Loss of
Rab5 drives non-autonomous cell proliferation through TNF and Ras
signaling in Drosophila. Dev Biol. 395:19–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gause WC, Wynn TA and Allen JE: Type 2
immunity and wound healing: Evolutionary refinement of adaptive
immunity by helminths. Nat Rev Immunol. 13:607–614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Samuelsson B: Arachidonic acid metabolism:
Role in inflammation. Z Rheumatol. 50 Suppl 1:S3–S6. 1991.
|
|
49
|
Chen X, Stauffer S, Chen Y and Dong J:
Ajuba Phosphorylation by CDK1 Promotes Cell Proliferation and
Tumorigenesis. J Biol Chem. 291:14761–14772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lin L, Zhang JH, Panicker LM and Simonds
WF: The parafibromin tumor suppressor protein inhibits cell
proliferation by repression of the c-myc proto-oncogene. Proc Natl
Acad Sci USA. 105:pp. 17420–17425. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bunnell TM, Burbach BJ, Shimizu Y and
Ervasti JM: β-Actin specifically controls cell growth, migration,
and the G-actin pool. Mol Biol Cell. 22:4047–4058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu D, Huang Y, Bu D, Liu AD, Holmberg L,
Jia Y, Tang C, Du J and Jin H: Sulfur dioxide inhibits vascular
smooth muscle cell proliferation via suppressing the Erk/MAP kinase
pathway mediated by cAMP/PKA signaling. Cell Death Dis.
5:e12512014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang Y, Chen C, Li Z, Guo W, Gegner JA,
Lin S and Han J: Characterization of the structure and function of
a new mitogen-activated protein kinase (p38beta). J Biol Chem.
271:17920–17926. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hsu YL, Wang MY, Ho LJ, Huang CY and Lai
JH: Up-regulation of galectin-9 induces cell migration in human
dendritic cells infected with dengue virus. J Cell Mol Med.
19:1065–1076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Adachi T, Sakurai T, Kashida H, Mine H,
Hagiwara S, Matsui S, Yoshida K, Nishida N, Watanabe T, Itoh K, et
al: Involvement of heat shock protein a4/apg-2 in refractory
inflammatory bowel disease. Inflamm Bowel Dis. 21:31–39. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sakurai T, Kashida H, Hagiwara S, Nishida
N, Watanabe T, Fujita J and Kudo M: Heat shock protein A4 controls
cell migration and gastric ulcer healing. Dig Dis Sci. 60:850–857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Z, Fan M, Candas D, Zhang TQ, Qin L,
Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et
al: Cyclin B1/Cdk1 coordinates mitochondrial respiration for
cell-cycle G2/M progression. Dev Cell. 29:217–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Han IS, Seo TB, Kim KH, Yoon JH, Yoon SJ
and Namgung U: Cdc2-mediated Schwann cell migration during
peripheral nerve regeneration. J Cell Sci. 120:246–255. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dawkins E and Small DH: Insights into the
physiological function of the β-amyloid precursor protein: Beyond
Alzheimer's disease. J Neurochem. 129:756–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Saitoh T, Sundsmo M, Roch JM, Kimura N,
Cole G, Schubert D, Oltersdorf T and Schenk DB: Secreted form of
amyloid beta protein precursor is involved in the growth regulation
of fibroblasts. Cell. 58:615–622. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ponugoti B, Xu F, Zhang C, Tian C, Pacios
S and Graves DT: FOXO1 promotes wound healing through the
up-regulation of TGF-β1 and prevention of oxidative stress. J Cell
Biol. 203:327–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang YL, Sun GY, Zhang Y, He JJ, Zheng S
and Lin JN: Tormentic acid inhibits H2O2-induced oxidative stress
and inflammation in rat vascular smooth muscle cells via inhibition
of NF-kB signaling pathway. Mol Med Rep. 14:3559–3564. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ohsawa R, Miyazaki H, Niisato N, Shiozaki
A, Iwasaki Y, Otsuji E and Marunaka Y: Intracellular chloride
regulates cell proliferation through the activation of
stress-activated protein kinases in MKN28 human gastric cancer
cells. J Cell Physiol. 223:764–770. 2010.PubMed/NCBI
|
|
64
|
Bear CE: Phosphorylation-activated
chloride channels in human skin fibroblasts. Febs Lett.
237:145–149. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sorrell JM and Caplan AI: Fibroblasts-a
diverse population at the center of it all. Int Rev Cell Mol Biol.
276:161–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Z, Yu B, Gu Y, Zhou S, Qian T, Wang
Y, Ding G, Ding F and Gu X: Fibroblast-derived tenascin-C promotes
Schwann cell migration through β1-integrin dependent pathway during
peripheral nerve regeneration. Glia. 64:374–385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pedley AM and Benkovic SJ: A new view into
the regulation of purine metabolism: The purinosome. Trends Biochem
Sci. 42:141–154. 2017. View Article : Google Scholar : PubMed/NCBI
|