Introduction
Worldwide, >350 million individuals are infected
with hepatitis B virus (HBV) and of those, ~1 million succumb to
liver cirrhosis, hepatitis or liver carcinoma (1). It is well known that the interaction
between the HBV and the host immune cells is crucial for
determining the clinical outcome. The cellular immune response
induced by the HBV is the main way the virus is cleared, and the
level of response produced is also the deciding factor in whether a
balance will be kept between viral replication and the immune
system. Immune regulation is one of the most important factors of
the anti-viral effect of the immune response (2,3).
Once infected with HBV, antigen-presenting cells (APCs) present HBV
antigens to naïve T cells by major histocompatibility complex
molecules, which the activates T cells (4). In addition, a co-stimulatory signal
is frequently required to make the T cells optimally active.
Co-stimulators can also exist in soluble forms.
Soluble (s) cluster of differentiation (CD)80, CD86, CD28 and
cytotoxic T-lymphocyte protein 4 (CTLA-4) were aberrantly expressed
in patients with chronic hepatitis B (5,6). The
expression of CD28 and CTLA-4 is involved in the pathogenesis of
patients infected with HBV that develop hepatic carcinoma (7). Inducible co-stimulator molecule
(ICOS) and programmed cell death protein 1 (PD-1) serve an
important role in T cell activation. A previous study revealed that
sICOS and sPD-1 are correlated with immune function and the
activation of T cells (8). Their
aberrant production may be associated with a number of different
diseases. sICOS is significantly increased in diffuse sclerosis
(9) and sPD-1 could promote T cell
activation and enhance its anti-tumor effect (10). The present study aims to discuss
the expression and effect of sICOS and sPD-1 in patients infected
with HBV.
Previous studies have demonstrated that there are
different isoforms of PD-1 as a result of alternative splicing of
mRNA and soluble forms of PD-1 and ICOS are present in the
circulation in order to maintain immune homeostasis. PD-1 and ICOS
have been identified in a number of diseases including hepatitis C,
rheumatoid arthritis and systemic lupus erythematosus (5,11,12).
However, the clinical significance and immunological roles of ICOS
and PD-1 in patients with HBV is unknown. The association of sPD-1
and sICOS with chronic HBV infection was investigated in the
present study.
Materials and methods
Subjects
For the present study 80 patients with chronic HBV
and 30 volunteers (normal controls) were recruited. The individuals
with chronic HBV infections were patients from The Affiliated
Hospital of North Sichuan Medical College (Sichuan, China) from
August 2012 to May 2013. Patient characteristics are presented in
Table I. All patients were
positive for the HBV large envelope protein, positive or negative
for HBV external core antigen (HBeAg), and negative for hepatitis
C, hepatitis D virus and human immunodeficiency virus. All patients
were without any other disease-associated liver damage. Patients
with concomitant illness and autoimmune disease were excluded. No
patients received anti-HBV agents or steroids one year prior to
samples being obtained. Each individual gave written informed
consent and the protocol was approved by the Clinical Research
Ethics Committee of the Affiliated Hospital of North Sichuan
Medical College.
 | Table I.Biochemical and immunological markers
in normal controls and in patients with chronic HBV. |
Table I.
Biochemical and immunological markers
in normal controls and in patients with chronic HBV.
| Marker | Control | HBeAg(−) | HBeAg(+) |
|---|
| No. of cases | 30 | 40 | 40 |
| Male/female, n | 17/13 | 24/16 | 27/13 |
| Mean age ± SD,
years | 42.7±16.4 | 40.2±16.1 | 42.7±14.6 |
| ALT median (IQ
range), units/ml | 11.3 (5.1–37.0) | 24.6
(8.3–1,135.8) | 57.4
(7.2–1,420.2) |
| AST median (IQ
range), units/ml | 10.8 (5.9–33.0) | 29.7
(17.3–1,094.4) | 55 (15.5–989.1) |
| HBsAg positive
rate | Negative | 100% | 100% |
| Mean lgHBV-DNA | Negative | 4.51±1.29 | 5.91±1.70 |
| Presence of auto
immune disease | No | No | No |
Patients were divided into the HBeAg(+) group and
the HBeAg(−) group. The HBeAg(+) group was divided into
immune-clearance phase and immune-tolerance phase groups.
Immune-clearance phase characteristics: HBsAg(+), HBeAg(+),
HBV-DNA(+), serum alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) continuously or repeatedly raised, or liver
histological examination revealed hepatitis lesions.
Immune-tolerance phase characteristics: HBsAg(+), HBeAg(+), HBV DNA
positive, but ALT and AST were in normal range, and liver
histological examination generally revealed no obvious
abnormalities.
Virus detection and biochemistry
assays
The HBV associate indexes (HBsAg, HBsAb, HBeAg,
HBeAb and HBcAb) were detected via Architect I2000sr immunology
analyzer (Abbott Pharmaceutical Co., Ltd., Lake Bluff, IL, USA).
The HBV DNA level was determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) with
hepatitis B virus nucleic acid determination kit (cat. no. 18441;
Xiamen Kehua Hengsheng, Co., Ltd, Fujian, China), using the
LightCycler® 480 System (Roche Diagnostics GmbH,
Mannheim, Germany) with a detection sensitivity of 1×103
IU/ml. Cycling conditions included an initial denaturation of 2 min
at 95°C followed by 40 cycles of 10 sec at 95°C and 30 sec at 60°C.
Each sample was run in triplicate and averaged. Serum ALT and AST
were measured by the Synchron LX20 auto-analyzer (Beckman Coulter,
Inc., Brea, CA, USA).
ELISA for sICOS and sPD-1
The serum concentration of sICOS and sPD-1 in the
patients with HBV and the control group was detected using ELISA
kits (sICOS; cat. no. SEA777Hu, Cayman Chemical Company, Ann Arbor,
MI, USA; sPD-1; cat. no. DY1086; R&D Systems, Inc.,
Minneapolis, MN, USA). The assay was conducted according to the
manufacturer's protocol.
RT-qPCR
Peripheral blood mononuclear cells (PBMC) of HBV
patients and the controls were obtained according to the
manufacturer's protocol for the PBMCs separation medium (cat. no.
LTS1077; Shanghai Haoyan Biotech Co., Ltd., Shanghai, China). Half
of the PBMCs/patient were stimulated with 20 µg/ml
phytohemagglutinin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and 1 µg/ml lipopolysaccharide (Sigma-Aldrich; Merck KGaA). RNA
extraction was performed using Trizol reagent and cDNA synthesis
with the iScript cDNA synthesis kit (Roche Diagnostics, Basel,
Switzerland) was performed according to the manufacturer's protocol
(Promega Corporation, Madison, WI, USA).
The following PCR primer sequences were used:
Forward, 5′-GTTCCCTGAGTTGTTTG-3′ and reverse,
5′-TCATCTTGAGGTGTCCC-3′ for ICOS; forward,
5′-CCGCCTTCTGTAATGGTTTGA-3′ and reverse,
5′-GGGCAGCTGTATGATCTGGAA-3′ for PD-1; Forward
5′-GCTCAGGGTGACAGGGAC-3′ and reverse, 5′-CAATGGTGGCATACTCCGTCT-3′
for PD-1Δex3 (encoding sPD-1). GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′) was used as an internal control to
normalize target mRNA levels.
The RT-qPCR was performed using
SYBRGreen® (Roche Diagnostics) as detection system. The
PCR reaction system (20 µl) consisted of the following: 0.4 µl of
each pair primer (10 nM), 2 µl of template DNA, 10 µl of 2X
SYBR-Green PCR master mix, and DNAse-RNse-free water to complete
the final 20 µl volume. Cycling conditions were the same as used
for HBV-DNA. Relative RNA levels were quantified using the
2−ΔΔCq formula (13).
Statistical analysis
The Kruskal-Wallis H test followed by bonferroni
post-hoc test was used to analyze the difference in sPD-1 and sICOS
levels between normal controls and patients. The Spearman's rank
correlation coefficient was used to assess the correlation between
sPD-1, sICOS, ALT, AST and HBV associate indexes. Results are
expressed as the median and interquartile range. The data were
analyzed with OriginPro statistical software (version 8.5;
OriginLab, Northampton MA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum concentration of sICOS and sPD-1
in patients with HBV and the controls
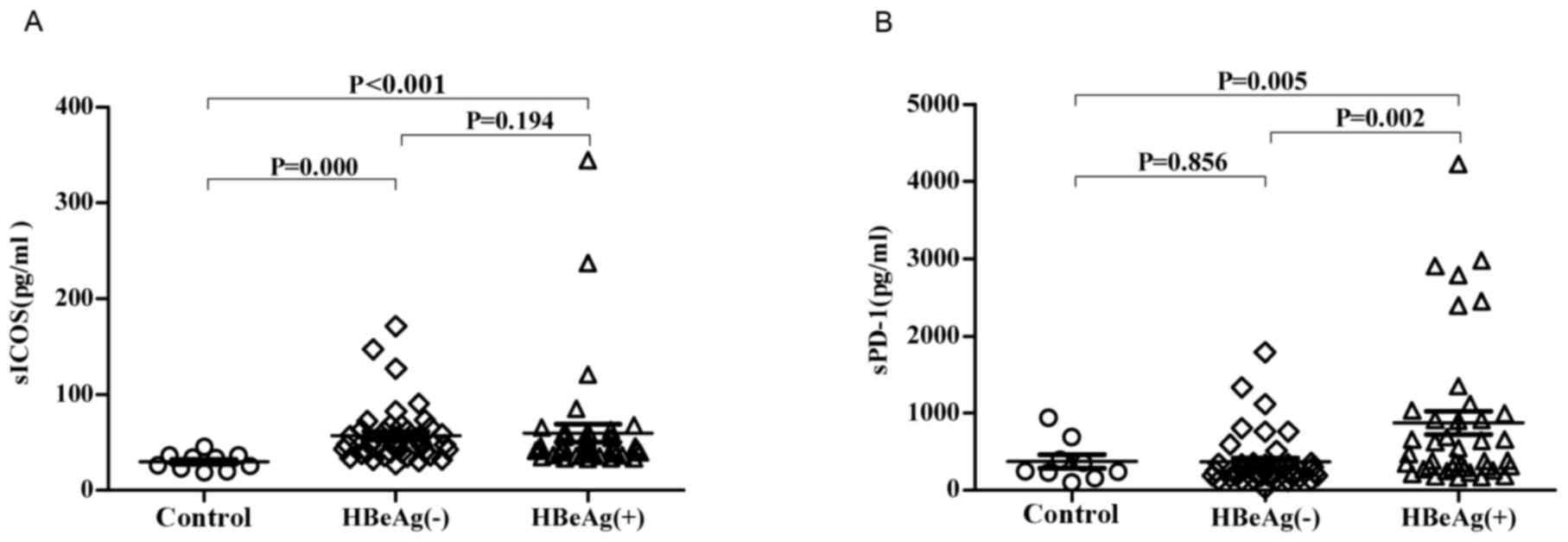
The concentrations of sICOS and sPD-1 in the
HBeAg(+) group were increased compared with the control group
(Fig. 1). There was no significant
difference in the concentration of sICOS in the HBeAg(−) group
compared with the HBeAg(+) group. The concentration of sPD-1 was
significantly increased in the HBeAg(+) group compared with the
HBeAg(−) group (P<0.05; Fig.
1B).
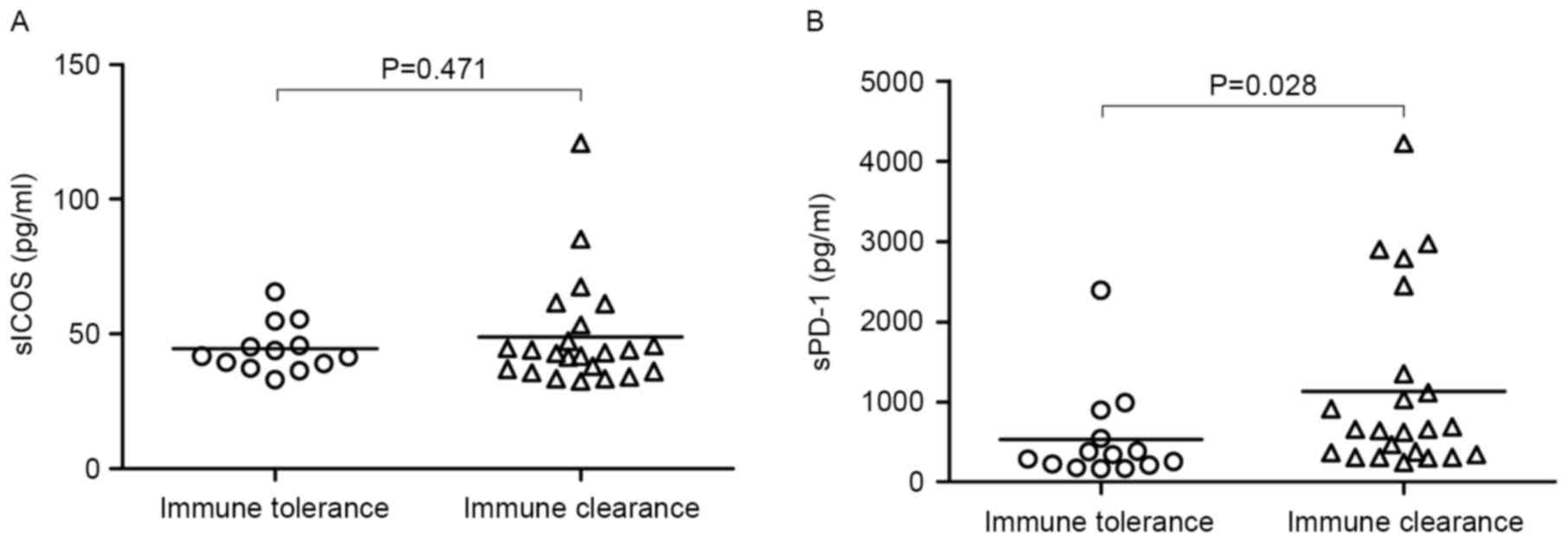
Serum concentration of sPD-1 and sICOS
in the immune tolerance and immune clearance phases
sPD-1 in the immune clearance phase was
significantly increased compared with the tolerance stage
(P<0.05) however there was no significant difference in the
concentration of sICOS between the different phases (Fig. 2). This indicates that sPD-1 is
associated with the co-stimulators involved in the reaction to HBV.
Their upregulation is not consistent with their receptor levels.
This result is negatively correlated to CD8+ lymphocyte numbers
(7,14).
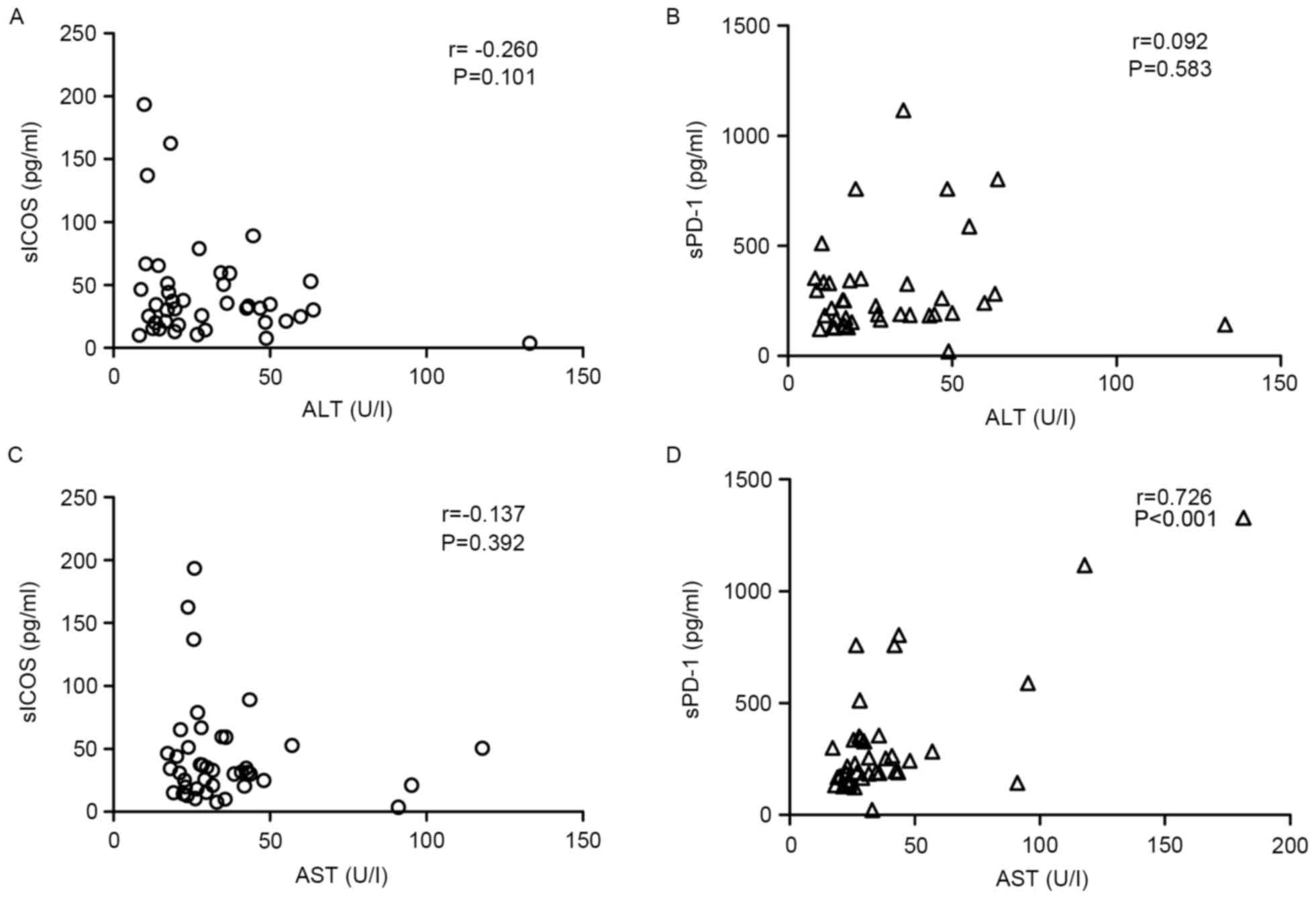
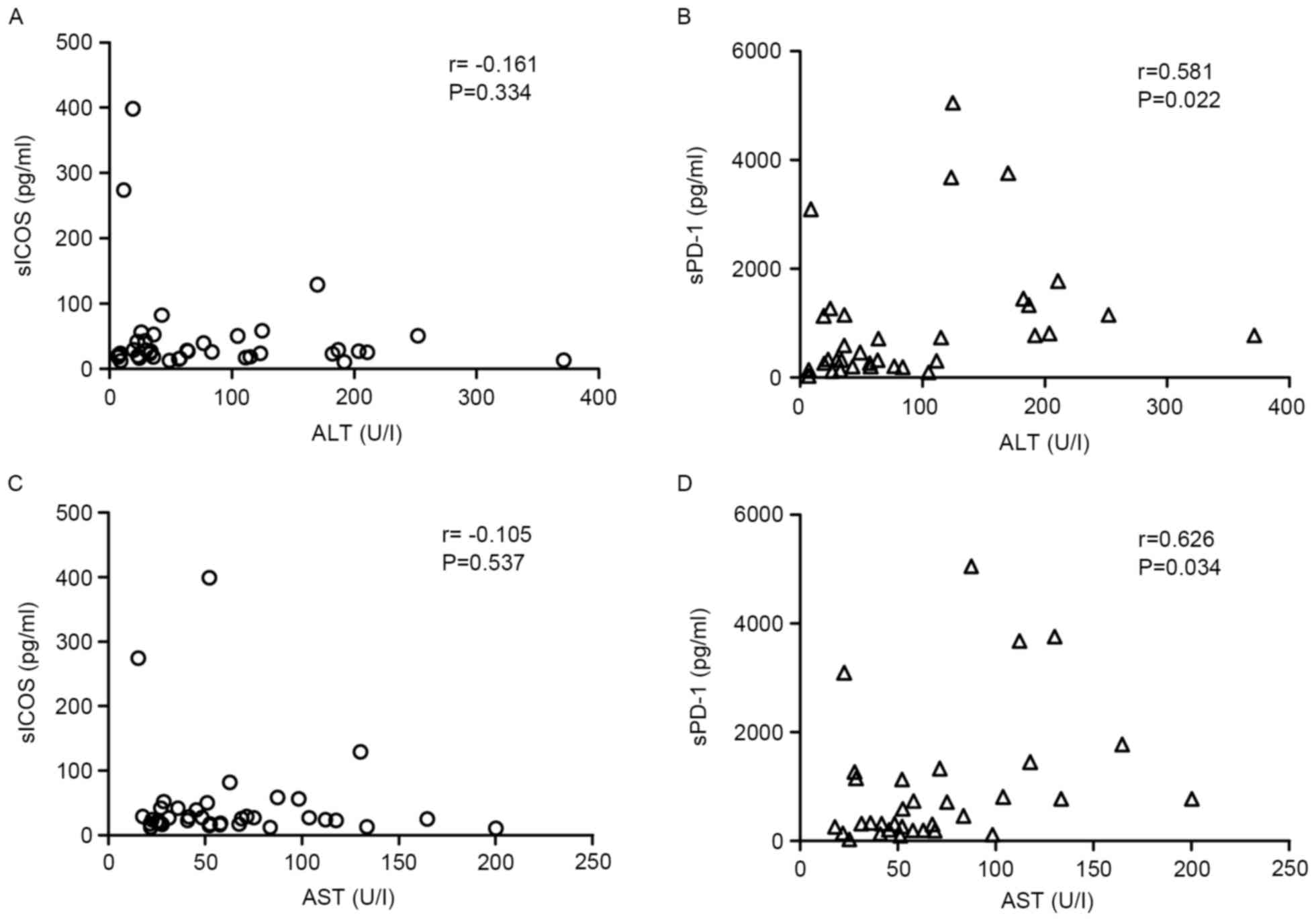
Correlation of sPD-1 or sICOS with
specific biochemical factors
Only sPD-1 demonstrated a positive correlation with
AST in HBeAg(−) patients (r=0.726, P<0.01), whereas sICOS
demonstrated no correlation with AST levels (Fig. 3). However, no correlation was
observed between sICOS or sPD-1 levels and ALT in HBeAg(−)
patients. In the HBeAg(+) group, sPD-1 exhibited a positive
correlation with AST and ALT (r>0.5; P<0.05; Fig. 4).
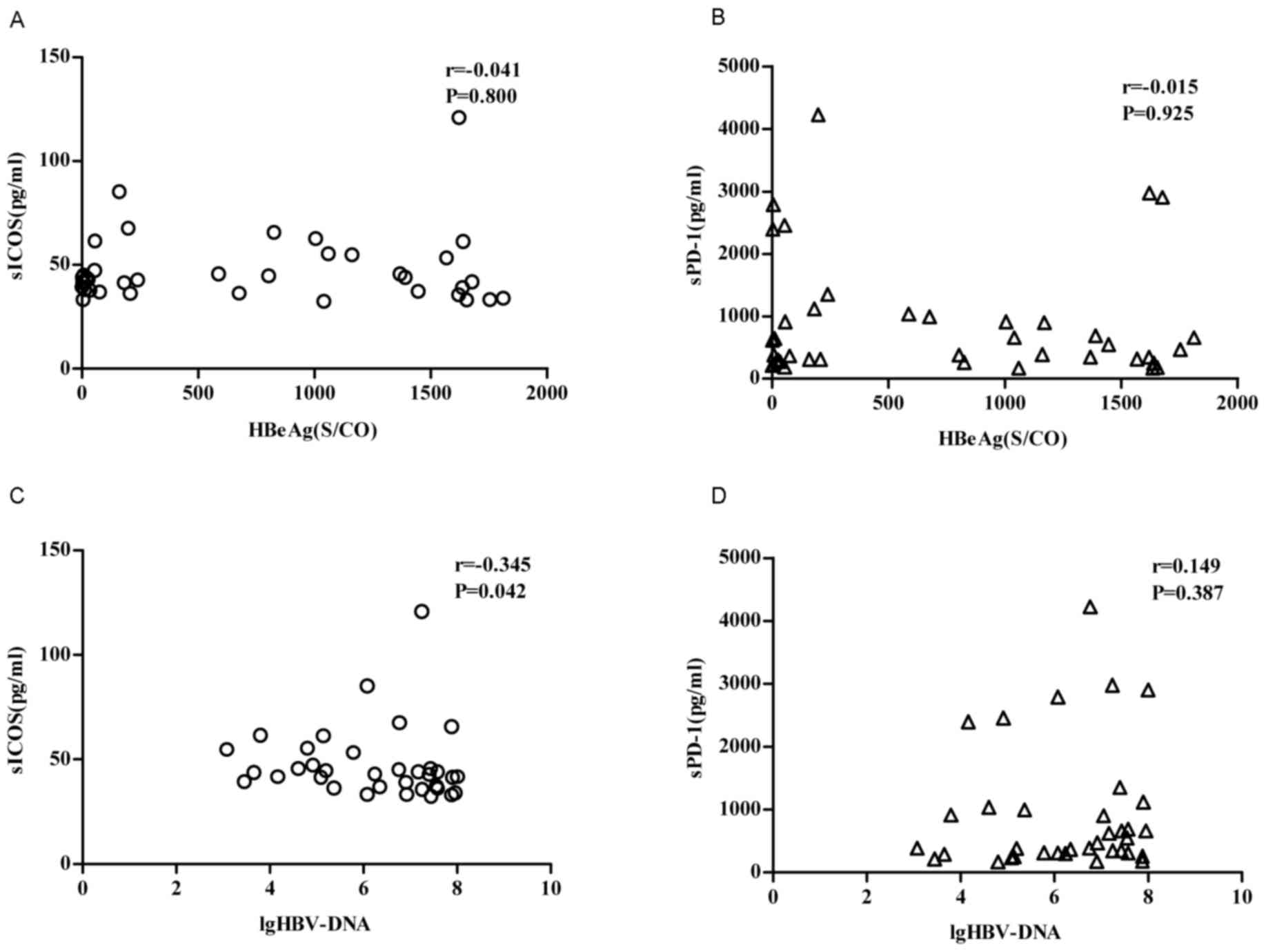
Correlation of sPD-1 or sICOS with
HBeAg levels and HBV-DNA levels
Only sICOS is poorly correlated with the log10
(lg10)-transformed value of HBV-DNA in patients with chronic HBV
(P<0.05, r=-0.345), whereas there was no correlation with HBeAg.
There was no correlation between sPD-1 and HBeAg
levels/lg10-transformed value of HBV-DNA (P>0.05; Fig. 5).
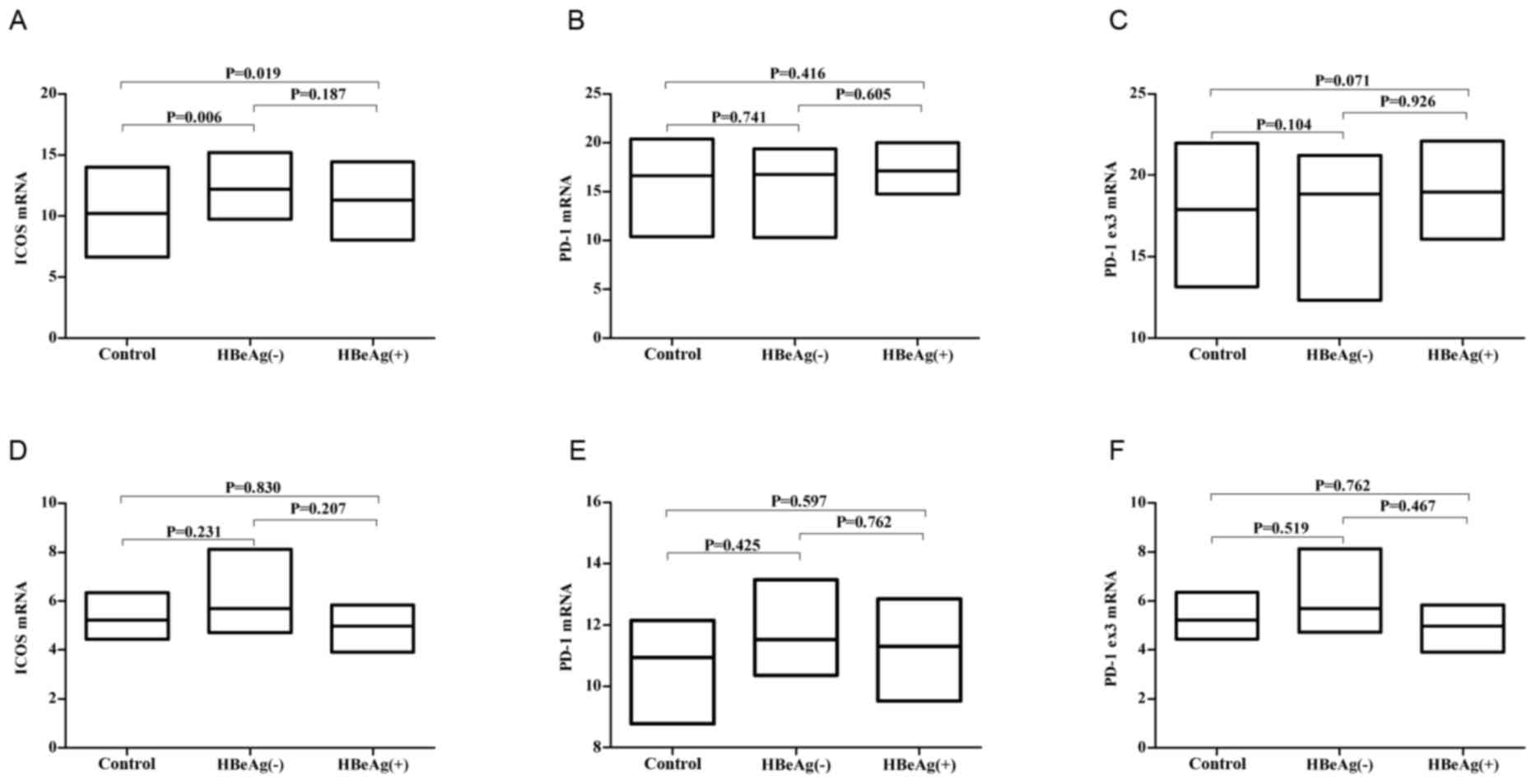
ICOS, PD-1 and PD-1Δex3 mRNA levels in
HBV patients' PBMCs cells
RT-qPCR demonstrated that the mRNA levels of ICOS in
PBMC was elevated in patients with chronic HBV compared with the
normal controls (Fig. 6A).
Considering that sICOS may be shed from the membranes of these
cells, the upregulation of these molecules may explain the high
levels of ICOS in HBV patients. Whereas the mRNA levels of PD-1 and
PD-1Δex3 (prior to and following stimulation) were no different
compared with the normal controls (Fig. 6B, C, E and F).
Discussion
APCs activate T cells in response to a variety of
signals. In patients with HBV, T cell activation is also important
for clearance of the virus. Co-stimulatory signals influence the
type of immune response and the intensity of the T cell activation
process (2,3). T cell dysfunction and abnormal
immunomodulation of molecules by APCs, in patients with HBV has
previously been demonstrated (15). There are numerous co-stimulatory
molecules, including CD28, CD80, CD86 and CTLA, all of which
possess membrane-bound and soluble forms (16). sPD-1 and sICOS are formed by
alternative mRNA splicing or shed from the membrane of T cells. The
structure of ICOS is similar to CD28, and the two molecules are
positive co-stimulators that activate T cells. PD-1 exhibits 23%
homology with CTLA-4 and, may suppress or terminate immune cell
proliferation and activation when combined with the PD-ligand 1
(L1) or PD-L2 (17,18). However to the best of the authors'
knowledge, the soluble co-stimulatory molecules in patients with
chronic HBV have not previously been investigated.
In the present study, it was demonstrated that the
serum concentrations of sPD-1 and sICOS were elevated in HBeAg(+)
patients compared with the normal controls. However in the HBeAg(−)
group, only sICOS increased. The small sample size of the present
study may have influenced the results and led to an incorrect
conclusion being drawn. When patients were separated into immune
tolerance and immune clearance groups, sPD-1 concentration
significantly rose in the clearance phase indicating that PD-1 may
be involved in the virus clearance process.
In the present study, sPD-1 and sICOS were
upregulated in the HBeAg(+) group compared with the normal control.
Although the function of sPD-1 and sICOS remains unknown the
results of the present study indicate an immune regulatory function
and this suggests that sPD-1 and sICOS may serve a role in the
pathogenesis of HBV. The soluble form may interfere with the
interaction between the two membrane bound forms and their ligands,
which may lead to immune dysregulation and a defective immune
response. Abnormal sICOS may compete with the ICOS-L to suppress T
cell activation and in turn cause T cell exhaustion. PD-1 and PD-L
has been demonstrated to negatively regulate the immune response in
primary and secondary immune reactions (19,20).
Therefore, the abnormal concentration of sPD-1 would enhance the
antiviral effects.
However, in the HBeAg(−) group, only sICOS
demonstrated a serious increase. In HBeAg(+) group, only sPD-1 is
significantly elevated compared with the HBeAg(−) group, whereas
there was no significant difference in sICOS. Considering the
numbers of patients enrolled in the present study, this phenomenon
requires further study. Different soluble co-stimulators do not
exhibit the same function in different HBV patient groups.
Patients with HBV were divided into immune tolerance
and clearance groups. sPD-1 and sICOS were elevated in the
clearance group. The correlation between the levels of sPD-1 and
sICOS, and clearance of the virus remains unclear and requires
further investigation. However, it was observed that sPD-1 and
sICOS concentrations may reflect the virus activity (3). In a PD-1 knock-out mouse, the
negative immune regulation induced by PD-1 is important to immune
tolerance. The extracellular domain of PD-1 specifically, could
combine with its ligand and inhibit the interaction between
PD-1/PD-L1, thus, maintaining immune homeostasis and enhancing T
cell activity (21). In the
present study, the upregulation of sPD-1 may prolong the life of
HBV specific T cells. The elevation of these cells should aid HBV
clearance by the immune system.
AST is frequently elevate in patients with active
HBV infections, and it is also positively correlated with sPD-1,
which demonstrates that sPD-1 is a useful molecular marker to
reflect the degree of liver injury and viral activity. From the
results of the present study, considering their weak
immunomodulatory function, sPD-1 and sICOS frequently do not alter
synchronously with other biochemical markers. As a result, the
correlation is significant but weak.
As sPD-1, but not sICOS, has been demonstrated to be
highly expressed in a number of diseases (22), sPD-1 may not be specific for
chronic HBV infections. Additionally, sICOS has not been
investigated previously. Therefore, these two markers are not
necessarily disease-specific molecules. However, due to the
correlation of sPD-1 and sICOS with the HBV DNA copies, the two
molecules may be partially associated with viral replication and
HBV pathogenesis, which may lead to the dysregulation of T-cell
co-stimulation.
To investigate the generation of sPD-1and sICOS in
patients with HBV, the expression of PD-1 and ICOS in PBMCs was
detected with qPCR. This result is not consistent with the soluble
forms of the molecules. This phenomenon may be explained by the
different speed of shedding from the membrane or the different
degradation speeds. As a result, the level of the soluble form is
not proportional to the level of the membrane form.
As PD-1 and ICOS exist at a high level in the cell
membrane, the soluble form may be shed and function as a regulator
in certain physiological and pathology processes. Although the
exact mechanism of how the soluble form is produced is not clear,
its upregulation in HBeAg(+) patients rather than other subtypes
may indicate a role in disease. Inhibiting PD-1/PD-1 L function
could be a useful way to recover T cell function and induce T cell
proliferation. In the present study, there was no significant
difference between HBeAg(+) patients and the healthy controls.
In conclusion, to the best of the authors' knowledge
the present study was the first to investigate the soluble form of
co-stimulator in serum of patients with HBV. The high levels of
sPD-1 and sICOS serve an important role in the pathological process
of HBV infection. These soluble molecules may be novel therapeutic
targets for HBV therapy. Further study should be carried out to
investigate the role of sPD-1 and sICOS in HBV infection.
References
|
1
|
Lok AS: The maze of treatments for
hepatitis B. N Engl J Med. 352:2743–2746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari C: HBV and the immune response.
Liver Int. 35 Suppl 1:S121–S128. 2015. View Article : Google Scholar
|
|
3
|
Lee JA, Kim YM, Hyun PM, Jeon JW, Park JK,
Suh GH, Jung BG and Lee BJ: Honeybee (Apis mellifera) venom
reinforces viral clearance during the early stage of infection with
porcine reproductive and respiratory syndrome virus through the
up-regulation of Th1-specific immune responses. Toxins (Basel).
7:1837–1853. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein J and Sato A: The HLA system. First
of two parts. N Engl J Med. 343:702–709. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang D, Zhou D, DU Q, Liang Q, Wang Q,
Fang L, Wang G, Fan Q, Liu B, Zhou J, et al: Aberrant production of
soluble inducible T-cell co-stimulator (sICOS) and soluble
programmed cell death protein 1 (sPD-1) in patients with chronic
hepatitis C. Mol Med Rep. 7:1197–1202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao J, Zhang L, Huang S, Chen P, Zou L,
Chen H, Xiang Y, Lai X and Ren G: Aberrant production of soluble
co-stimulatory molecules CTLA-4 and CD28 in patients with chronic
hepatitis B. Microb Pathog. 51:262–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Zhao C, Peng Q, Shi J and Gu G:
Expression levels of CD28, CTLA-4, PD-1 and Tim-3 as novel
indicators of T-cell immune function in patients with chronic
hepatitis B virus infection. Biomed Rep. 2:270–274. 2014.PubMed/NCBI
|
|
8
|
Nurieva RI, Mei XM, Forbush K, Bevan MJ
and Dong C: B7h is required for T cell activation, differentiation,
and effector function. Proc Natl Acad Sci USA. 100:pp. 14163–14168.
2003; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yanaba K, Asano Y, Noda S, Akamata K,
Aozasa N, Taniguchi T, Takahashi T, Ichimura Y, Toyama T, Sumida H,
et al: Increased production of soluble inducible costimulator in
patients with diffuse cutaneous systemic sclerosis. Arch Dermatol
Res. 305:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu X, Zhang H, Xing Q, Cui J, Li J, Li Y,
Tan Y and Wang S: PD-1(+) CD8(+) T Cells are exhausted in tumours
and functional in draining lymph nodes of colorectal cancer
patients. Br J Cancer. 111:1391–1399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Her M, Kim D, Oh M, Jeong H and Choi I:
Increased expression of soluble inducible costimulator ligand
(ICOSL) in patients with systemic lupus erythematosus. Lupus.
18:501–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan B, Nie H, Liu A, Feng G, He D, Xu R,
Zhang Q, Dong C and Zhang JZ: Aberrant regulation of synovial T
cell activation by soluble costimulatory molecules in rheumatoid
arthritis. J Immunol. 177:8844–8850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Ye B, Liu X, Li X, Kong H, Tian L and Chen
Y: T-cell exhaustion in chronic hepatitis B infection: Current
knowledge and clinical significance. Cell Death Dis. 6:e16942015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bengsch B, Martin B and Thimme R:
Restoration of HBV-specific CD8+ T cell function by PD-1 blockade
in inactive carrier patients is linked to T cell differentiation. J
Hepatol. 61:1212–1219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Magistrelli G, Jeannin P, Elson G, Gauchat
JF, Nguyen TN, Bonnefoy JY and Delneste Y: Identification of three
alternatively spliced variants of human CD28 mRNA. Biochem Biophys
Res Commun. 259:34–37. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hutloff A, Dittrich AM, Beier KC,
Eljaschewitsch B, Kraft R, Anagnostopoulos I and Kroczek RA: ICOS
is an inducible T-cell co-stimulator structurally and functionally
related to CD28. Nature. 397:263–266. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.PubMed/NCBI
|
|
19
|
Jiang W: Blockade of B7-H1 enhances
dendritic cell-mediated T cell response and antiviral immunity in
HBV transgenic mice. Vaccine. 30:758–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shankar EM, Che KF, Messmer D, Lifson JD
and Larsson M: Expression of a broad array of negative
costimulatory molecules and Blimp-1 in T cells following priming by
HIV-1 pulsed dendritic cells. Mol Med. 17:229–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng HY, Kang PJ, Chuang YH, Wang YH, Jan
MC, Wu CF, Lin CL, Liu CJ, Liaw YF, Lin SM, et al: Circulating
programmed death-1 as a marker for sustained high hepatitis B viral
load and risk of hepatocellular carcinoma. PLoS One. 9:e958702014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thio CL, Mosbruger TL, Kaslow RA, Karp CL,
Strathdee SA, Vlahov D, O'Brien SJ, Astemborski J and Thomas DL:
Cytotoxic T-lymphocyte antigen 4 gene and recovery from hepatitis B
virus infection. J Virol. 78:11258–11262. 2004. View Article : Google Scholar : PubMed/NCBI
|