Introduction
Bone formation and resorption are mediated by
osteoblasts and osteoclasts, respectively, and regulate the balance
of normal bone metabolism. Interruption of this balance may result
in increased resorption compared with formation and lead to excess
bone loss, causing a variety of diseases, including myeloma bone
disease, osteoporosis and rheumatoid arthritis (1,2). As
isolating and culturing bone cells is difficult, research and
molecular analysis of osteoclastogenesis has stagnated for a long
period. However, developments in techniques over the past decade
have allowed for further investigation (3,4).
There are multiple biomolecules involved in the signaling pathways
of osteoclastogenesis. Among these, the most important factors are
receptor activator for nuclear factor-κB ligand (RANKL) and
macrophage colony-stimulating factor (M-CSF).
Research has demonstrated that RANKL and its
receptor, RANK, are necessary for osteoclastogenesis (5). These proteins activate the
reconstitution of dynamic differentiation processes, including cell
fusion (5,6). In addition, the proteins are
considered to be a possible factor involved in controlling the
differentiation process. RANKL may also activate the expression of
transcriptional factors such as c-Fos, microphthalmia-associated
transcription factor and nuclear factor of activated T-cells
cytoplasmic 1 (NFATc1), which are important for osteoclastogenesis
(7). Therefore, the RANKL-RANK
signaling pathway is a key signaling component involved in
osteoclastogenesis.
RANK mediates signaling by recruiting adaptor
molecules, including proteins of the tumor necrosis factor (TNF)
receptor-associated factor (TRAF) family (8,9).
Studies have revealed that TRAF1 binds to RANK (10,11).
TRAF6 also binds to RANK, which further induces trimerization of
TRAF6 and subsequently activates nuclear factor (NF)-κB and
mitogen-activated kinases (MAPKs) (12,13).
NF-κB is associated with the biomolecular progress of
osteoclastogenesis induced by RANK and is a basic component of
osteoclastogenesis activated by TRAF6 (14).
M-CSF is another essential cytokine involved in
osteoclastogenesis besides RANKL (15,16).
Research has demonstrated that M-CSF serves an important role in
the proliferation and survival of osteoclast precursor cells
(17), upregulates the expression
levels of RANK, and may participate in the progression of
differentiation by activating c-Fos, protein kinase B and
extracellular signal-regulated kinase (ERK) pathways, which may
interact with RANKL signals (18,19).
Studies have demonstrated that bromodomain and extra
terminal domain proteins (BET) serve an important role in different
types of cancer, including cancer of the bones (20,21).
I-BET151 is a quinoline class of BET protein inhibitors, which has
been demonstrated to exhibit activity against several types of
cancer (22). Studies revealed
that I-BET151 induced B-cell lymphoma like 11-dependent apoptosis
and cell cycle arrest of human melanoma cells (23), and suppressed expression of
inflammatory genes and matrix degrading enzymes in rheumatoid
arthritis synovial fibroblasts (24). Research has also demonstrated that
I-BET151 suppresses pathologic bone loss in TNF-induced
inflammatory osteolysis (21).
Despite numerous reports of I-BET151, few have focused on the role
of I-BET151 in osteoclastogenesis. In the present study, the
effects of I-BET151 on osteoclastogenesis and the underlying
molecular signaling pathways involved were investigated.
Materials and methods
Cell culture
A mouse macrophage cell line, RAW264.7
(TIB-71™), was purchased from American Type Culture
Collection (Manassas, VA, USA). RAW264.7 cells were cultured in
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). RANKL (PeproTech, Inc., Rocky Hill, NJ, USA) was diluted
to 100 ng/ml in aquae sterilisata. I-BET151 (MedChemExpress,
Monmouth Junction, NJ, USA) was dissolved in dimethyl sulfoxide at
a concentration of <0.1% (Sigma-Aldrich; Merck KGaA). RAW264.7
cells were seeded in 6-well plates (2×107 cells/well) in
a humidified incubator at 5% CO2 and 37°C, supplemented
with 100 ng/ml RANKL, and treated with different concentrations of
I-BET151 (25). Cells were
cultured for 7 days to induce osteoclast differentiation. The study
was divided into 6 groups: Control; RANKL (100 ng/ml); RANKL (100
ng/ml) and I-BET151 (50 nM); RANKL (100 ng/ml) and I-BET151 (100
nM); RANKL (100 ng/ml) and I-BET151 (200 nM); and RANKL (100 ng/ml)
and I-BET151 (400 nM).
TRACP staining
TRACP staining was used to determine the effect of
I-BET151 on osteoclastogenesis and the percentage of TRACP positive
multinucleated cells was calculated. Cells were fixed using freshly
made, refrigerated, 3% paraformaldehyde (PFA; Sigma-Aldrich; Merck
KGaA) and 2% sucrose in phosphate-buffered saline (PBS;
Sigma-Aldrich, Merck KGaA) for 10 min and stained for TRACP using a
tartrate-resistant acid phosphatase stain kit according to
manufacturer's protocol (Nanjing Jiangcheng Bioengineering
Institute, Nanjing, China), after 7 days culture in the presence of
RANKL and different concentrations of I-BET151. TRACP positive
multinucleated cells (>3 nuclei) were counted under 8 fields of
view for each sample and regarded as osteoclasts (26). Cells were observed using a Zeiss
Axio Observer D1 microscope with ×100 magnification and images were
captured and analyzed with Zeiss ZEN software version 2012 (Zeiss
GmbH, Jena, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RAW264.7 cells (2×104) were incubated
with RANKL (100 ng/ml) and different concentrations of I-BET151 for
4 days. RT-qPCR was employed to determine the expression levels of
osteoclastic-specific marker genes and GAPDH was used as a control.
RNA extraction and reverse-transcription were performed as
described previously (27). Total
RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A High
Capacity cDNA Reverse Transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used to convert RNA into cDNA. qPCR
was conducted using a ABI 7500 real-time PCR instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with SYBR Premix Ex Taq
(Takara Biotechnology Co., Ltd., Dalian, China). Primers used in
PCR were as follows: Primers for TRACP were: Forward,
5′-ACACAGTGATGCTGTGTGGCAACTC-3′ and reverse,
5′-CCAGAGGCTTCCACATATATGATGG-3′; Primers for MMP9 were: Forward,
5′-AGTTTGGTGTCGCGGAGCAC-3′ and reverse, 5′-TACATGAGCGCTTCCGGCAC-3′;
Primers for CtsK were: Forward, 5′-GGCCAACTCAAGAAGAAAAC-3′ and
reverse, 5′-GTGCTTGCTTCCCTTCTGG-3; Primers for c-Src were: Forward,
5′-CCAGGCTGAGGAGTGGTACT-3′ and reverse, 5′-CAGCTTGCGGATCTTGTAGT-3′;
Primers for GAPDH were: Forward, 5′-AACTTTGGCATTGTGGAAGG-3′ and
reverse, 5′-ACACATTGGGGGTAGGAACA-3′. DNA was denatured at 94°C for
10 min, followed by initial denaturation with 30 cycles at 94°C for
1 min, 60°C for 1 min and 72°C for 2 min, and finally ended up with
an extension step at 72°C for 5 min. Relative quantification of
RT-qPCR product was performed using the comparative
2−ΔΔCq method (28).
Western blotting
Western blotting was used to determine the
expression levels of TRAF6, NFATc l, and the influence of I-BET151
on the NF-κB signaling pathway (p65 and IκB-α) and MAPK signaling
pathway [ERK, Jun N-terminal kinase (JNK) and p38]. A Nuclear
Extraction kit was purchased from Cayman Chemical Company (Ann
Arbor, MI, USA) to use for measurement of p65. β-actin was used as
a loading control. Samples extracted from the cells as previously
described (29) were loaded on 10%
SDS-PAGE, prior to transfer onto polyvinylidene difluoride
membranes. Membranes were blocked with 5% non-fat milk in TBS
containing 0.1% Tween-20 for 2 h at room temperature. Subsequently,
membranes were probed with primary antibodies at 4°C overnight and
a horseradish peroxidase (HRP) conjugated secondary antibody for 2
h at room temperature (Cell Signaling Technology, Inc., Danvers,
MA, USA). The membranes were incubated with an Enhanced
Chemiluminescence detection kit (Amersham Pharmacia Biotech AB,
Uppsala, Sweden) and were exposed to X-ray film. The films were
scanned and proteins were quantified using Quantity One software
version 4.2.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Antibodies used in western blotting were as follows: Anti-TRAF6
(ab33915; 1:1,000; Abcam, Cambridge, UK); NFATC1 antibody (MA3-024;
1:2,000; Thermo Fisher Scientific, Inc.); anti-NF-κB p65 (ab16502;
1:1,000); anti-IκB-α (E130; ab32518; 1:1,000); anti-ERK1+ERK2
(ab17942; 1:1,000); anti-ERK 1/2 (phospho-Thr202/Tyr204; ab214362;
1:1,000); anti-JNK1+JNK2+JNK3 (ab179461; 1:1,000);
anti-JNK1+JNK2+JNK3 (phospho T183+T183+T221; ab124956; 1:1,000);
anti-p38 (ab31828; 1:1,000); anti-p38 (phospho Y182; ab47363;
1:1,000); anti-β-actin (ab8226; 1:1,000); and anti-mouse IgG
VeriBlot for IP secondary (HRP-conjugated; ab131368; 1:2,000) (all
from Abcam).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Independent continuous variables were compared using a Student's
t-test for comparison of two groups or a one-way analysis of
variance followed by Tukey post hoc test for multiple comparison.
P<0.05 was considered to indicate a statistically significant
difference. All calculations were made using SPSS software version
18.0 (SPSS, Inc., Chicago, IL, USA).
Results
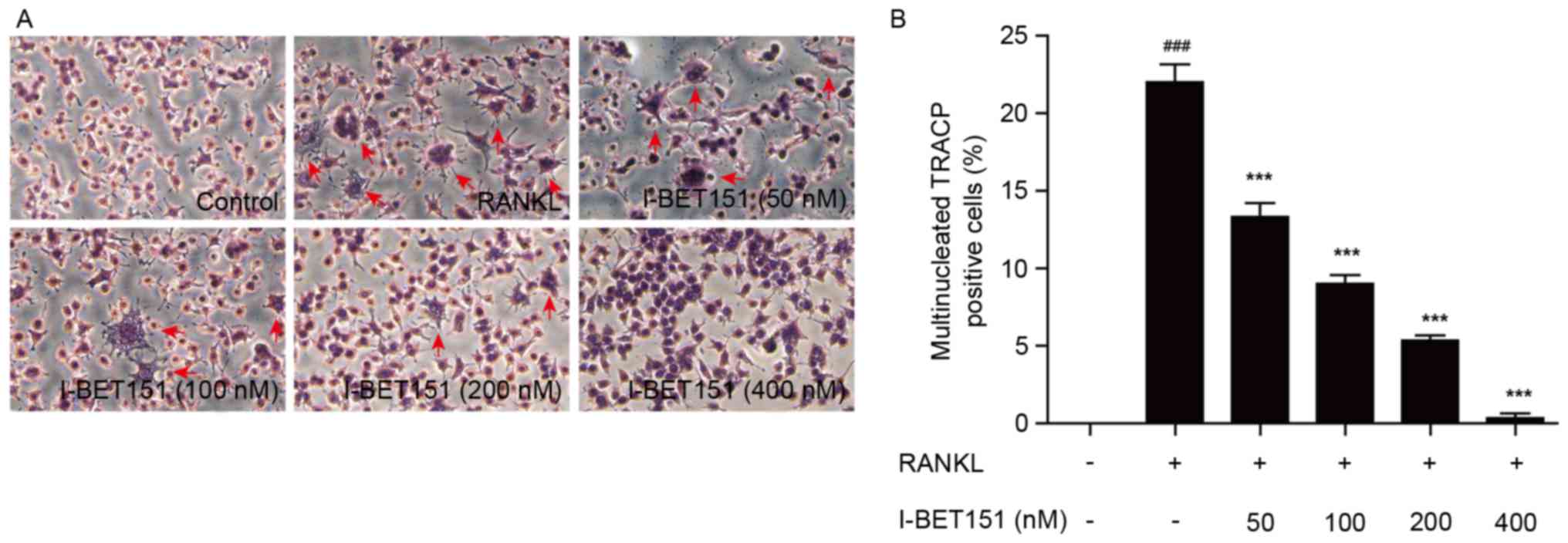
TRACP staining
TRACP staining was used to determine the effect of
I-BET151 on osteoclastogenesis and the percentage of TRACP
multinucleated positive cells was calculated. Results revealed that
in the RANKL group, multiple cell fusion increased the cell volumes
and cell synapse, and resulted in multinucleated cells, which was
inhibited by I-BET151 (Fig. 1). In
the control group, osteoclastogenesis proceeded normally; however,
this was enhanced in the RANKL group. When treated with I-BET151,
it was apparent that osteoclastogenesis induced by RANKL was
dose-dependently inhibited (Fig.
1). Fig. 1B demonstrates the
percentage TRACP positive cells. Compared with the control group,
the percentage of TRACP positive cells in groups 2–5 was increased
(Fig. 1). However, the percentage
of TRACP positive cells was almost completely abolished following
treatment with 400 nM I-BET151. The percentage of TRACP positive
cells in all groups treated with I-BET151 was significantly
decreased compared with the RANKL group (P<0.001; Fig. 1) and the inhibitory effect was dose
dependent.
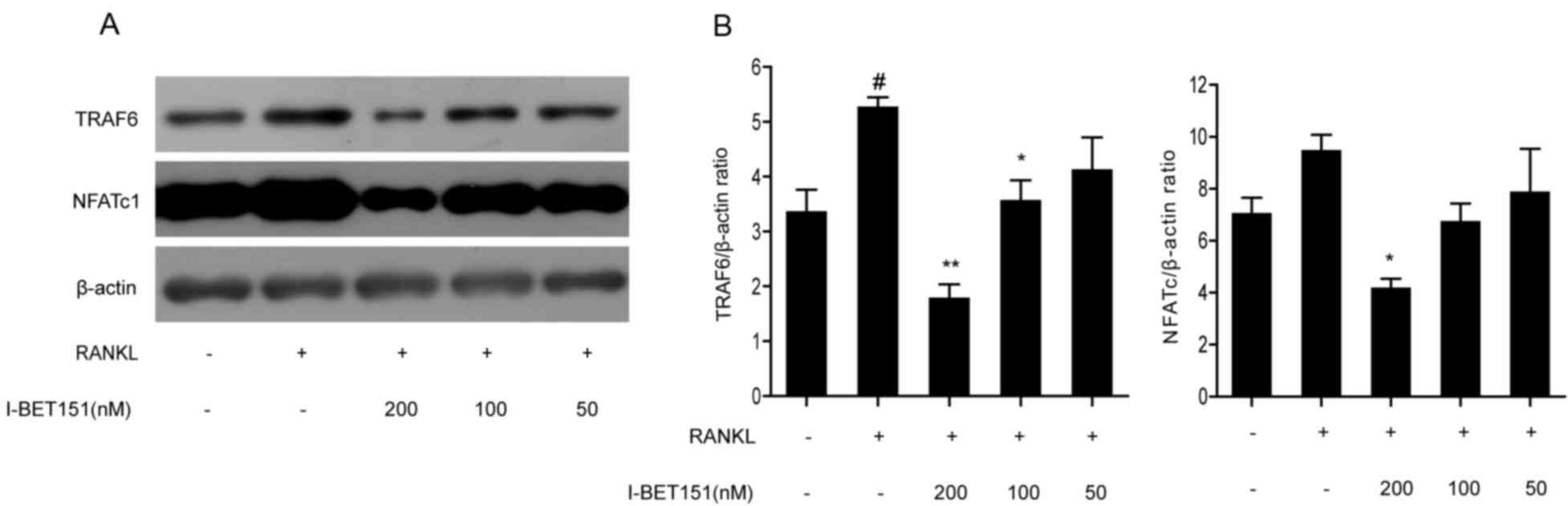
Effect of I-BET151 on the RANKL
signaling pathway
The expression levels of TRAF6 and NFATc l, which
are upstream and downstream effectors of the RANKL signaling
pathway, respectively, were measured by western blotting (Fig. 2). TRAF6 and NFATc l were
dose-dependently inhibited by I-BET151. Densitometric analysis of
the TRAF6/β-actin ratio in the I-BET151 100 and 200 nM groups were
significantly decreased compared with the RANKL group, whereas the
NFATc l/β-actin ratio was only significantly decreased compared
with the RANKL group following treatment with 200 nM I-BET151.
TRAF6/β-actin and NFATc l/β-actin in the 50 nM I-BET151 group
demonstrated no significant difference compared with the RANKL
group. The NFATc l/β-actin ratio and TRAF6/β-actin ratio in the
RANKL group was significant increased compared with the control
group. The inhibition effect of both TRAF6/β-actin and NFATc
l/β-actin by I-BET151 were dose-dependent.
Effect of I-BET151 on the NF-κB and
MAPK signaling pathways
The expression levels of p65 and IκB-α (involved in
the NF-κB signaling pathway) and ERK, JNK and p38 (involved in the
MAPK signaling pathway) were evaluated by western blotting. Results
revealed that nuclear expression of p65 was significantly inhibited
by I-BET151 (Fig. 3A and B) at
concentrations of 50, 100 and 200 nM compared with the RANKL group,
and the effect increased as the dose increased. In addition, under
concentrations of 100 and 200 nM I-BET151, IκB-α was significantly
inhibited compared with cells treated with RANKL alone (Fig. 3A and C). However, expression of
IκB-α after treatment with 50 nM I-BET151 did not demonstrate a
significant difference compared with the RANKL group (Fig. 3C). Phosphorylation of JNK and p38
was significantly inhibited by 50, 100 and 200 nM I-BET151 compared
with the RANKL group (Fig. 3D, E and
F). By contrast, only 50 nM I-BET151 markedly inhibited the
expression levels of p-ERK compared with the RANKL group; however,
no significant difference was observed following treatment with
I-BET151 (Fig. 3D and G).
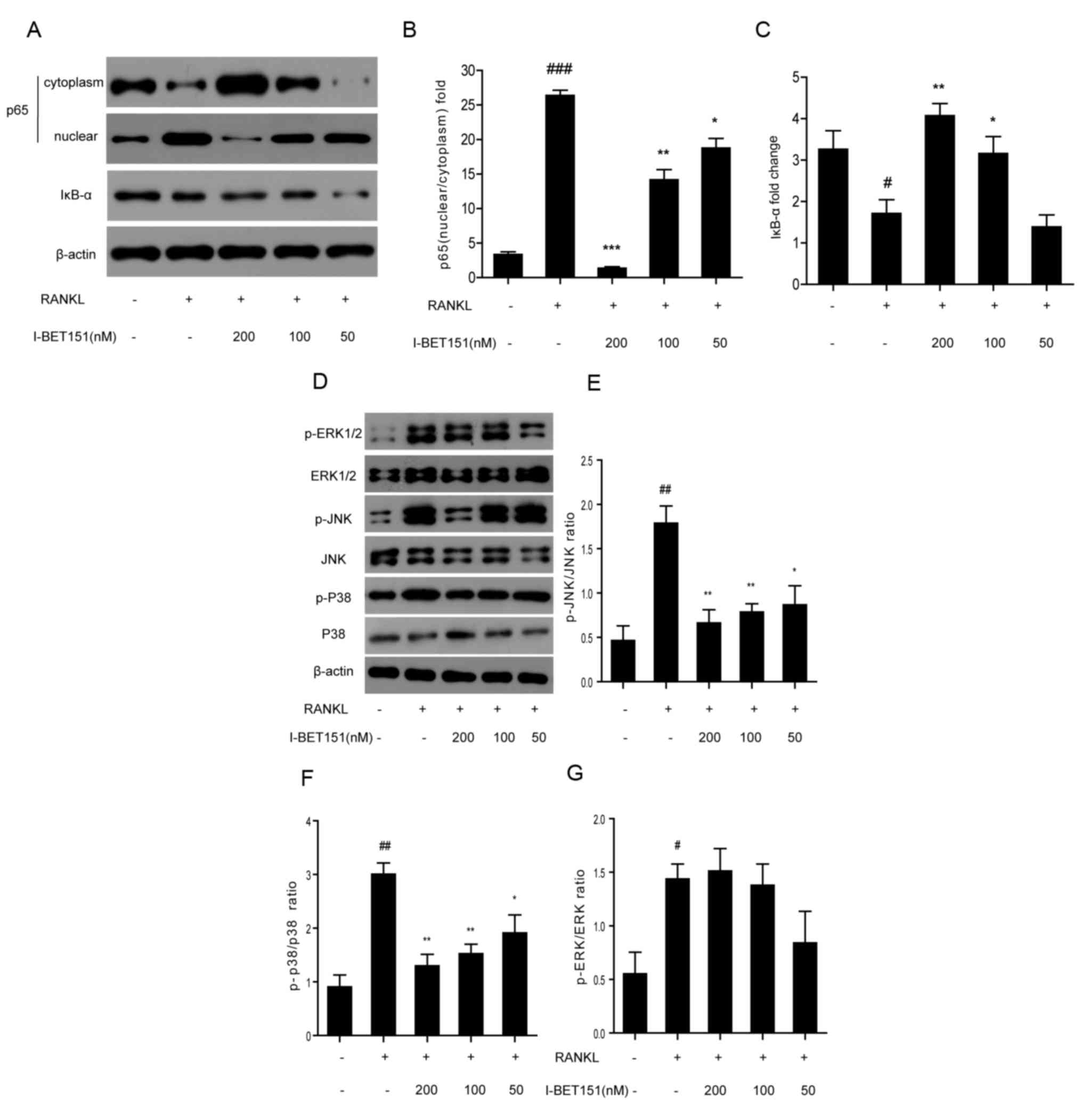 | Figure 3.(A) Western blotting of p65 and IκB-α.
RAW264.7 cells treated with or without RANKL. and with or without
I-BET151 at 200, 100 or 50 nM. Densitometric analysis of (B)
nuclear/cytoplasmic p65 and (C) IκB-α. (D) Western blotting of
total or phosphorylated ERK, JNK and p38. Densitometric analysis of
(E) p-JNK/JNK, (F) p-p38/p38 and (G) p-ERK/ERK.
#P<0.05, ##P<0.01,
###P<0.001 vs. control group; *P<0.05,
**P<0.01, ***P<0.01 vs. RANKL group. RANKL, receptor
activator of nuclear factor-κB ligand; IκB-α, nuclear factor of κ
light polypeptide gene enhancer in B-cells inhibitor-α; ERK,
extracellular signal-regulated kinase; JNK, Jun N-terminal kinase;
p-JNK, phosphorylated Jun N-terminal kinase; p-p38, phosphorylated
p38; p-ERK, phosphorylated extracellular signal-regulated
kinase. |
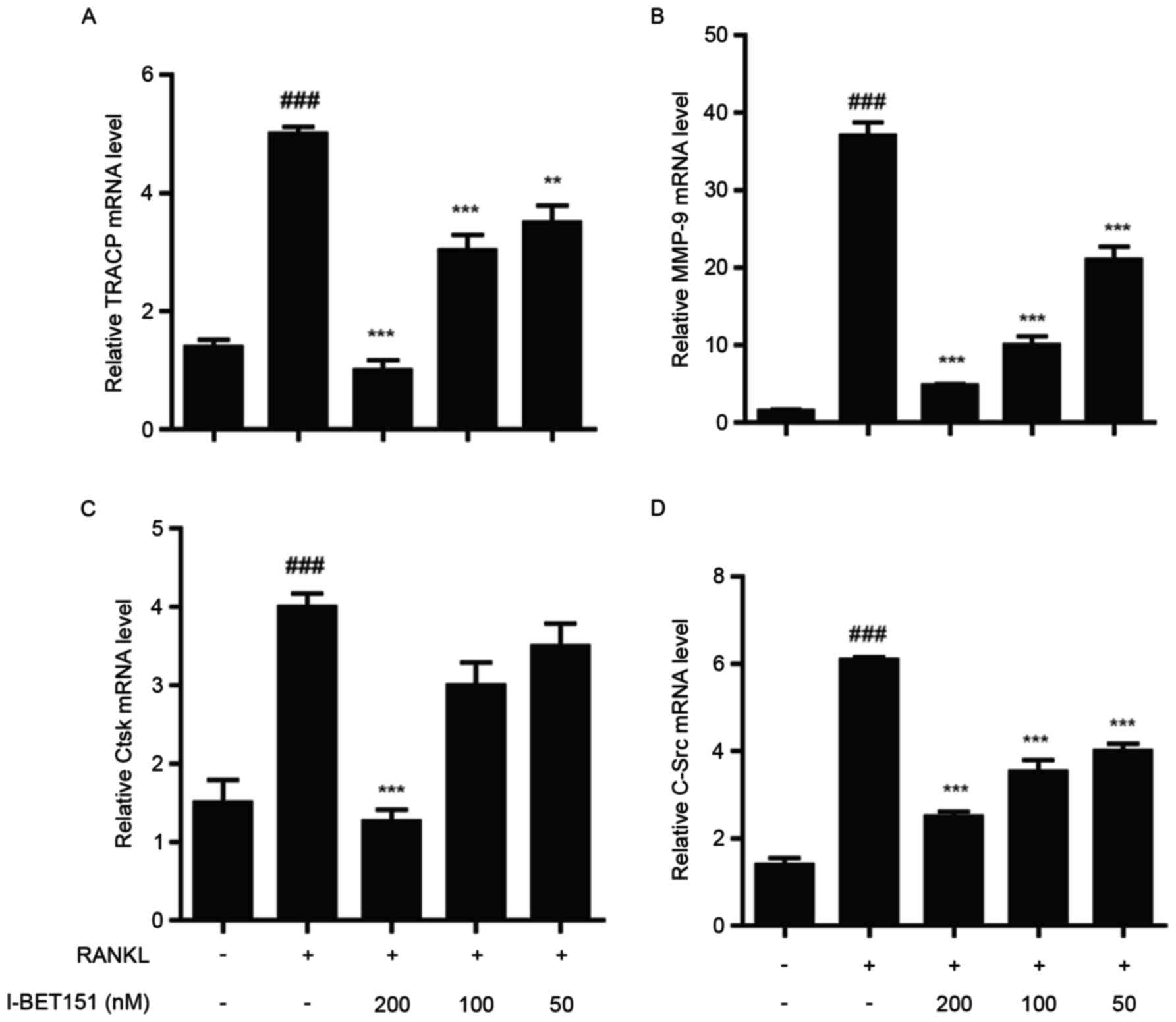
Effect of I-BET151 on the expression
of osteoclast-specific genes
The mRNA expression levels of the genes encoding
osteoclast-specific proteins TRACP, matrix metalloproteinase-9
(MMP9), cathepsin K (CtsK) and c-Src were measured using RT-qPCR.
The results revealed that all genes were dose-dependently inhibited
by I-BET151 compared with the RANKL group. With the exception of
CtsK, the inhibitory effects on TRACP, MMP9, and c-Src at all
concentrations of I-BET151 were statistically significant compared
with the RANKL group (Fig. 4). For
Ctsk, only 200 nM I-BET151 led to significant inhibition in
expression compared with the RANKL group.
Discussion
A delicate balance exists in the process of bone
remodeling between bone formation and resorption (30). Excessive bone resorption may lead
to over activation of osteoclasts and be a risk of various lytic
bone diseases, including rheumatoid arthritis, psoriatic arthritis
and osteoporosis (31). RANKL
serves a role in the process of osteoclastogenesis. In the RANKL
signaling pathway, a number of biomolecules are involved and have
their own role, including TRAF6, NF-κB and MAPKs. NF-κB is
essential for the initial induction or autoamplification of NFATc1,
which may activate other osteoclast-specific genes including TRACP,
CtsK, calcitonin receptor and MMP9. Although there are various
studies focusing on I-BET151, few report the effects of BET on the
inhibition of osteoclastogenesis. Park-Min et al (21) reported that I-BET151 suppresses
pathologic bone loss in TNF-induced inflammatory osteolysis and
suppresses osteoclastogenesis; however, the detailed effects of
I-BET151 on the RANKL signaling pathway, and its associated
proteins and genes involved in this process, remain to be
identified. In the present study, the effects of I-BET151 on
osteoclastogenesis were investigated by determining the expression
levels of TRAF6 and NFATcl that are involved in RANKL pathway, and
the effects of I-BET151 on osteoclast-specific gene expression.
According to the TRACP staining results, I-BET151
inhibited RANKL-induced osteoclastogenesis and the inhibition was
demonstrated to be dose dependent. Also, the percentage of TRACP
multinucleated-positive cells was reduced when treated with
I-BET151, and the effect was significant compared with the RANKL
group. Expression of TRAF6 and NFATc, which are upstream and
downstream effectors of the RANKL pathway were dose-dependently
inhibited by I-BET151, which was consistent with the research by
Park-Min et al (21). In
the present study, at concentrations of 100 and 200 nM I-BET151,
the inhibitory effect of TRAF6 was significant, as was the
inhibitory effect for NFATcl at 200 nM I-BET151. Studies have
demonstrated that there are several inhibitors of the RANKL
signaling pathway. Meng et al (25) reported that another BET inhibitor,
JQ1, may significantly suppress RANKL-induced osteoclast markers,
such as c-Fos, NFATcl, TRAP and CtsK, as well as toll-like receptor
(TLR)2/4, NF-κB phosphorylation and nuclear translocation. Li et
al (29) studied the effect of
sinomenine on TLR4/TRAF6 expression, and the results revealed that
sinomenine reduces the expression levels of RANK adaptor molecule
TRAF6 and downregulates RANKL-induced NF-κB activation.
Further effects of I-BET151 on expression of p65 and
IκB-α (involved in NF-κB pathway) and ERK, JNK and p38 (involved in
MAPK pathway) were also evaluated in the present study. Results
revealed that nuclear expression of p65 was significantly inhibited
by I-BET151 at all concentrations. Degradation of IκB-α and
phosphorylation of JNK and p38 was also significantly inhibited by
I-BET151, with the exception of IκB-α expression after treatment
with I-BET151 at 50 nM, which did not exhibit a significant
difference compared with the RANKL group. These results were also
consistent with the effects of JQ1 (25) and sinomenine (27,29).
However, I-BET151 did not exhibit a significant inhibitory effect
on ERK, and this result was consistent with the effect of
sinomenine (29).
Klein et al (24) reported that I-BET151 may suppress
cytokine and TLR ligand-induced secretion of MMP1, MMP3,
interleukin (IL)-6 and IL-8, and mRNA expression of ≥70% of the
genes induced by TNF-α and IL-1β. In the present study, the mRNA
expression levels of the genes encoding osteoclast-specific
proteins TRACP, MMP9, CtsK and c-Src were measured. The results
demonstrated that all genes were dose-dependently inhibited by
I-BET151. With the exception of CtsK, the inhibitory effects of
TRACP, MMP9 and c-Src were significant compared with the RANKL
group.
In conclusion, the influence of I-BET151 on
osteoclastogenesis in RAW264.7 cells was investigated in the
present study. The results revealed that the BET inhibitor,
I-BET151, significantly suppressed osteoclastogenesis of RAW264.7
cells, possibly via the RANKL signaling pathway. Based on the
present literature, to the best of the authors' knowledge, there is
no previous study focusing on the effect of I-BET151 on the
osteoclastogenesis of RAW264.7 cells and the possible associated
pathways, including RANKL, NF-κB and MAPK.
Acknowledgements
The work of the present study was supported by the
Science and Technology Planning Project of Health and Family
Planning Commission of Jiangxi Province in 2016 (grant nos.
20161066 and 20161067).
References
|
1
|
Weivoda MM, Ruan M, Hachfeld CM, Pederson
L, Howe A, Davey RA, Zajac JD, Kobayashi Y, Williams BO, Westendorf
JJ, et al: Wnt signaling inhibits osteoclast differentiation by
activating canonical and noncanonical cAMP/PKA pathways. J Bone
Miner Res. 31:65–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longo V, Brunetti O, D'Oronzo S, Dammacco
F and Silvestris F: Therapeutic approaches to myeloma bone disease:
An evolving story. Cancer Treat Rev. 38:787–797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delaisse JM, B A, Ali NN, et al: The
effects of both cysteine proteinase and collagenase inhibitors on
dentine resorption by isolated osteoclasts-Bone. Bone. 44:45–46.
2014.
|
|
4
|
Bara JJ, Richards RG, Alini M and Stoddart
MJ: Concise review: Bone marrow-derived mesenchymal stem cells
change phenotype following in vitro culture: Implications for basic
research and the clinic. Stem Cells. 32:1713–1723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klein-Nulend J, Bakker AD, Bacabac RG,
Vatsa A and Weinbaum S: Mechanosensation and transduction in
osteocytes. Bone. 54:182–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhai ZJ, Li HW, Liu GW, Qu XH, Tian B, Yan
W, Lin Z, Tang TT, Qin A and Dai KR: Andrographolide suppresses
RANKL-induced osteoclastogenesis in vitro and prevents inflammatory
bone loss in vivo. Br J Pharmacol. 171:663–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cicek M, Vrabel A, Sturchio C, Pederson L,
Hawse JR, Subramaniam M, Spelsberg TC and Oursler MJ: TGF-β
inducible early gene 1 regulates osteoclast differentiation and
survival by mediating the NFATc1, AKT and MEK/ERK signaling
pathways. Plos One. 6:e175222011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Amelio P, Isaia G and Isaia GC: The
osteoprotegerin/RANK/RANKL system: A bone key to vascular disease.
J Endocrinol Invest. 32(4 Suppl): S6–S9. 2014.
|
|
9
|
Jules J, Wang SQ, Shi ZQ, Liu JZ, Wei S
and Feng X: The IVVY motif and tumor necrosis factor
receptor-associated factor (TRAF) sites in the cytoplasmic domain
of the receptor activator of nuclear factor κB (RANK)
cooperate to induce osteoclastogenesis. J Biol Chem.
290:23738–23750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galibert L, Tometsko ME, Anderson DM,
Cosman D and Dougall WC: The involvement of multiple tumor necrosis
factor receptor (TNFR)-associated factors in the signaling
mechanisms of receptor activator of NF-kappaB, a member of the TNFR
superfamily. J Biol Chem. 273:34120–34127. 1999. View Article : Google Scholar
|
|
11
|
Wong BR, Josien R, Lee SY, Vologodskaia M,
Steinman RM and Choi Y: The TRAF family of signal transducers
mediates NF-kappaB activation by the trance receptor. J Biol Chem.
273:28355–28359. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Tamashiro S, Baritaki S, Penichet
M, Yu Y, Chen H, Berenson J and Bonavida B: TRAF6 activation in
multiple myeloma: A potential therapeutic target. Clin Lymphoma
Myeloma Leuk. 12:155–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi N, Kadono Y, Naito A, Matsumoto
K, Yamamoto T, Tanaka S and Inoue J: Segregation of TRAF6-mediated
signaling pathways clarifies its role in osteoclastogenesis. Embo
Journal. 20:1271–1280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan H, Mi B, Li Y, Wu W, Tan P, Fang Z,
Li J, Zhang Y and Li F: Decitabine represses osteoclastogenesis
through inhibition of RANK and NF-κB. Cell Signal.
27:969–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Vries TJ, Schoenmaker T, Aerts D,
Grevers LC, Souza PP, Nazmi K, van de Wiel M, Ylstra B, Lent PL,
Leenen PJ and Everts V: M-CSF priming of osteoclast precursors can
cause osteoclastogenesis-insensitivity, which can be prevented and
overcome on bone. J Cell Physiol. 230:210–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wikto-rjedrzejczak W, Bartocci A, Ferrante
AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW and Stanley ER: Total
absence of colony-stimulating factor 1 in the macrophage-deficient
osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 87:pp.
4828–4832. 1990; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HJ, Yoon HJ, Yoon KA, Gwon MR, Jin
Seong S, Suk K, Kim SY and Yoon YR: Lipocalin-2 inhibits osteoclast
formation by suppressing the proliferation and differentiation of
osteoclast lineage cells. Exp Cell Res. 334:301–309. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goettsch C, Babelova A, Trummer O, Erben
RG, Rauner M, Rammelt S, Weissmann N, Weinberger V, Benkhoff S,
Kampschulte M, et al: NADPH oxidase 4 limits bone mass by promoting
osteoclastogenesis. J Clin Invest. 123:4731–4738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ross FP and Teitelbaum SL: Alphavbeta3 and
macrophage colony-stimulating factor: Partners in osteoclast
biology. Immunol Rev. 208:88–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lamoureux F, Baud'huin M, Calleja LR,
Jacques C, Berreur M, Rédini F, Lecanda F, Bradner JE, Heymann D
and Ory B: Selective inhibition of BET bromodomain epigenetic
signalling interferes with the bone-associated tumour vicious
cycle. Nature Communications. 5:2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park-Min KH, Lim E, Lee MJ, Park SH,
Giannopoulou E, Yarilina A, Van der Meulen M, Zhao B, Smithers N,
Witherington J, et al: Inhibition of osteoclastogenesis and
inflammatory bone resorption by targeting BET proteins and
epigenetic regulation. Nat Commun. 5:54182014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaidos A, Caputo V, Gouvedenou K, Liu B,
Marigo I, Chaudhry MS, Rotolo A, Tough DF, Smithers NN, Bassil AK,
et al: Potent antimyeloma activity of the novel bromodomain
inhibitors I-BET151 and I-BET762. Blood. 123:697–705. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallagher SJ, Mijatov B, Gunatilake D,
Tiffen JC, Gowrishankar K, Jin L, Pupo GM, Cullinane C, Prinjha RK,
Smithers N, et al: The epigenetic regulator I-BET151 induces
BIM-dependent apoptosis and cell cycle arrest of human melanoma
cells. J Invest Dermatol. 134:2795–2805. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klein K, Kabala PA, Grabiec AM, Gay RE,
Kolling C, Lin LL, Gay S, Tak PP, Prinjha RK, Ospelt C and
Reedquist KA: The bromodomain protein inhibitor I-BET151 suppresses
expression of inflammatory genes and matrix degrading enzymes in
rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis.
75:422–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng S, Zhang L, Tang Y, Tu Q, Zheng L, Yu
L, Murray D, Cheng J, Kim SH, Zhou X and Chen J: BET inhibitor JQ1
blocks inflammation and bone destruction. J Dent Res. 93:657–662.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, Li Z, Yang Z, Zheng C, Jing J, Chen
Y, Ye X, Lian X, Qiu W, Yang F, et al: Caffeic acid
3,4-dihydroxy-phenethyl ester suppresses receptor activator of
NF-κB ligand-induced osteoclastogenesis and prevents
ovariectomy-induced bone loss through inhibition of
mitogen-activated protein kinase/activator protein 1 and
Ca2+-nuclear factfactor of activated T-cells cytoplasmic
1 signaling pathways. J Bone Miner Res. 27:1298–1308. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He LG, Duan H, Li XL, Wang S, Zhang Y, Lei
L, Xu J, Liu S and Li X: Sinomenine down-regulates TLR4/TRAF6
expression and attenuates lipopolysaccharide-induced
osteoclastogenesis and osteolysis. Eur J Pharmacol. 779:66–79.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, He L, Hu Y, Duan H, Li X, Tan S, Zou
M, Gu C, Zeng X, Yu L, et al: Sinomenine suppresses osteoclast
formation and mycobacterium tuberculosis H37Ra-induced bone loss by
modulating RANKL signaling pathways. Plos One. 8:2013.
|
|
30
|
Kular J, Tickner J, Chim SM and Xu JK: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deal C: Bone loss in rheumatoid arthritis:
Systemic, periarticular and focal. Curr Rheumatol Rep. 14:231–237.
2012. View Article : Google Scholar : PubMed/NCBI
|