Introduction
Open globe injury (OGI) is a severe eye disease that
frequently causes unilateral visual loss. Ocular trauma is a public
health problem in developing countries (1,2). A
previous study indicated that the annual prevalence of ocular
trauma was 4.9 per 100,000 in the Western Sicily Mediterranean
area, which investigated a 5 year period from January 2001 to
December 2005 (3). In addition,
the incidence of OGI is increased in men compared with women
(4). OGI primarily occurs in the
15–44 and 0–14 age groups (5). A
total of ~19 million cases of unilateral blindness or decreased
vision are caused by ocular trauma each year (6). Clinically, OGI is frequently
associated with corneal injury and iris prolapse (7), retinal detachment (8), glaucoma (9) and endophthalmitis (10). At present, surgery is the principal
means of treatment for OGI (11,12).
Computed tomography (CT) and B-scan ultrasonography
are important clinical tests for the diagnosis of OGI. Although CT
may provide information for the diagnosis of OGI (13), it is insufficient for making the
decision of immediate treatment (14). B-scan ultrasonography is able to
locate retinal detachment points, retinal tears and vitreous
traction, and thus may be beneficial for further medical treatment
(15). The aforementioned methods
focus solely on ocular trauma in OGI. However, other parts of the
visual system, including the connecting pathways and the visual
cortex, are frequently overlooked. Little is known about the
underlying mechanisms of neural alterations in the OGI.
Resting-state functional magnetic resonance imaging
(fMRI) is able to evaluate intrinsic brain activity in subjects at
rest (16). It has been widely
used in visual studies associated with brain functional
alterations. A previous study reported decreased functional
connectivity within the occipital visual cortices and a correlation
with other vision-associated brain regions in patients with early
blindness (17). An additional
study demonstrated that patients with early blindness exhibited
stronger connectivity in the primary somatosensory area (S1) and
primary visual cortex (V1) compared with patients with late
blindness (18). In addition, a
previous report demonstrated that patients with early blindness
exhibited markedly decreased gray matter volumes in the optic tract
and visual cortex (19). Although
there have been numerous studies into visual and brain function
alterations in patients with blindness, less is known about the
spontaneous brain activity alterations in patients with acute
unilateral vision loss caused by OGI.
Voxel-wise degree centrality (DC) is a measurement
that illustrates the network architecture of functional
connectivity within the human brain connectome at the voxel level
(20). Distinct from the amplitude
of low-frequency fluctuation (ALFF) (21) and regional homogeneity (ReHo)
(22) techniques, it does not
require the definition of regions of interest. The DC method is
able to provide insights into the functional connectivity of the
entire brain. The DC method has been successfully used to evaluate
the pathological mechanisms underlying a number of diseases,
including autism (23), obesity
(24) and Parkinson's disease
(25). A previous study
investigated strabismus and optic neuritis through whole-brain
voxel-based analysis of diffusion tensor imaging (26,27).
In addition, ALFF and ReHo were previously used to analyzed
patients with acute OGI (28,29).
The present study evaluated functional network brain activity
alterations in patients with acute unilateral vision loss caused by
OGI, and associations with clinical features.
Subjects and methods
Subjects
A total of 18 patients with acute unilateral OGI (16
male and 2 female; 8 right eye injury and 8 left eye injury; age
range, 18–65 years) were recruited from the ophthalmology
departments of the First Affiliated Hospital of Nanchang University
and Xiangya Hospital between August 2015 and January 2016. Acute
unilateral OGI was diagnosed with the following critera: i) Severe
ocular trauma; ii) acute vision loss; iii) corneal and scleral
rupture; iv) decreased intraocular pressure; v) incomplete eyeball
wall visualized using orbital CT or orbital MRI; and vi)
contralateral eye best-corrected visual acuity (VA) ≥1.0.
Exclusion conditions were as follows: i) Patients
with other eye diseases (including cataracts, glaucoma, pterygium
and strabismus, ocular infection and inflammation, hereditary optic
neuropathy, ischemic diseases, demyelinating diseases, intraocular
placeholder lesions, toxic lesions, vascular lesions and ischemic
optic neuropathy); ii) central nervous system diseases and systemic
disorders; iii) diabetes and cardiovascular diseases; and iv)
addictions (including drugs or alcohol).
A total of 18 healthy controls (HCs; 16 males and 2
females) with matched age, sex and education were recruited for the
present study. All HCs met the following conditions: i) Normal
brain parenchyma on head MRI scan; ii) no ocular or central nervous
system diseases; iii) naked eye or the best-corrected VA >1.0;
and iv) no MRI scanning contraindications (for example, implanted
metal devices).
The research methods of the present study followed
the Declaration of Helsinki and conformed to the principles of
medical ethics. The present study was approved by the Ethics
Committees of the First Affiliated Hospital of Nanchang University
and Xiangya Hospital. All subjects participated voluntarily and
were informed of the purposes, methods and procedures, and all
subjects signed an informed consent form.
MRI data acquisition
MRI scanning was performed on a 3-Tesla MR scanner
(Trio; Siemens AG, Munich, Germany). High-resolution T1-weighted
images were acquired as described previously (30). A total of 240 functional images
covering the whole brain in one subject were obtained.
fMRI data preprocessing
All functional data were prefiltered using MRIcro
(www.MRIcro.com) and preprocessed using SPM8
(www.fil.ion.ucl.ac.uk/spm), DPARSFA
(rfmri.org/DPARSF) and the Resting-state Data
Analysis Toolkit (www.restfmri.net), as described previously (30).
Degree centrality
The voxel-wise functional network was generated as
described previously (30). Based
on the voxel-wise functional network, DC was calculated as the
counting of significant suprathresholded correlations (or the
degree of the binarized adjacency matrix) for each subject. The
voxel-wise DC map for each individual was converted into a z-score
map, as described previously (30).
Statistical analysis
For demographic and clinical measurements, the data
were presented as the mean ± standard deviation. The differences in
clinical features between the patients and HCs were calculated
using independent two-sample t-tests.
Independent t-tests with generalized linear model
analysis was performed using the SPM8 toolkit to investigate the
group differences in DC values between patients with OGI and HCs.
P<0.05 was considered to indicate a statistically significant
difference, with Gaussian random field theory correction. Pearson
correlation analysis was used to calculate the association between
mean DC values and clinical features. Statistical tests were
performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Demographics and visual
measurements
There were no significant differences in weight
(P=0.423), age (P=0.990), best-corrected VA-right (P<0.001) and
best-corrected VA-left (P<0.001) between the two groups
(Table I).
 | Table I.Demographic information and clinical
features of subjects in the OGI and HC groups. |
Table I.
Demographic information and clinical
features of subjects in the OGI and HC groups.
| Feature | OGI | HC | t | P-value |
|---|
| Sex,
male/female | 16/2 | 16/2 | N/A | N/A |
| Agea, years | 44.17±13.94 | 44.11±12.78 | 0.012 | 0.990 |
| Weighta, kg | 55.83±6.04 | 54.39±4.42 | 0.813 | 0.423 |
| Handedness | 20 R | 20 R | N/A | >0.999 |
| Duration of OGI,
ha | 24.83±31.72 | N/A | N/A | N/A |
| Best-corrected
VA-righta | 0.56±0.57 | 1.16±0.18 | −4.262 | <0.001 |
| Best-corrected
VA-lefta | 0.64±0.53 | 1.17±0.18 | −4.023 | <0.001 |
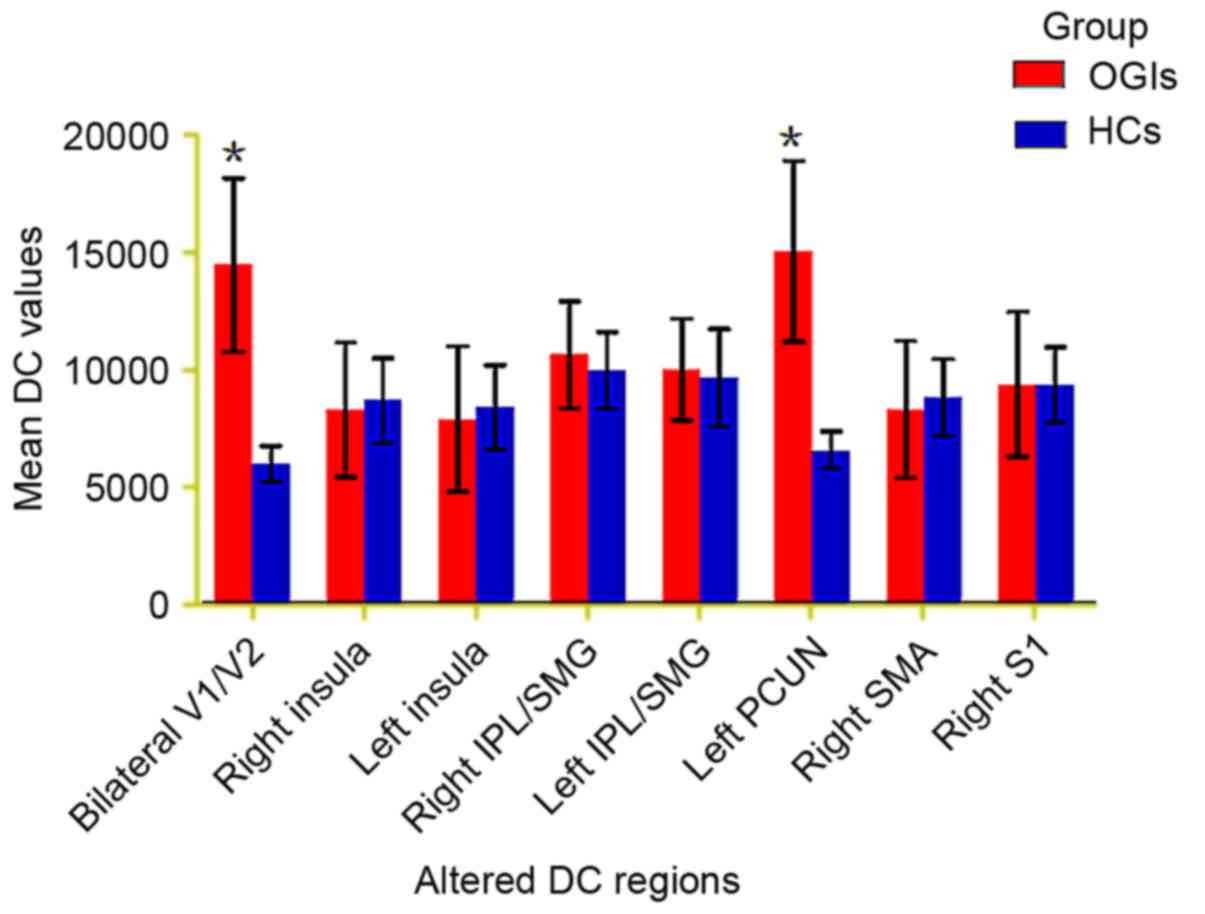
DC differences
Compared with HCs, DC values of patients with acute
OGI were increased in the bilateral visual cortex (V1/V2) and left
precuneus (PCUN) regions, although they were decreased in the right
insula, left insula, right inferior parietal lobule
(IPL)/supramarginal gyrus (SMG), left IPL/SMG, right supplementary
motor area (SMA) and S1 (Fig. 1;
Table II; z>2.3; cluster-wise
P<0.05 corrected). The mean altered DC values between patients
with OGIs and HCs are presented in Fig. 2.
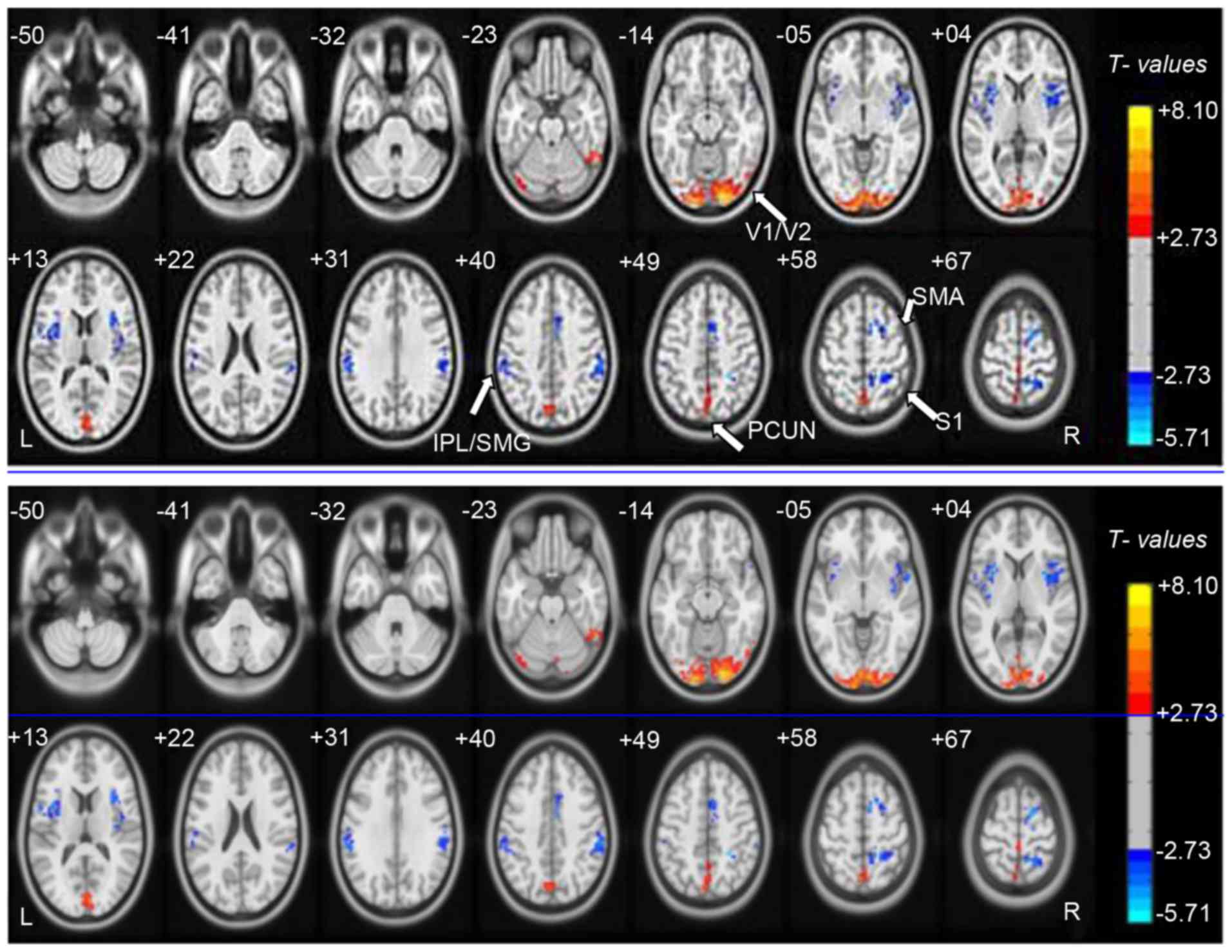 | Figure 1.Voxel-wise comparison of DC in the
OGI and HC groups. Significant differences in DC were observed in
the bilateral V1/V2, left PCUN, right insula, left insula, right
IPL/SMG, left IPL/SMG, right SMA and S1. Red, increased DC values;
blue, decreased DC values. DC, Degree centrality; OGI, open globe
injury; HC, healthy control; V1/V2, primary visual cortex; PCUN,
precuneus; IPL, inferior parietal lobule; SMG, supramarginal gyrus;
SMA, supplementary motor area; S1, postcentral gyrus. |
 | Table II.Brain regions with significant
differences in DC between the OGI and HC groups. |
Table II.
Brain regions with significant
differences in DC between the OGI and HC groups.
|
| MNI
coordinates |
|
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Brain area | x | y | z | Voxel no. | BA | L/R/B | Peak T values |
|---|
| OGI<HC |
|
|
|
|
|
|
|
| Insula | 33 | 6 | 6 | 241 | 13 | R | −5.503 |
| Insula | −30 | 12 | −3 | 167 | 13 | L | −4.948 |
| IPL/SMG | 60 | −30 | 36 | 147 | 40 | R | −4.741 |
| IPL/SMG | −60 | −33 | 27 | 119 | 40 | L | −4.382 |
| SMA | 15 | −6 | 66 | 176 | 6 | R | −4.970 |
| S1 | 21 | −51 | 63 | 128 | 5 | R | −5.711 |
| OGI>HC |
|
|
|
|
|
|
|
| V1/V2 | 27 | −99 | −12 | 879 | 17,18 | B | 8.076 |
| PCUN | −3 | 0 | 75 | 278 | 7 | L | 5.935 |
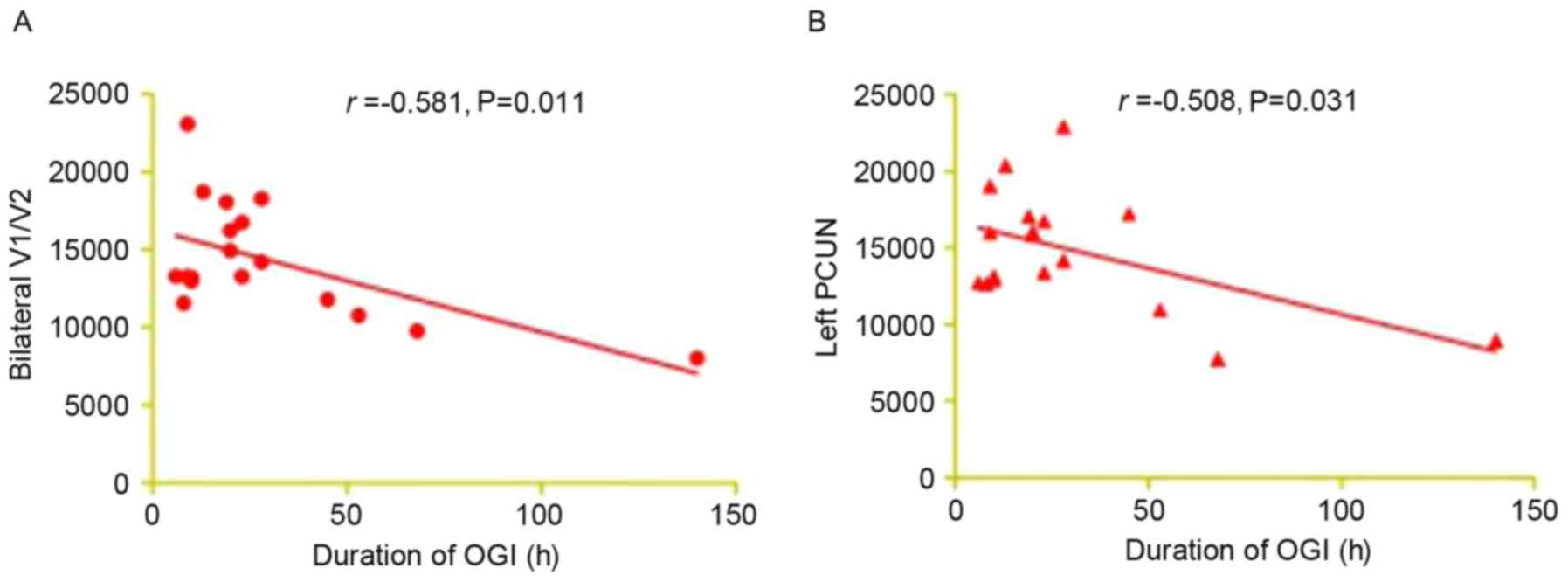
Correlation analysis of DC values and
clinical outcomes in the OGI group
In the acute OGI group, it was observed that the
duration of OGI was negatively correlated with the DC signal value
of the bilateral V1/V2 (r=-0.581; P=0.011; Fig. 3A) and the left PCUN (r=-0.508;
P=0.031; Fig. 3B).
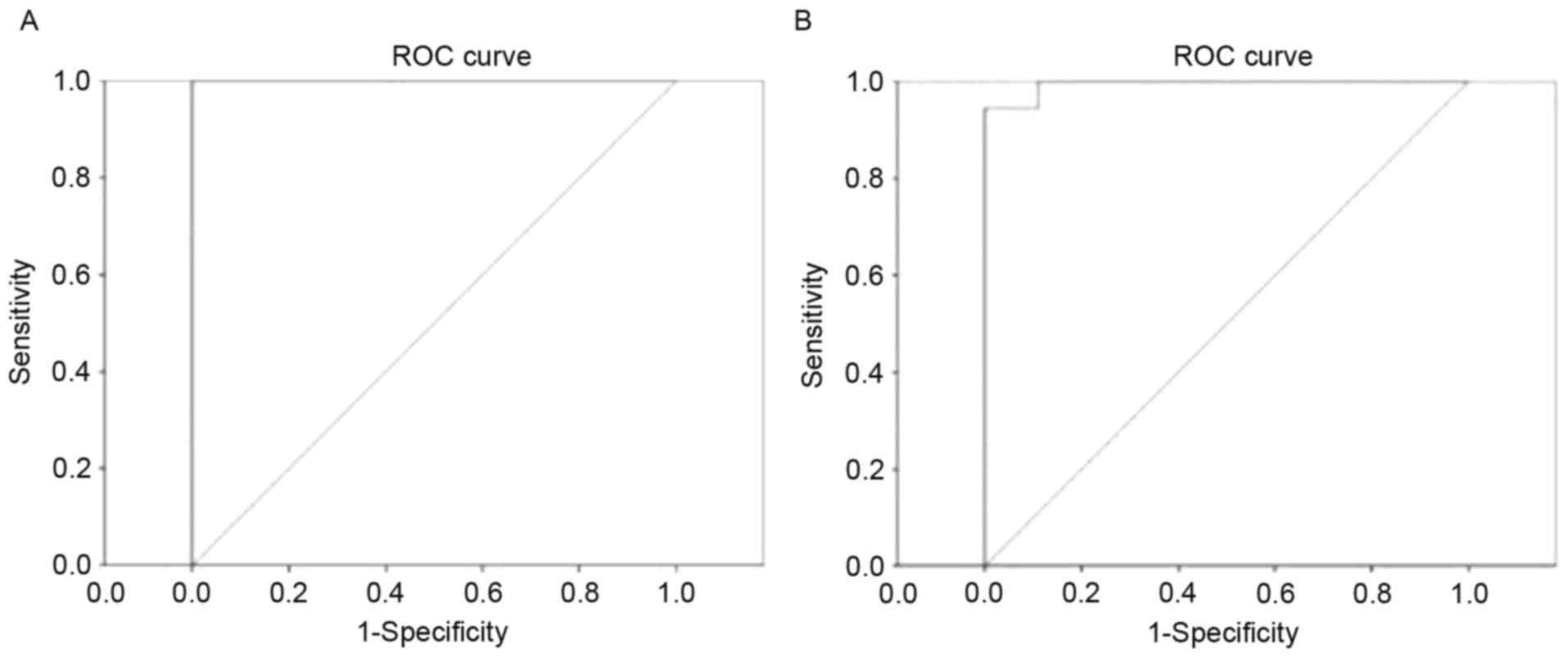
Receiver operating characteristic
(ROC) curve
It was proposed that DC differences between patients
with OGI and HCs may be useful diagnostic markers. The mean DC
values of the different brain regions were used for ROC curves
analysis. The area under the curve values were: Bilateral V1/V2,
1.000 and left PCUN, 0.994, respectively (Fig. 4).
Discussion
To the best of our knowledge, the present study was
the first to evaluate the effects of acute OGI on functional
networks and brain-activity changes using the DC technique.
Compared with HCs, patients with acute unilateral OGI exhibited
increased DC values in the bilateral V1/V2 and left PCUN, and
decreased DC values in the right insula, left insula, right
IPL/SMG, left IPL/SMG, right SMA and S1. It was observed that the
duration of OGI was negatively correlated with the DC signal value
of the bilateral V1/V2 (r=-0.581; P=0.011) and left PCUN (r=-0.508;
P=0.031).
The primary visual cortex, also termed V1 (striate
cortex or Brodmann area 17) (31)
is located in the occipital lobe involved in the processing of
visual information. The extrastriate areas are located next to the
primary visual cortex, including functional areas V2, V3, V4 and V5
(32). The extrastriate areas
receive visual information from the primary visual cortex and
transmit the information to other brain areas (33). A previous study reported that
visual acuity exerts a marked effect on the V1 blood oxygen
level-dependent (BOLD) response (34). An additional study demonstrated
that central vision loss may lead to cortical atrophy of V1
(35). Increased regional
homogeneity in the occipital areas was reported in patients with
early blindness (36). Consistent
these previous findings, it was observed in the present study that
patients with acute unilateral OGI exhibited significantly
increased DC values in the bilateral V1/V2, which may reflect the
compensation of the visual cortex in acute unilateral vision loss
associated with OGI. It was additionally demonstrated that the
duration of OGI exhibited a negative correlation with the DC signal
value of the bilateral V1/V2 (r=-0.581; P=0.011). This suggested
that a stronger visual compensatory function may occur in V1/V2
during the early phase of acute OGI.
The PCUN, located forward of the occipital lobe,
contributes to visuospatial information processing (37) and memory (38). A previous study reported that the
PCUN is activated during visuospatial activities (39). In the present study, it was
observed that patients with acute unilateral OGI had increased DC
values in the left PCUN, which may reflect the compensation of the
PCUN in acute unilateral visual loss associated with OGI.
Additionally, it was observed that the duration of OGI was
negatively correlated with the DC signal value of the left PCUN.
Therefore, a stronger compensatory function may occur in the PCUN
during the early phase of acute OGI.
The insula, located in the lateral sulcus (40), is divided into two part. The insula
serves roles in emotion and cognition (41–43).
A previous study reported that increased activity of the insula is
associated with emotional regulation (44). Dysfunction of the insula has been
observed in negative emotional experiences (45) and anxiety-prone subjects (46). In the present study, it was
demonstrated that DC values in the right insula and left insula
were decreased in patients with OGI, which may reflect impaired
emotional processing caused by acute unilateral OGI.
The SMA, located in front of the primary motor
cortex, is involved in the control of movement (47,48).
A previous study demonstrated that the SMA served an important role
in the orchestration of movements (49). An additional study demonstrated the
role of injury to the upper motor neuron in supplementary motor
area syndrome (50). In the
present study, it was observed that patients with acute unilateral
OGI exhibited increased DC values in the right SMA, indicating that
OGI may be associated with the dysfunction of movement.
In conclusion, the results of the present study
demonstrated that patients with OGI had dysfunctional activity in
specific regions of the brain, which may be associated with
compensation for vision loss in acute OGI. The present findings may
provide a basis for identifying the downstream impact of OGI on
brain network organization. However, the sample size of the present
study was relatively small. In addition, the clinical
characteristics were not strictly defined. Right and left
eye-injured patients were included in the present study, which may
have affected the DC results. In future studies, differences will
be distinguished and brain function activity alterations measured
more accurately.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170,823, 81460092
and 81400372), the Science and Technology Program Project of Hunan
Province Science and Technology Department (grant nos. 2015JC3011
and 2015JC3118), the Jiangxi Province Voyage Project (grant no.
2014022), the Natural Science Key Project of Jiangxi Province
(grant no. 20161ACB21017), the Youth Science Foundation of Jiangxi
Province (grant no. 20151BAB215016), and the Technology and Science
Foundation of Jiangxi Province (grant no. 20151BBG70223).
References
|
1
|
Chaikitmongkol V, Leeungurasatien T and
Sengupta S: Work-related eye injuries: Important occupational
health problem in northern Thailand. Asia Pac J Ophthalmol (Phila).
4:155–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vasu U, Vasnaik A, Battu RR, Kurian M and
George S: Occupational open globe injuries. Indian J Ophthalmol.
49:43–47. 2001.PubMed/NCBI
|
|
3
|
Cillino S, Casuccio A, Di Pace F,
Pillitteri F and Cillino G: A five-year retrospective study of the
epidemiological characteristics and visual outcomes of patients
hospitalized for ocular trauma in a Mediterranean area. BMC
Ophthalmol. 8:82008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Liu Z, Liu Y, Zhao L, Xu S, Su G
and Zhao J: Determination of visual prognosis in children with open
globe injuries. Eye (Lond). 28:852–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao H, Li L and Zhang M: Epidemiology of
patients hospitalized for ocular trauma in the Chaoshan region of
China, 2001–2010. PLoS One. 7:e483772012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Négrel AD and Thylefors B: The global
impact of eye injuries. Ophthalmic Epidemiol. 5:143–169. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nawani N, Vazirani J, Ojha H and Sangwan
VS: Conjunctival pedicle flap in management of open globe injury
with corneal tissue loss. BMJ Case Rep. 2016:pii:
bcr20152137032016. View Article : Google Scholar
|
|
8
|
Stryjewski TP, Andreoli CM and Eliott D:
Retinal detachment after open globe injury. Ophthalmology.
121:327–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osman EA: Glaucoma after open globe
injury. Saudi J Ophthalmol. 29:222–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Zhang MN, Jiang CH, Yao Y and
Zhang K: Endophthalmitis following open globe injury. Br J
Ophthalmol. 94:111–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng K, Hu YT and Ma Z: Prognostic
indicators for no light perception after open-globe injury: Eye
injury vitrectomy study. Am J Ophthalmol. 152:654–662.e2. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heidari E and Taheri N: Surgical treatment
of severely traumatized eyes with no light perception. Retina.
30:294–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arey ML, Mootha VV, Whittemore AR, Chason
DP and Blomquist PH: Computed tomography in the diagnosis of occult
open-globe injuries. Ophthalmology. 114:1448–1452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allon G, Beiran I, Seider N and Blumenthal
EZ: The role of computed tomography in the immediate workup of open
globe injury. Eur J Ophthalmol. 26:503–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andreoli MT, Yiu G, Hart L and Andreoli
CM: B-scan ultrasonography following open globe repair. Eye (Lond).
28:381–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Biswal BB: Resting state fMRI: A personal
history. Neuroimage. 62:938–944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Yu C, Liang M, Li J, Tian L, Zhou
Y, Qin W, Li K and Jiang T: Whole brain functional connectivity in
the early blind. Brain. 130:2085–2096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujii T, Tanabe HC, Kochiyama T and Sadato
N: An investigation of cross-modal plasticity of effective
connectivity in the blind by dynamic causal modeling of functional
MRI data. Neurosci Res. 65:175–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan WJ, Wu G, Li CX, Lin F, Sun J and Lei
H: Progressive atrophy in the optic pathway and visual cortex of
early blind Chinese adults: A voxel-based morphometry magnetic
resonance imaging study. Neuroimage. 37:212–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuo XN, Ehmke R, Mennes M, Imperati D,
Castellanos FX, Sporns O and Milham MP: Network centrality in the
human functional connectome. Cereb Cortex. 22:1862–1875. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang X, Cai FQ, Hu PH, Zhong YL, Zhang Y,
Wei R, Pei CG, Zhou FQ and Shao Y: Disturbed spontaneous
brain-activity pattern in patients with optic neuritis using
amplitude of low-frequency fluctuation: A functional magnetic
resonance imaging study. Neuropsychiatr Dis Treat. 11:3075–3083.
2015.PubMed/NCBI
|
|
22
|
Shao Y, Cai FQ, Zhong YL, Huang X, Zhang
Y, Hu PH, Pei CG, Zhou FQ and Zeng XJ: Altered intrinsic regional
spontaneous brain activity in patients with optic neuritis: A
resting-state functional magnetic resonance imaging study.
Neuropsychiatr Dis Treat. 11:3065–3073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Martino A, Zuo XN, Kelly C, Grzadzinski
R, Mennes M, Schvarcz A, Rodman J, Lord C, Castellanos FX and
Milham MP: Shared and distinct intrinsic functional network
centrality in autism and attention-deficit/hyperactivity disorder.
Biol Psychiatry. 74:623–632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garcia-Garcia I, Jurado MÁ, Garolera M,
Marqués-Iturria I, Horstmann A, Segura B, Pueyo R, Sender-Palacios
MJ, Vernet-Vernet M, Villringer A, et al: Functional network
centrality in obesity: A resting-state and task fMRI study.
Psychiatry Res. 233:331–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lou Y, Huang P, Li D, Cen Z, Wang B, Gao
J, Xuan M, Yu H, Zhang M and Luo W: Altered brain network
centrality in depressed Parkinson's disease patients. Mov Disord.
30:1777–1784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai H, Yin D, Hu C, Morelli JN, Hu S, Yan
X and Xu D: Whole-brain voxel-based analysis of diffusion tensor
MRI parameters in patients with primary open angle glaucoma and
correlation with clinical glaucoma stage. Neuroradiology.
55:233–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Li HJ, Zhang Y, Peng DC, Hu PH,
Zhong YL, Zhou FQ and Shao Y: Microstructural changes of the whole
brain in patients with comitant strabismus: Evidence from a
diffusion tensor imaging study. Neuropsychiatr Dis Treat.
12:2007–2014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang X, Li HJ, Ye L, Zhang Y, Wei R,
Zhong YL, Peng DC and Shao Y: Altered regional homogeneity in
patients with unilateral acute open-globe injury: A resting-state
functional MRI study. Neuropsychiatr Dis Treat. 12:1901–1906. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan G, Huang X, Ye L, Wu AH, He LX, Zhong
YL, Jiang N, Zhou FQ and Shao Y: Altered spontaneous brain activity
patterns in patients with unilateral acute open globe injury using
amplitude of low-frequency fluctuation: A functional magnetic
resonance imaging study. Neuropsychiatr Dis Treat. 12:2015–2020.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai F, Gao L, Gong H, Jiang F, Pei C,
Zhang X, Zeng X and Huang R: Network centrality of resting-state
fMRI in primary angle-closure glaucoma before and after surgery.
PLoS One. 10:e01413892015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tootell RB, Hadjikhani NK, Vanduffel W,
Liu AK, Mendola JD, Sereno MI and Dale AM: Functional analysis of
primary visual cortex (V1) in humans. Proc Natl Acad Sci USA.
95:pp. 811–817. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Orban GA: Higher order visual processing
in macaque extrastriate cortex. Physiol Rev. 88:59–89. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laramée ME, Bronchti G and Boire D:
Primary visual cortex projections to extrastriate cortices in
enucleated and anophthalmic mice. Brain Struct Funct.
219:2051–2070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cunningham SI, Weiland JD, Bao P,
Lopez-Jaime GR and Tjan BS: Correlation of vision loss with
tactile-evoked V1 responses in retinitis pigmentosa. Vision Res.
111:197–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burge WK, Griffis C, Nenert R, Elkhetali
A, Decarlo K, Ver Hoef W, Ross A and Visscher M: Cortical thickness
in human V1 associated with central vision loss. Sci Rep.
6:232682016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu C, Liu Y, Li W, Wang D, Jiang T, Zhang
Y and Yu C: Increased regional homogeneity of blood oxygen
level-dependent signals in occipital cortex of early blind
individuals. Neuroreport. 22:190–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mahayana IT, Tcheang L, Chen CY, Juan CH
and Muggleton NG: The precuneus and visuospatial attention in near
and far space: A transcranial magnetic stimulation study. Brain
Stimul. 7:673–679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bonni S, Veniero D, Mastropasqua C, Ponzo
V, Caltagirone C, Bozzali M and Koch G: TMS evidence for a
selective role of the precuneus in source memory retrieval. Behav
Brain Res. 282:70–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oshio R, Tanaka S, Sadato N, Sokabe M,
Hanakawa T and Honda M: Differential effect of double-pulse TMS
applied to dorsal premotor cortex and precuneus during internal
operation of visuospatial information. NeuroImage. 49:1108–1115.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Naidich TP, Kang E, Fatterpekar GM, Delman
BN, Gultekin SH, Wolfe D, Ortiz O, Yousry I, Weismann M and Yousry
TA: The insula: Anatomic study and MR imaging display at 1.5 T.
AJNR Am J Neuroradiol. 25:222–232. 2004.PubMed/NCBI
|
|
41
|
Gu X, Hof PR, Friston KJ and Fan J:
Anterior insular cortex and emotional awareness. J Comp Neurol.
521:3371–3388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seth AK, Suzuki K and Critchley HD: An
interoceptive predictive coding model of conscious presence. Front
Psychol. 2:3952012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gasquoine PG: Contributions of the insula
to cognition and emotion. Neuropsychol Rev. 24:77–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grecucci A, Giorgetta C, Bonini N and
Sanfey AG: Reappraising social emotions: The role of inferior
frontal gyrus, temporo-parietal junction and insula in
interpersonal emotion regulation. Front Hum Neurosci. 7:5232013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Steward T, Picó-Pérez M, Mata F,
Martinez-Zalacain I, Cano M, Contreras-Rodriguez O,
Fernandez-Aranda F, Yucel M, Soriano-Mas C and Verdejo-Garcia A:
Emotion regulation and excess weight: Impaired affective processing
characterized by dysfunctional insula activation and connectivity.
PLoS One. 11:e01521502016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stein MB, Simmons AN, Feinstein JS and
Paulus MP: Increased amygdala and insula activation during emotion
processing in anxiety-prone subjects. Am J Psychiatry. 164:318–327.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Halsband U, Ito N, Tanji J and Freund HJ:
The role of premotor cortex and the supplementary motor area in the
temporal control of movement in man. Brain. 116:243–266. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eccles JC: The initiation of voluntary
movements by the supplementary motor area. Arch Psychiatr Nervenkr
(1970). 231:423–441. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Salardini A, Narayanan NS, Arora J,
Constable T and Jabbari B: Ipsilateral synkinesia involves the
supplementary motor area. Neurosci Lett. 523:135–138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Florman JE, Duffau H and Rughani AI: Lower
motor neuron findings after upper motor neuron injury: Insights
from postoperative supplementary motor area syndrome. Front Hum
Neurosci. 7:852013. View Article : Google Scholar : PubMed/NCBI
|