Introduction
Selective serotonin reuptake inhibitors (SSRIs) were
previously considered to increase the occurrence of seizures
(1). Previously, clinical and
experimental results indicated that SSRIs alleviate the
susceptibility to seizures (2–5).
This effect is attributed to elevated levels of extracellular
serotonin. However, the underlying molecular mechanism of this
increase remains unclear. Our previous study demonstrated a
downregulation of hippocampal extracellular serotonin levels in
epileptic rats and impaired serotonergic neuronal function in raphe
nucleus (6). The membrane bound
serotonin transporter (SERT) serves an important role in modulating
the metabolism of 5-hydroxytryptamine (5-HT). SSRIs target SERT in
the raphe nucleus, decreasing serotonin reuptake and increasing the
synaptic availability of serotonin. Therefore, the present study
hypothesized that abnormal SERT expression may be present in
epileptic models.
Paroxetine has been demonstrated to regulate the
expression of B-cell lymphoma-2 (Bcl-2) and brain derived
neurotropic factor (BDNF), which are associated with cell apoptosis
and proliferation (7). In
addition, mesial temporal lobe epilepsy (MTLE), the most common
form of refractory epilepsy, is characterized by hippocampal
sclerosis, including cell apoptosis and glial proliferation.
Therefore, the present study hypothesized that paroxetine
alleviates seizures by regulating both Bcl-2/BDNF and SERT.
The mechanism in which paroxetine may regulate SERT,
Bcl-2 and BDNF in epilepsy remains to be fully elucidated. In
recent years, an increasing number of studies, including clinical
and animal experiments, demonstrated that microRNAs (miRNAs) serve
an important role in the pathophysiology of epilepsy (8–11).
Epileptic models are generally accompanied by selective alterations
in miRNAs that regulate neuronal death, ion channels and
inflammation (12–15). In a genome wide miRNA profiling
study, microRNA (miR)-16 was increased in hippocampal tissue
collected from patients with MTLE (15). One study revealed that paroxetine
upregulates miR-16 expression in the raphe nucleus (7), and another demonstrated that Bcl-2
expression was negatively associated with miR-16 expression
(16). These results were obtained
using animal models of depression or tumor cells.
The present study focused on pilocarpine-induced
chronic epileptic rats. Firstly, the effects of paroxetine on
spontaneous recurrent seizures (SSRs) and hippocampal apoptosis was
investigated. Secondly, SERT, Bcl-2 and BDNF expression levels were
evaluated using western blotting, and miR-16 expression was
evaluated using reverse transcription-quantitative polymerase chain
reaction. Finally, the underlying molecular mechanism of miR-16 in
the pathogenesis of epilepsy was investigated.
Materials and methods
Pilocarpine model of chronic
epilepsy
The present study was performed in accordance with
the Guide for the Care and Use of Animal Experimentation of Fujian
Medical University and the Fujian Medical University Animal Ethics
Committee (Fuzhou, China) specifically approved this study. Surgery
was performed using 10% chloral hydrate anesthesia and efforts were
made to minimize suffering. Male adult (8–10 weeks) Sprague Dawley
rats (220–250 g) were housed under standard conditions
(temperature, 22–26°C; 12-h light/dark cycle; humidity, 45–50%) and
had free access to food and water. Thirty rats were divided into
two groups (6 rats in the vehicle group and 24 rats in the
epileptic group). One week prior to the induction of status
epilepticus (SE), surface electrodes were implanted into the skulls
of the rats under 10% chloral hydrate anesthesia as previously
described (6). A frontal electrode
was implanted above the frontal cortex [coordinates, 2.5 mm
frontal; 2.0 mm left and 0.5 mm deep from the bregma (17)], a second electrode was fixed to the
surface of the skull as a ground electrode and a third electrode
was fixed behind the ear as a reference electrode. Following the
implantation procedure, animals were intraperitoneally (i.p.)
injected with gentamicin to prevent infection and were allowed to
recover from surgery for 1 week prior to experimentation. Twenty
minutes prior to injection of pilocarpine, the muscarinic
antagonist, atropine, was administered i.p. (1 mg/kg) to reduce the
adverse peripheral effects of pilocarpine. The rats were injected
i.p. with pilocarpine (30 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) 16–18 h after the administration of lithium
(127 mg/kg, i.p.). After the drugs were administered, the
progressive evolution of seizure behavior was observed and rated
according to the Racine scale (18). The Racine scale was used to rate
the stage of epilepsy: Stage 1 was characterized by behavioral
arrest; stage 2 by head nodding, gnawing, and mild tremors; stage 3
by unilateral forelimb clonus; stage 4 by bilateral forelimb
clonus; and stage 5 by severe seizures with prolonged loss of
postural control or prolonged clonus. Only animals that developed
stage IV and V seizures were used. SE was defined as the
persistence of stage IV and V seizures for longer than 30 min
(18). Electroencephalogram (EEG)
potentials and the behavior of the animals were monitored using a
video monitoring system (Biopac Systems Inc., Goleta, CA, USA)
three times a day for 2 h each session for 8 weeks after the
establishment of SE. During the chronic period, the SSRs were
evaluated based on frequency (times/week) and stage (19). EEG discharges with amplitudes
exceeding 50 µV, which was typically twice the basal EEG discharge
amplitude, and spikes (≤70 msec) and sharp waves (70–200 msec) were
counted as seizure discharges.
Intervention
A total of 18 rats survived SE induction however,
four weeks after the induction of SE only 14 rats had survived.
These 14 epileptic rats were divided into two sub-groups: SE and SE
+ paroxetine. The former group received normal saline (NS) as a
control, and the latter received paroxetine. Paroxetine (5
mg/kg/day) or NS was injected i.p. for 4 weeks; only 12 rats
survived during the final experiment.
Brain region isolation and
morphological examinations
At the end of the experiments, the rats were deeply
anesthetized (10% chloral hydrate, 2 ml/kg) and transcardially
perfused with 0.1 mmol/l PBS (pH 7.4). If the tissue required
fixation, the rats were perfused with PBS followed by 4%
paraformaldehyde. One portion of the hippocampal tissue was used
for terminal deoxynucleotidyl transferase dUTP nick-end labelling
TUNEL/horseradish peroxidase (HRP) staining to visualize apoptotic
cells. Another portion of the hippocampal tissue and a sample of
the raphe nucleus tissue were used to evaluate expressions of SERT,
Bcl-2 and BDNF via western blotting. A second set of
hippocampal/raphe nucleus tissue samples was used to analyze
expression of miR-16 via reverse transcription-quantitative
polymerase chain reaction (RT-qPCR).
Immunohistochemical staining
The unilateral hippocampal tissue from the six rats
in each of the control, SE and SE + paroxetine groups was rapidly
isolated, fixed with 4% paraformaldehyde for 48 h at 4°C and
embedded in paraffin. Next, the paraffin-embedded tissue was cut
into sections including the cornu ammonis (CA) 1–3 and the
dentate gyrus (DG) regions of the hippocampus. Subsequently,
the slices were dewaxed in a series of alcohols and incubated with
proteinase K (20 µg/ml) for 10 min at room temperature (22–28°C),
terminal deoxynucleotidyl transferase (50 µl) at 37°C for 60 min
and an anti-biotin HRP solution at 37°C for 30 min. Finally,
diaminobenzidine was used for color development and hematoxylin was
used for counterstaining at 37°C for 15 min. The stained brain
sections were observed using a Leica DM2500 microscope (Leica
Microsystems GmbH, Wetzlar, Germany), and images were captured
using a digital camera and Leica software version 3.7 (Leica
Microsystems GmbH). For quantification, five fields of view at ×400
magnification were randomly examined, and the number of brown cells
in each field was counted by independent blinded operators. Brown
spots were counted irrespective of whether they contained a blue
nucleus. An immunohistochemical score (IHS) was calculated by
multiplying the number of immunoreactive cells (quantity score) by
the staining intensity (staining intensity score). Quantity scores
were estimated as follows: No staining, 0; 1–10% of cells, 1;
11–50%, 2; 51–80%, 3; 81–100%, 4. Staining intensity was rated on a
scale of 0–3 where: 0, negative; 1, weak; 2, moderate; and 3,
strong. The IHS ranged from 0 to 12.
Western blotting
Proteins were extracted from the hippocampal and
raphe nucleus tissue using cytoplasmic extracts (Beyotime Institute
of Biotechnology, Jiangsu, China) and 10X PMSF (100:1). Protein
concentration was detected using a bicinchoninic acid working
solution (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. A total of 30 µg protein/lane was
separated via 10% SDS-PAGE and transferred onto a nitrocellulose
membrane. After blocking of the membranes with 5% skim milk powder
for 2 h at room temperature, they were incubated at 4°C overnight
in primary antibodies against the following target proteins: SERT
(cat. no. ab172884, 1:1,000, polyclonal rabbit; Abcam, Cambridge,
UK), Bcl-2 (cat. no. AB112-1, 1:1,000, monoclonal rabbit; Beyotime
Institute of Biotechnology), BDNF (1:1,000, polyclonal rabbit, cat.
no. ab75040; Abcam, Cambridge, MA, USA), and β-actin (cat. no.
EM32011-02, 1:1,000, monoclonal mouse; Beijing Emarbio Science and
Technology, Beijing, China; www.emarbio.com). Subsequently, the membranes were
washed and incubated in species-specific peroxidase-conjugated
secondary antibodies for 2 h at room temperature. The secondary
antibodies (all 1:6,000; HRP-conjugated) used to distinguish SERT,
Bcl-2, BDNF (anti-rabbit, cat. no. A0208; Beyotime Institute of
Biotechnology) and β-actin (anti-mouse, cat. no. A0216; Beyotime
Institute of Biotechnology) were all produced in goats. The
specific bands were detected using an Enhanced Chemiluminescence
system (GE Healthcare, Chicago, IL, USA) and a Bio-Rad
electrophoresis image analyzer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and analyzed using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
RT-qPCR
Total RNA was extracted from hippocampal and raphe
nucleus tissue samples using TRIzol® Reagent (cat. no.
15596-026; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions and quantified
using a spectrophotometer (NanoDrop2000/2000C; Thermo Fisher
Scientific, Inc.). Subsequently, the RNA was reverse transcribed
into cDNA using M-MLV Reverse Transcriptase (cat. no. M1705;
Promega Corporation, Madison, WI, USA) according to the
manufacturer's instructions and amplified using a Real-Time PCR
Mx3000p Instrument (Agilent Technologies, Inc., Santa Clara, CA,
USA). RT-qPCR was performed using SYBR® Premix Ex Taq™
(with a pre-denaturation at 95°C for 30 sec, followed by 40 cycles
of denaturation at 95°C for 5 sec, primer annealing at 60°C for 30
sec; acquisition of the dissolve curve at 95°C for 15 sec; at 60°C
for 30 sec; at 95°C for 15 sec) (cat. no. DRR041B; Takara
Biotechnology Co., Ltd., Dalian, China). The primers for the target
gene (forward primer, cat. no. SSD809230873; downstream primer,
cat. no. SSD089261711; and reverse primer, cat. no. SSD809230181)
and the reference gene U6 (forward primer, cat. no. SSD0904071006;
downstream primer, cat. no. SSD0904071007; and reverse primer, cat.
no. SSD904071008) were designed and synthesized by Guangzhou
RiboBio Co., Ltd (Guangzhou, China). After PCR, a melting curve was
obtained to assess the quality of the reaction. The relative
expression of miRNA was calculated as follows: 2−∆∆Cq
(∆Ct = Cq (TG) - Cq (RG); ∆∆Cq = ∆Cq (experimental) - ∆Cq (control)
(20).
Materials
Atropine, pilocarpine hydrochloride, paroxetine,
trypsin, paraformaldehyde and the monoclonal antibody for BDNF were
purchased from Sigma-Aldrich; Merck KGaA. The specific antibodies
for Bcl-2, SERT and β-actin were purchased from Abcam. The
TUNEL/HRP kit was purchased from Roche Applied Science (Penzberg,
Germany). All other reagents were purchased from Biyuantian
(Jiangsu, China).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using GraphPad Prism v6.0
software (GraphPad Software, Inc., La Jolla, CA, USA). One-way
analysis of variance was performed with multiple comparisons
between the groups using an Dunnett's post hoc tests and
comparisons between the attack levels were performed using the
Mann-Whitney test method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Behavioral alterations in chronic
epileptic rats
At 3–10 min following the administration of
pilocarpine, the animals exhibited masticatory movements,
salivation, sniffing movements, tremors and partial seizures. At
15–30 min following pilocarpine injection, the animals developed SE
that persisted for longer than 30 min. SE was successfully induced
in all the epileptic group rats. The acute phase was followed by a
quiescent phase of 2–7 days in which the animals behaved normally
except for anorexia and hypokinesis. SSR-like activity was observed
8–27 days after the induction of SE. A total of 14 rats survived
the induction of SE at 4 weeks; subsequently, 7 rats received
paroxetine treatment and the other 7 rats were untreated. At the
end of the experiment, 6 rats survived in each of the
paroxetine-treated and untreated groups (total, n=12 rats). The
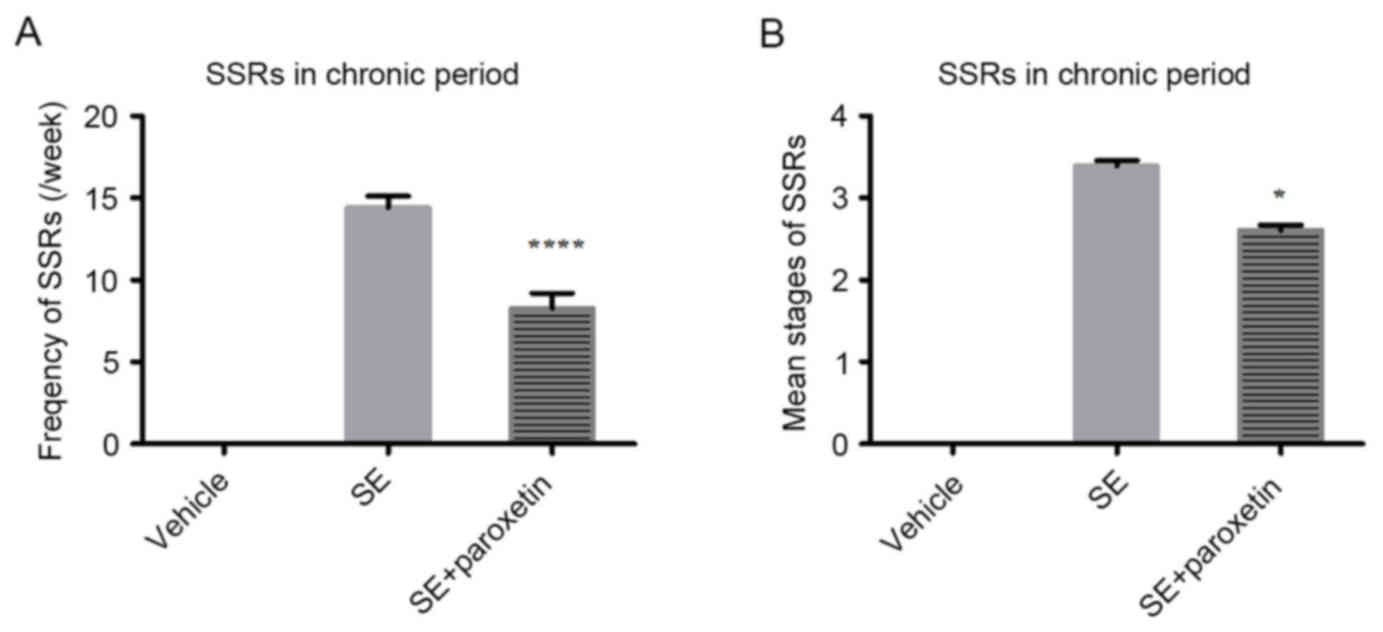
frequency per week (P<0.0001; Fig.
1A) and the mean stage of the SSRs (P<0.05; Fig. 1B) were significantly decreased
after paroxetine intervention compared with the SE group. The
mortality of the rats may have been due to pilocarpine-induced
epilepsy.
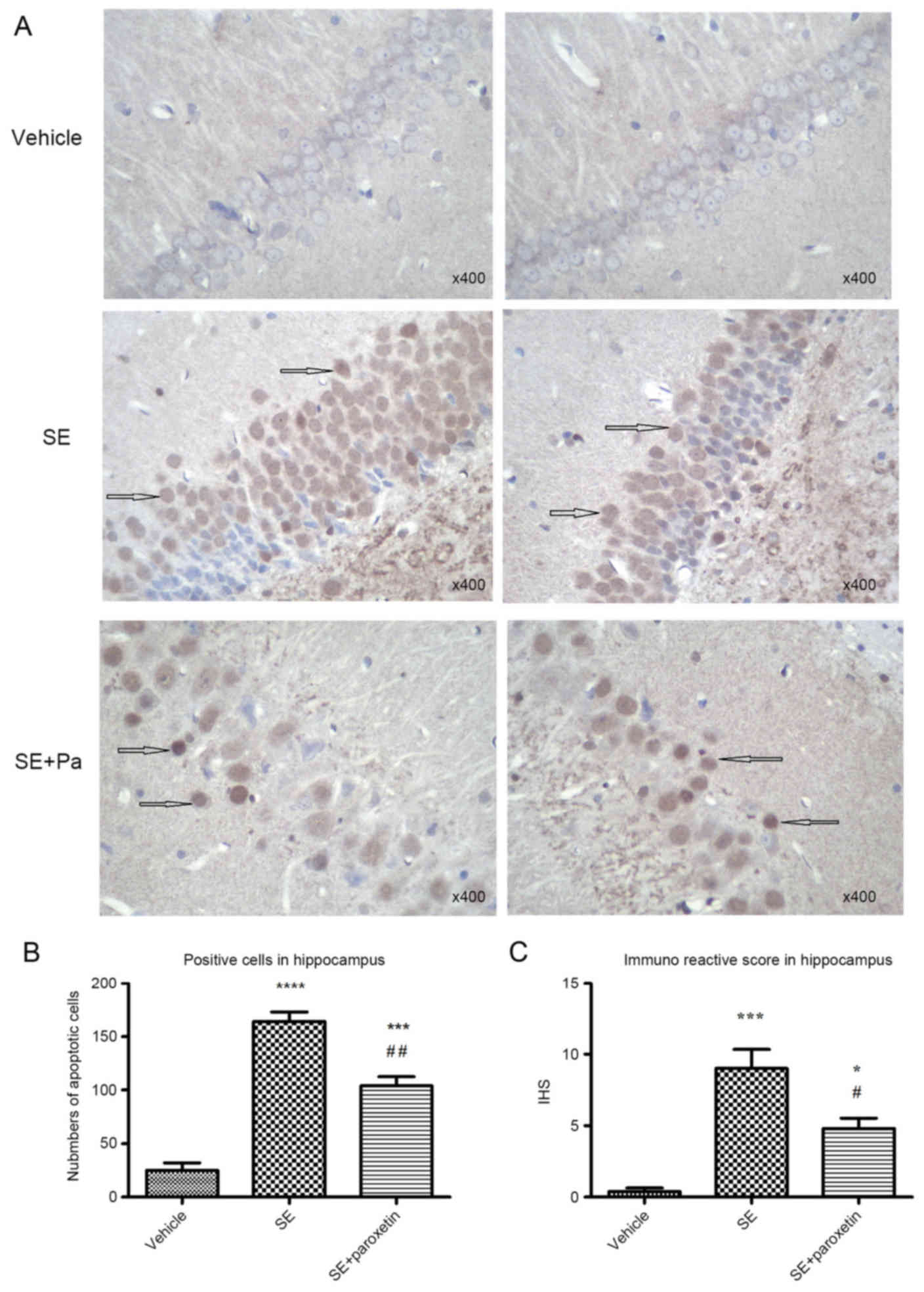
TUNEL/HRP staining in the
hippocampus
Tissue sections of the hippocampus from the
experimental groups were stained with TUNEL/HRP in order to
evaluate apoptosis. With this assay, apoptotic neurons in the DG
region were positively stained (brown) in the cytoplasm. In the
vehicle group, positively stained neurons were sparse, and those
that were positive only exhibited light positive staining (Fig. 2A). The number and IHS score of
apoptotic neurons were increased in the SE group compared with the
vehicle group (P<0.0001 and P<0.001, respectively; Fig. 2B and C). Following paroxetine
intervention, the number of apoptotic neurons and IHS score
significantly decreased compared with the SE group (P<0.001 and
P<0.05, respectively; Fig. 2B and
C).
Expression of SERT, Bcl-2 and BDNF
proteins
SERT was expressed in both the raphe nucleus and the
hippocampus in all experimental groups (Fig. 3A). In the hippocampus, SERT
expression in the SE group was decreased compared with the vehicle
group (P<0.01; Fig. 3A and B),
but this effect was reversed by paroxetine, with SERT expression
being significantly increased in the SE + paroxetine group compared
with the SE group (P<0.05; Fig. 3A
and B). In the raphe nucleus, SERT expression was decreased in
the SE + paroxetine group compared with the SE group (P<0.05;
Fig. 3A and C). The pattern of
differences in Bcl-2 expression in the hippocampus was similar to
that of SERT expression. Bcl-2 expression levels in the SE group
were decreased compared with the vehicle group (P<0.0001;
Fig. 3A and D), but following
paroxetine intervention, Bcl-2 expression levels were significantly
increased compared with the SE group (P<0.01; Fig. 3A and D). Additionally, in the
hippocampus, BDNF expression levels in the SE group were decreased
compared with the vehicle group (P<0.05; Fig. 3A and E); however, paroxetine
intervention did not significantly alter BDNF expression compared
with the SE group (Fig. 3A and
D).
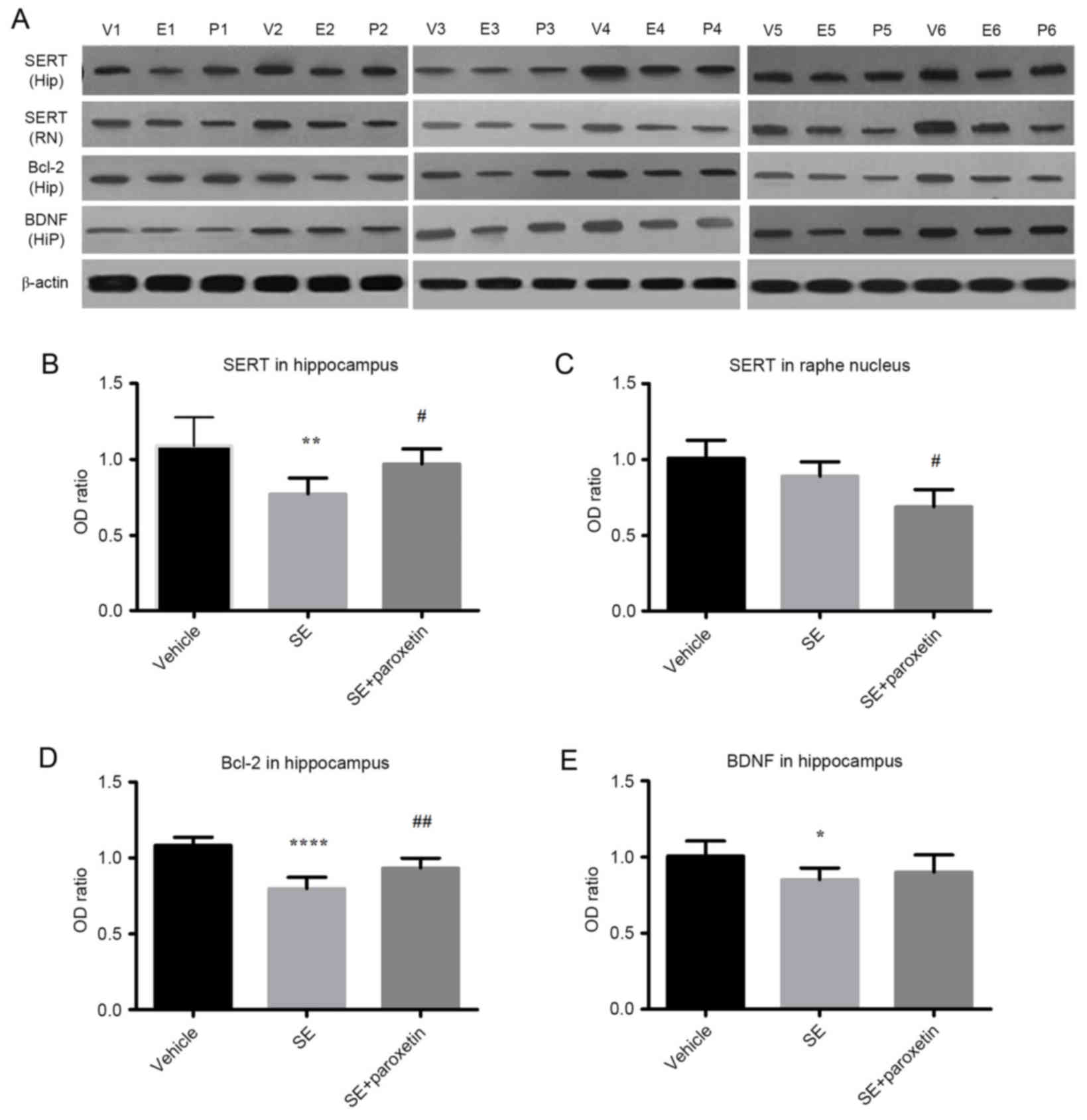 | Figure 3.Protein expression levels of SERT,
Bcl-2 and BDNF detected by western blotting. (A) Blot images of
samples from 6 animals per experimental group (V1-6, vehicle group;
E1-6, SE group; P1-6, SE + paroxetine group). Quantification of
protein signals was performed for (B) SERT expression in the
hippocampus, (C) SERT expression in the raphe nucleus, (D) Bcl-2 in
the hippocampus and (E) BDNF expression in the hippocampus. Data
are presented as the mean ± standard deviation. *P<0.05,
**P<0.01 and ****P<0.0001 vs. vehicle; #P<0.05
and ##P<0.01 vs. SE. SERT, serotonin transporter;
Bcl-2, B-cell lymphoma-2; BDNF, brain derived neurotropic factor;
SE, status epilepticus; Hip, hippocampus; RN, raphe nucleus. |
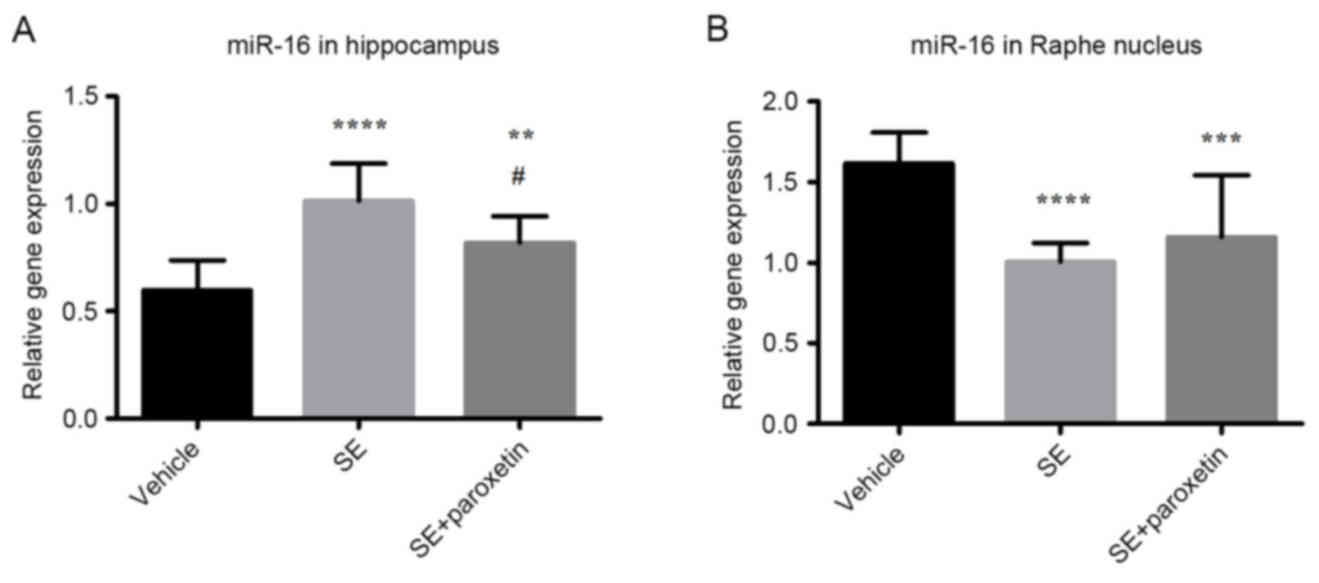
Expression of miR-16
In the hippocampus, miR-16 expression in the SE
group was increased compared with the vehicle group (P<0.0001;
Fig. 4A). Following paroxetine
administration, miR-16 expression was significantly decreased
compared with the SE group (P<0.05; Fig. 4A); however, miR-16 expression
remained higher in the SE + paroxetine group than in the vehicle
group (P<0.001; Fig. 4A). In
the raphe nucleus, miR-16 expression in the SE group was decreased
compared with the vehicle group (P<0.0001; Fig. 4B), and this increased expression
was not significantly altered following paroxetine administration
(Fig. 4B).
Discussion
Previous studies have demonstrated that serotonin
serves an important role in epilepsy (21–23).
In general, drugs that increase the level of extracellular
serotonin, such as SSRIs/tryptophan and 5-hydroxytryptophan (5-HT)
(21,24,25),
alleviate seizures, whereas drugs such as 5,7-dihydroxytryptamine
that reduce the level of serotonin may aggravate seizures (26,27).
However, the effects of SSRIs on seizures remain controversial.
Previous clinical tests suggested that long-term treatment of
depression with SSRIs increases the incidence of epilepsy. The rate
of epileptogenesis in rats has been demonstrated to be enhanced by
chronic SSRI treatment (28).
However, clinical and animal experiments have demonstrated that
SSRIs may decrease seizures, and these drugs are considered safe
for use in epilepsy (3–5,25).
In the present study, four weeks of paroxetine treatment alleviated
seizures in pilocarpine-induced chronic epileptic rats. Further
studies will be required to determine the longer-term effects of
SSRI treatment on epilepsy.
The molecular mechanism underlying the therapeutic
role of SSRIs in epilepsy remains unclear. Our previous study
revealed that in pilocarpine-induced epileptic rats, the level of
extracellular serotonin in the hippocampus decreased, as did the
number of 5-HTP-positive neurons in the raphe nucleus (6). SERT, which modulates 5-HT metabolism,
is considered important for epilepsy, especially when it is
accompanied by depression (29–32).
Therefore, we hypothesized that SERT is abnormally expressed in
pilocarpine-induced epileptic rats, although one study demonstrated
the absence of a significant change in mRNA expression levels of
SERT in this model (28). The
present study demonstrated that SERT is expressed not only in the
raphe nucleus but also in the hippocampus. No significant decreases
in SERT expression in the raphe nucleus was observed in
pilocarpine-induced chronic epileptic rats. Theoretically, it
should decrease due to impairment of the raphe nucleus. It was
hypothesized that the absence of an alteration in SERT expression
reflects a form of self-regulation to ensure the availability of
serotonin. Following paroxetine intervention, SERT was
downregulated in the raphe nucleus, decreasing reuptake and thus
increasing synaptic 5-HT availability. Additionally, it was
downregulated in the hippocampus in epileptic rats. This result is
consistent with the results of Martinez et al (31), who demonstrated that SERT activity
in the insula and fusiform gyrus was reduced in patients with
temporal lobe epilepsy accompanied by depression. Following
paroxetine intervention, SERT was upregulated in the hippocampus,
indicating increased reuptake and therefore an increased level of
serotonin in the hippocampus. Therefore, SERT expression
alterations in pilocarpine-induced chronic epileptic rats differed
across brain regions, and paroxetine treatment modulated the
expression of SERT to increase the level of extracellular serotonin
in the hippocampus.
The question remains as to why SERT expression
levels are altered in epilepsy. Previous studies have focused on
the epigenetic and genetic pathogenesis of epilepsy (33–36),
under the assumption that one gene modulates a number of proteins
and one protein may be regulated by various different genes. miRNA
is a one example, as selective alterations in miRNAs that regulate
neuronal cell death, ion channels and inflammation have been
identified in epileptic patients and in experimental epileptogenic
models (8,10,11,13,37,38).
In a genome wide miRNA profiling study, miR-16 expression was
increased in the hippocampus of patients with MTLE (9). Similarly, the present study
demonstrated that miR-16 was upregulated in the hippocampus in
pilocarpine-induced chronic epileptic rats. However, following
paroxetine intervention, it was downregulated. By contrast, in the
raphe nucleus, miR-16 was downregulated, demonstrating that the
alteration in miR-16 expression in chronic epileptic rats had brain
tissue specificity. The pattern of change in miR-16 expression was
opposite to that of SERT. In addition, miR-16 has been reported to
target SERT, and in experimental models of depression, paroxetine
may upregulate miR-16 expression in the raphe nucleus (7). Therefore, it may be hypothesized that
miR-16 may have a role in regulating the gene expression of SERT in
the raphe nucleus and hippocampus of chronic epileptic rats.
Another question that remains is whether other
proteins are targeted by miR-16. Recent experimental results have
suggested that miR-16 may regulate the cell cycle and apoptosis in
tumors (16,39,40),
including T lymphoblastic lymphoma/leukemia, breast cancer, glioma
and hepatocellular carcinoma. For example, Mobarra et al
(41) revealed that miR-16
overexpression reduces Cyclin D1 and Bcl-2 expression and increases
apoptosis in breast cancer cells. Recent studies have demonstrated
that miR-16 may target BDNF (42,43).
In general, miR-16 overexpression may downregulate BDNF and
therefore inhibit cell proliferation, including in depression
models and SH-SY5Y cells (42,43).
Temporal lobe epilepsy is characterized by hippocampal sclerosis,
including neuronal apoptosis and glial proliferation. As an
antiapoptotic protein, Bcl-2 has been demonstrated to regulate
mitochondrial permeability and caspase-3 activity in epilepsy
(44,45), whereas the association between BDNF
and seizures remains controversial. One study revealed that
upregulating BDNF may increase epilepsy susceptibility (46), and another reported that
upregulating BDNF alleviates seizures in pilocarpine-induced
epileptic mice (47). This
suggested that continuously injecting an appropriate amount of BDNF
into the hippocampus may alleviate kainic acid-induced seizures via
the promotion of neuronal regeneration and therefore demonstrated
that BDNF serves a protective role in neuronal apoptosis (47). In the present study, obvious
neuronal apoptosis and downregulation of Bcl-2 and BDNF expression
were observed in pilocarpine-induced chronic epileptic rats.
Paroxetine alleviated neuronal apoptosis and upregulated Bcl-2
expression. In the present study, Bcl-2 exhibited an opposite trend
of expression than miR-16, and therefore it may be hypothesized
that miR-16 overexpression downregulated Bcl-2 expression and
increased neuronal apoptosis in chronic epileptic rats. However,
the association between BDNF and miR-16 in epileptic rats remains
uncertain.
The mechanism by which miR-16 targets SERT, Bcl-2
and BDNF requires further study. In addition, one protein may be
regulated by a number of miRNAs. For example, SERT is regulated by
miR-16, in addition to miR-55 and other miRNAs (48,49).
Therefore, further studies are required to determine whether SERT,
Bcl-2 and BDNF are primarily targeted by miR-16.
In conclusion, the present study demonstrated that
seizures and hippocampal apoptosis in chronic epileptic rats may be
alleviated by paroxetine treatment, which may be associated with
alterations in SERT and Bcl-2/BDNF protein expression. The
alterations in miR-16 expression may provide a potential
explanation for the modulation of apoptosis. Further study is
required to determine the underlying molecular mechanisms.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371426), the
Health Department Youth Foundation of Fujian Province (grant no.
2013-1-26) and was sponsored by the Key Clinical Specialty
Discipline Construction Program of Fujian, China. Thanks to Dr
Edward C. Mignot (Shandong University) for linguistic advice.
References
|
1
|
Curran S: Effect of paroxetine on seizure
length during electroconvulsive therapy. Acta Psychiatr Scand.
92:239–240. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alper K, Schwartz KA, Kolts RL and Khan A:
Seizure incidence in psychopharmacological clinical trials: An
analysis of Food and Drug Administration (FDA) summary basis of
approval reports. Biol Psychiatry. 62:345–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Payandemehr B, Ghasemi M and Dehpour AR:
Citalopram as a good candidate for treatment of depression in
patients with epilepsy. Epilepsy Behav. 44:96–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiha AA, de Cristóbal J, Delgado M,
Fernández de la Rosa R, Bascuñana P, Pozo MA and García-García L:
Subacute administration of fluoxetine prevents short-term brain
hypometabolism and reduces brain damage markers induced by the
lithium-pilocarpine model of epilepsy in rats. Brain Res Bull.
111:36–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vermoesen K, Massie A, Smolders I and
Clinckers R: The antidepressants citalopram and reboxetine reduce
seizure frequency in rats with chronic epilepsy. Epilepsia.
53:870–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin WH, Huang HP, Lin MX, Chen SG, Lv XC,
Che CH and Lin JL: Seizure-induced 5-HT release and chronic
impairment of serotonergic function in rats. Neurosci Lett.
534:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Launay JM, Mouillet-Richard S, Baudry A,
Pietri M and Kellermann O: Raphe-mediated signals control the
hippocampal response to SRI antidepressants via miR-16. Transl
Psychiatry. 1:e562011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Yu JT and Tan L, Tian Y, Ma J, Tan
CC, Wang HF, Liu Y, Tan MS, Jiang T and Tan L: Genome-wide
circulating microRNA expression profiling indicates biomarkers for
epilepsy. Sci Rep. 5:95222015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li MM, Jiang T, Sun Z, Zhang Q, Tan CC, Yu
JT and Tan L: Genome-wide microRNA expression profiles in
hippocampus of rats with chronic temporal lobe epilepsy. Sci Rep.
4:47342014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Henshall DC: MicroRNA and epilepsy:
Profiling, functions and potential clinical applications. Curr Opin
Neurol. 27:199–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu K, Xie YY, Zhang C, Ouyang DS, Long HY,
Sun DN, Long LL, Feng L, Li Y and Xiao B: MicroRNA expression
profile of the hippocampus in a rat model of temporal lobe epilepsy
and miR-34a-targeted neuroprotection against hippocampal neurone
cell apoptosis post-status epilepticus. BMC Neurosci. 13:1152012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Omran A, Peng J, Zhang C, Xiang QL, Xue J,
Gan N, Kong H and Yin F: Interleukin-1β and microRNA-146a in an
immature rat model and children with mesial temporal lobe epilepsy.
Epilepsia. 53:1215–1224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reschke CR and Henshall DC: microRNA and
epilepsy. Adv Exp Med Biol. 888:41–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashhab MU, Omran A, Kong H, Gan N, He F,
Peng J and Yin F: Expressions of tumor necrosis factor alpha and
microRNA-155 in immature rat model of status epilepticus and
children with mesial temporal lobe epilepsy. J Mol Neurosci.
51:950–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kan AA, van Erp S, Derijck AA, de Wit M,
Hessel EV, O'Duibhir E, de Jager W, Van Rijen PC, Gosselaar PH, de
Graan PN and Pasterkamp RJ: Genome-wide microRNA profiling of human
temporal lobe epilepsy identifies modulators of the immune
response. Cell Mol Life Sci. 69:3127–3145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin K, Farahani M, Yang Y, Johnson GG,
Oates M, Atherton M, Douglas A, Kalakonda N and Pettitt AR: Loss of
MIR15A and MIR16-1 at 13q14 is associated with increased TP53 mRNA,
de-repression of BCL2 and adverse outcome in chronic lymphocytic
leukaemia. Br J Haematol. 167:346–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paxinos G and Watson C: The Rat Brain in
Stereotactic Coordinates. 5th edition. Elsevier Academic Press;
Boston, MA: pp. 1612005
|
|
18
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Veliskova J: Behavioral characterization
of seizures in ratsModels of Seizures and Epilepsy. Elsevier
Academic Press; Burlington: pp. 601–611. 2006, View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bagdy G, Kecskemeti V, Riba P and Jakus R:
Serotonin and epilepsy. J Neurochem. 100:857–873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Theodore WH: Does serotonin play a role in
epilepsy? Epilepsy Curr. 3:173–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gidal BE: Serotonin and epilepsy: The
story continues. Epilepsy Curr. 13:289–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Airaksinen EM: Uptake of taurine, GABA,
5-HT, and dopamine by blood platelets in progressive myoclonus
epilepsy. Epilepsia. 20:503–510. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bateman LM, Li CS, Lin TC and Seyal M:
Serotonin reuptake inhibitors are associated with reduced severity
of ictal hypoxemia in medically refractory partial epilepsy.
Epilepsia. 51:2211–2214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trindade-Filho EM, de Castro-Neto EF, de A
Carvalho R, Lima E, Scorza FA, Amado D, Naffah-Mazzacoratti Mda G
and Cavalheiro EA: Serotonin depletion effects on the pilocarpine
model of epilepsy. Epilepsy Res. 82:194–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
da Fonseca NC, Joaquim HP, Talib LL, de
Vincentiis S, Gattaz WF and Valente KD: Hippocampal serotonin
depletion is related to the presence of generalized tonic-clonic
seizures, but not to psychiatric disorders in patients with
temporal lobe epilepsy. Epilepsy Res. 111:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cardamone L, Salzberg MR, Koe AS, Ozturk
E, O'Brien TJ and Jones NC: Chronic antidepressant treatment
accelerates kindling epileptogenesis in rats. Neurobiol Dis.
63:194–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esmail EH, Labib DM and Rabie WA:
Association of serotonin transporter gene (5HTT) polymorphism and
juvenile myoclonic epilepsy: A case-control study. Acta Neurol
Belg. 115:247–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang K, Su J, Hu Z, Lang R, Sun X, Li X,
Wang D, Wei M and Yin J: Serotonin transporter (5-HTT) gene
polymorphisms and susceptibility to epilepsy: A meta-analysis and
meta-regression. Genet Test Mol Biomarkers. 17:890–897. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martinez A, Finegersh A, Cannon DM, Dustin
I, Nugent A, Herscovitch P and Theodore WH: The 5-HT1A receptor and
5-HT transporter in temporal lobe epilepsy. Neurology.
80:1465–1471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schenkel LC, Bragatti JA, Torres CM,
Martin KC, Gus-Manfro G, Leistner-Segal S and Bianchin MM:
Serotonin transporter gene (5HTT) polymorphisms and temporal lobe
epilepsy. Epilepsy Res. 95:152–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobow K and Blümcke I: The methylation
hypothesis: Do epigenetic chromatin modifications play a role in
epileptogenesis? Epilepsia. 52(Suppl 4): S15–S19. 2011. View Article : Google Scholar
|
|
34
|
Serikawa T, Mashimo T, Kuramoro T, Voigt
B, Ohno Y and Sasa M: Advances on genetic rat models of epilepsy.
Exp Anim. 64:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ran X, Li J, Shao Q, Chen H, Lin Z, Sun ZS
and Wu J: EpilepsyGene: A genetic resource for genes and mutations
related to epilepsy. Nucleic Acids Res. 43(Database issue):
D893–D899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weber YG, Nies AT, Schwab M and Lerche H:
Genetic biomarkers in epilepsy. Neurotherapeutics. 11:324–333.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moon J, Lee ST, Choi J, Jung KH, Yang H,
Khalid A, Kim JM, Park KI, Shin JW, Ban JJ, et al: Unique
behavioral characteristics and microRNA signatures in a drug
resistant epilepsy model. PloS one. 9:e856172014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jimenez-Mateos EM and Henshall DC:
Epilepsy and microRNA. Neuroscience. 238:218–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang S, Zou X, Zhu JN, Fu YH, Lin QX,
Liang YY, Deng CY, Kuang SJ, Zhang MZ, Liao YL, et al: Attenuation
of microRNA-16 derepresses the cyclins D1, D2 and E1 to provoke
cardiomyocyte hypertrophy. J Cell Mol Med. 19:608–619. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li W, Qi Z, Wei Z, Liu S, Wang P, Chen Y
and Zhao Y: Paeoniflorin inhibits proliferation and induces
apoptosis of human glioma cells via microRNA-16 upregulation and
matrix metalloproteinase-9 downregulation. Mol Med Rep.
12:2735–2740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mobarra N, Shafiee A, Rad SM, Tasharrofi
N, Soufi-Zomorod M, Hafizi M, Movahed M, Kouhkan F and Soleimani M:
Overexpression of microRNA-16 declines cellular growth,
proliferation and induces apoptosis in human breast cancer cells.
In Vitro Cell Dev Biol Anim. 51:604–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bai M, Zhu X, Zhang Y, Zhang S, Zhang L,
Xue L, Yi J, Yao S and Zhang X: Abnormal hippocampal BDNF and
miR-16 expression is associated with depression-like behaviors
induced by stress during early life. PLoS One. 7:e469212012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun YX, Yang J, Wang PY, Li YJ, Xie SY and
Sun RP: Cisplatin regulates SH-SY5Y cell growth through
downregulation of BDNF via miR-16. Oncol Rep. 30:2343–2349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kilany A, Raouf ER, Gaber AA, Aloush TK,
Aref HA, Anwar M, Henshall DC and Abdulghani MO: Elevated serum
Bcl-2 in children with temporal lobe epilepsy. Seizure. 21:250–253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Henshall DC, Clark RS, Adelson PD, Chen M,
Watkins SC and Simon RP: Alterations in bcl-2 and caspase gene
family protein expression in human temporal lobe epilepsy.
Neurology. 55:250–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Scharfman H: Does BDNF contribute to
temporal lobe epilepsy? Epilepsy Curr. 2:92–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kuramoto S, Yasuhara T, Agari T, Kondo A,
Jing M, Kikuchi Y, Shinko A, Wakamori T, Kameda M, Wang F, et al:
BDNF-secreting capsule exerts neuroprotective effects on epilepsy
model of rats. Brain Res. 1368:281–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song MF, Dong JZ, Wang YW, He J, Ju X,
Zhang L, Zhang YH, Shi JF and Lv YY: CSF miR-16 is decreased in
major depression patients and its neutralization in rats induces
depression-like behaviors via a serotonin transmitter system. J
Affect Disord. 178:25–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zurawek D, Kusmider M, Faron-Gorecka A,
Gruca P, Pabian P, Solich J, Kolasa M, Papp M and
Dziedzicka-Wasylewska M: Reciprocal microrna expression in
mesocortical circuit and its interplay with serotonin transporter
define resilient rats in the chronic mild stress. Mol Neurobiol.
Sep 22–2016.(Epub ahead of print). PubMed/NCBI
|