Introduction
The human skin surrounds the external surface of the
human body and acts as a protector against environmental factors,
including ultraviolet (UV) irradiation, destructive agents and
viral infections (1,2). The skin consists of two layers, the
epidermis and dermis. The epidermis forms an epidermal barrier
through keratinocyte differentiation (3). The dermis consists of an
extracellular matrix that is predominantly composed of type I
collagen synthesized by fibroblasts (4–6). The
skin loses these two functions as it ages, due to intrinsic aging
and extrinsic factors (7).
Intrinsic factor-induced skin aging is a natural skin aging
process, caused by the loss of function of age-associated genes due
to heredity or the passage of time (8). Extrinsic factor-induced skin aging is
aging or photoaging, caused by environmental conditions,
predominantly from exposure to UV radiation (9). UV-induced oxidative stress is
accelerated by deep wrinkles caused by the loss of collagen
production (10,11). Therefore, an endogenous
anti-oxidant defense system is required for protection against the
effects of photodamage, which contribute to skin aging (12).
Arctiin, a lignin compound isolated from Arctium
lappa, also known as greater burdock, possesses a variety of
anti-viral, anti-inflammatory and anti-proliferative effects in
mammalian cells (13–15). Arctiin has recently been
demonstrated to improve procollagen type I synthesis and exhibits a
protective effect against ultraviolet B (UVB) radiation (16,17).
An extract of A. lappa or isolated arctiin induces collagen
synthesis in the dermis (18).
However, the underlying mechanism of collagen synthesis, induced by
arctiin treatment, remains unknown.
MicroRNAs (miRNAs) are small non-coding RNAs that
function as important regulators of gene expression in skin aging
and differentiation (19–22). These miRNAs are 16–35 nucleotides
(nts) in length (median, 21–22 nts) that interact with the 3′
untranslated region (3′ UTR) of target messenger RNAs (mRNAs).
Consequently, the complementary interaction of miRNA and its target
mRNA inhibits protein translation at the post-transcriptional level
(23,24). In skin aging, these miRNAs serve a
key role by targeting skin aging-associated gene expression and UV
protective-associated gene expression in the dermis (25–27).
A previous study revealed that miRNAs serve an important role in
anti-aging functions and skin stress responses of skin-derived
cells, including keratinocytes and dermal fibroblasts (28). Additionally, several miRNAs have
been reported to regulate melanogenesis, skin aging, and
differentiation of melanocytes, keratinocytes, and dermal
fibroblasts (29,30).
In a previous study, the authors revealed that
arctiin inhibited microRNA-378b (miR-378b) expression in
UVB-irradiated normal human dermal fibroblasts (nHDFs) (16). Therefore, in the present study, it
was investigated whether this alteration of miR-378b contributes to
the enhanced procollagen synthesis induced by treatment with
arctiin in nHDFs. A detailed mechanism by which arctiin stimulates
collagen synthesis in nHDFs, by inhibiting miR-378b expression
that, in turn, increases expression of its target sirtuin-6 (SIRT6)
through reduced miRNA-mediated repression is demonstrated.
Materials and methods
Cell culture, chemicals, and
reagents
nHDFs (Lonza Group, Ltd., Basel, Switzerland) were
cultured in Dulbecco's Modified Eagle medium containing 10% fetal
bovine serum (both HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) and antibiotics in a humidified incubator under 5%
CO2 at 37°C. Arctiin was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) and dissolved in dimethyl
sulfoxide.
Arctiin treatment and UVB
exposure
For arctiin treatment alone, nHDFs (1×105
cells in 60 mm culture dish and 3×103 cells in 96-well
plates) were incubated with arctiin at 37°C for 24 h, and then a
cell viability assay and reverse transcription-quantitative
polymerase chain reaction were performed. At UVB exposure, nHDFs
(1×105 cells in 60 mm culture dish and 3×103
cells in 96-well plates) was pre-incubated with arctiin at 37°C for
3 h, and then 30 mJ/cm2 UVB was exposed to
arctiin-pretreated nHDFs. After UVB exposure, the nHDFs were
incubated at 37°C for 24 h and then western blotting and RT-qPCR
were performed.
Cell viability assay
The cytotoxic effects of arctiin on nHDFs were
determined using a water-soluble tetrazolium salt (WST-1) assay
(EZ-Cytox cell viability assay kit; ITS Bio, Seoul, Korea). nHDFs
were seeded at a density of 3×103 cells in 96-well
plates and incubated for 24 h. The cells were then incubated with
0–40 µM arctiin for 24 h. The WST-1 assay solution was added to the
cells at a 1/10 volume of the total culture medium and were
incubated at 37°C for 1 h. Cell viability was evaluated by
measuring the absorbance at 450 nm, using an iMark microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transfection of a miR-378b mimic and
anti-miR-378b
An miR-378b mimic (100 nM;
5′-ACUGGACUUGGAGGCAGAA-3′), an anti-miR-378b (100 nM;
5′-ACUGGACUUGGAGGCAGAA-3′), and 100 nM scrambled control
(AccuTarget™ negative control siRNA; Bioneer Corporation, Daejeon,
Korea) were purchased from Qiagen GmbH (Hilden, Germany). miRNAs
were dissolved in nuclease-free water (USB Biochemicals;
Affymetrix, Inc., Santa Clara, CA, USA). nHDFs were seeded at a
density of 1×105 cells in 60 mm culture dish and
incubated for 24 h. Cells were transfected with the miRNA mimic,
anti-miRNA of miR-378b, or the negative control using
Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol.
RNA preparation and RT-qPCR
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The purity and concentration of the RNA
samples were estimated using MaestroNano® (Maestrogen,
Inc., Las Vegas, NV, USA). cDNA was synthesised from total RNA
using the SuperScript™ III Reverse Transcriptase kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. To evaluate collagen type 1α 1 chain (COL1A1)
mRNA expression, qPCR was performed using COL1A1 primers (human
COL1A1 forward: 5′-AGGGCCAAGACATC-3′; and reverse:
5′-AGATCACGTCATCGCACAACA-3′) and EvaGreen premix (Solis BioDyne,
Tartu, Estonia) with the StepOnePlus™ real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Expression was normalized to β-actin (human β-actin forward:
5′-GGATTCCTATGTGGGCGACGA-3′ and reverse:
5′-GCTCGGTGAGGATCTTCATG-3′). Additionally, cDNAs for miR-378b
detection were synthesized using the miScript II RT Kit (Qiagen
Inc., Valencia, CA, USA) according to the manufacturer's protocol.
qPCR was performed using a miR-378b-specific primer, Hs_miR-1290_1
miScript Primer Assay (Qiagen Inc., Valencia, CA, USA), and the
miScript SYBR-Green PCR Kit (Qiagen Inc., Valencia, CA, USA) with
the StepOnePlus™ real-time PCR system. MiR-378b expression was
normalized to U6 small nuclear RNA. The 2−ΔΔCq method
was used to calculate the relative expression level of COL1A1 and
miR-378b (31). Cycling conditions
were as follows: An initial predenaturation step at 94°C for 5 min,
followed by 40 cycles of denaturation at 94°C for 30 sec, annealing
at 60°C for 30 sec, extension at 72°C for 30 sec and a final
extension step at 72°C for 5 min. All experiments were repeated
three times. Data was analysed using Microsoft Excel 2016
(Microsoft Corporation, Redmond, WA, USA) and presented as the mean
± standard deviation.
Western blot analysis
Cells were analysed using radioimmunoprecipitation
assay buffer (50 mM Tris-Cl pH=8.0, 150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate, and 0.1% SDS) containing protease inhibitors
(EASYpack; Roche Applied Science, Mannheim, Germany). The protein
concentration was measured using the Bradford assay (Bio-Rad
Laboratories, Inc.). A total of 50 µg cellular protein was
dissolved in SDS sample buffer. Proteins were separated by SDS-PAGE
on a 10% gel and transferred to polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked in
5% skimmed milk buffer (in 50 mM Tris, 150 mM NaCl and 0.1% Tween
20) for 1 h at room temperature and were probed with rabbit
anti-SIRT6 (1:2,000; catalog no. 12486; Cell Signalling Technology,
Inc., Danvers, MA, USA) or anti-β-actin (1:10,000; N-21; catalog
no. sc-130656; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
primary antibodies at 4°C for 18 h. Subsequently, these proteins
were visualized using an anti-mouse IgG horseradish peroxidase
(HRP) (1:5,000; catalog no. 7076; Cell Signalling Technology, Inc.)
and anti-rabbit IgG HRP (1;3,000; catalog no. 7074; Cell Signalling
Technology, Inc.) secondary antibodies at 25°C for 1 h. A
SuperSignal™ West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific, Inc.) and a ChemiDoc Touch Imaging system (Bio-Rad
Laboratories) were used to visualise protein bands.
Statistical analysis
Statistical significance was calculated using
one-way analysis of variance with Tukey's post-hoc test. P<0.05
was considered to indicate a statistically significant difference
using Microsoft Excel 2016 (Microsoft Corporation). Data are
presented as the mean ± standard error.
Results and Discussion
Arctiin reduces miR-378b expression in
nHDFs
Arctiin has been reported to possess a variety of
biological effects, including anti-viral, anti-inflammatory and
anti-proliferative, in mammalian cells (32,33).
Binic et al (34) revealed
that arctiin induces collagen synthesis in nHDFs. These results
have provided a novel insight into the anti-aging effect of arctiin
on the skin. However, the underlying mechanism of collagen
induction by treatment with arctiin remains unknown. In a previous
study, it was revealed that arctiin markedly inhibited miR-378b in
UVB-irradiated nHDFs (16).
Therefore, in the present study, the miRNA-dependent mechanism with
which COL1A1 is induced in arctiin-treated nHDFs was
investigated.
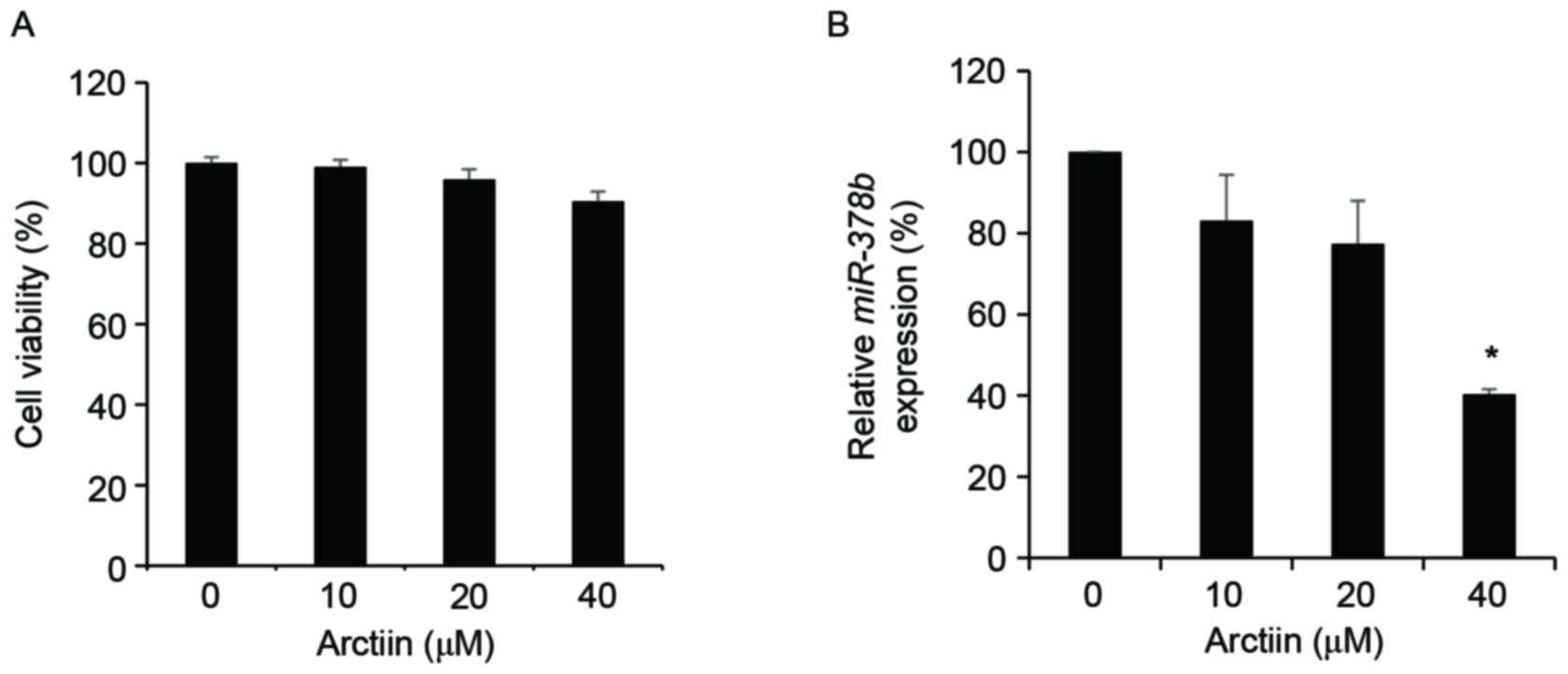
Cell viability in nHDFs was not affected at a
concentration of 0–40 µM arctiin (Fig.
1A). Furthermore, in nHDFs, miR-378b levels decreased by
treatment with arctiin in a dose-dependent manner (Fig. 1B). Treatment with 40 µM arctiin
significantly downregulated miR-378b by 40.34% compared with
control cells (P<0.05). Therefore, in the subsequent experiments
a dose of arctiin <40 µM was used.
Arctiin increases COL1A1 expression
through the miR-378b-SIRT6 axis
Our ongoing study revealed that miR-378b repressed
COL1A1 expression, interfering with SIRT6 expression (data
unpublished). Therefore, it is hypothesized that miR-378b
downregulates COL1A1 indirectly by interfering with the translation
of SIRT6 mRNA. Additionally, SIRT6 silencing is implicated
in the regulation of COL1A1 expression and skin aging in human
dermal fibroblasts (35).
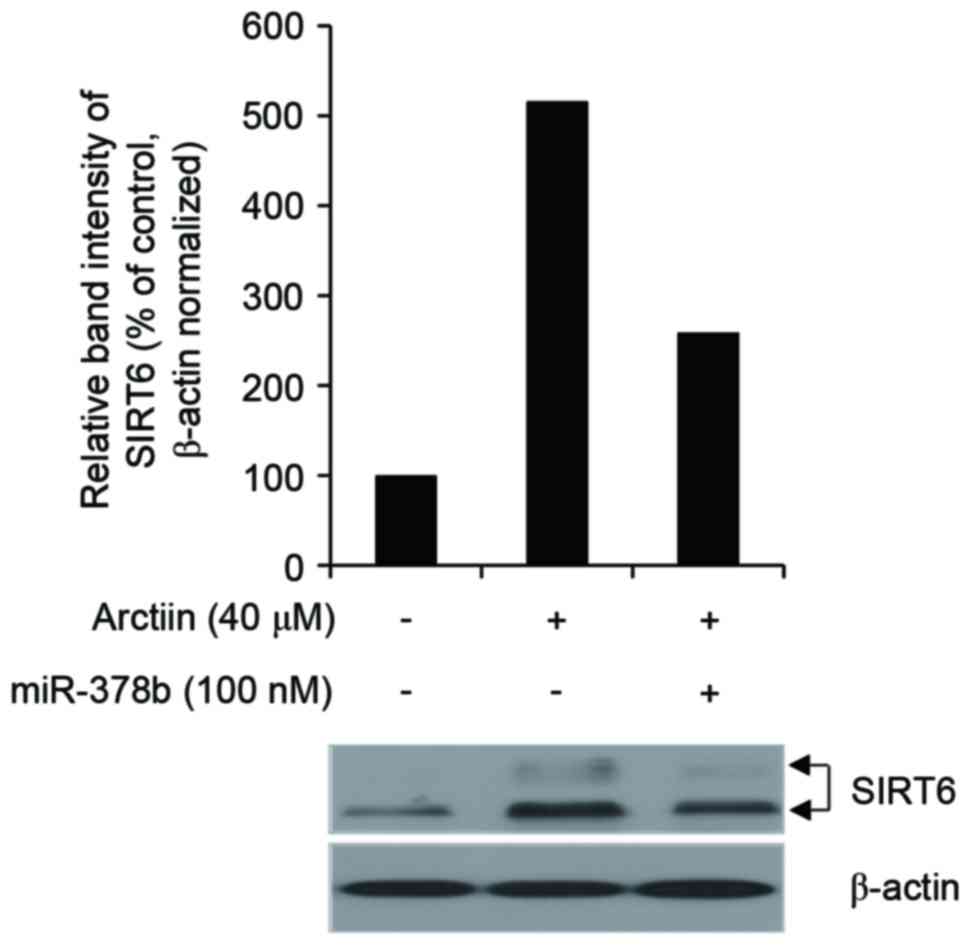
Treatment with 40 µM of arctiin increased SIRT6 protein expression
in nHDFs in Fig. 2, demonstrating
that arctiin regulates SIRT6 in nHDFs. Furthermore, miR-378b
inhibited the arctiin-mediated increase in SIRT6 protein expression
(Fig. 2). These results indicate
that arctiin elevates SIRT6 protein expression indirectly, through
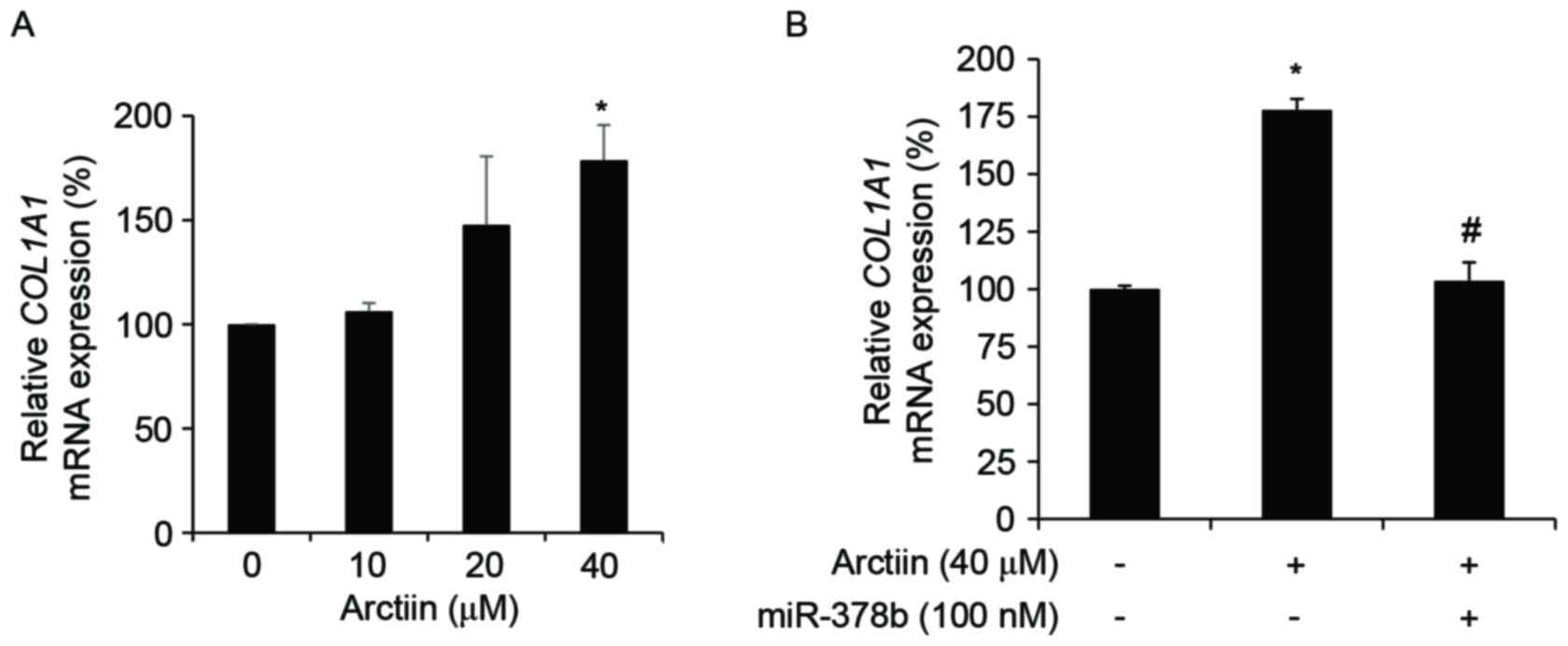
inhibition of miR-378b. Furthermore, the arctiin-mediated
increase of COL1A1 gene was antagonized by the addition of
exogenous miR-378b (Fig.
3B). Arctiin regulates COL1A1 via the miR-378b-SIRT6
axis.
Arctiin protects against UVB-induced
decrease in COL1A1 levels in nHDFs
Previous studies have indicated that arctiin has
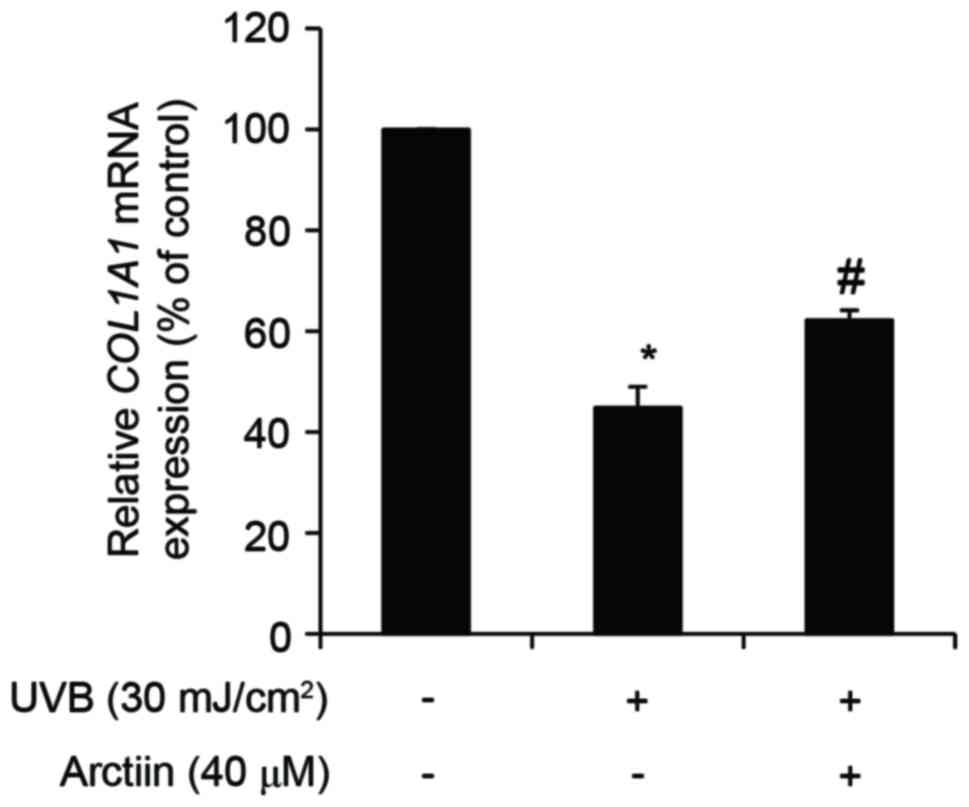
protective effects against UVB radiation in nHDFs (16). It was examined whether this
protective effect of arctiin against UVB opposed the
photoaging-dependent decrease in COL1A1 levels in nHDFs. nHDFs were
pretreated with 40 µM arctiin for 6 h and then exposed to 100
mJ/cm2 UVB radiation. Pre-treatment with arctiin
alleviated the UVB-induced inhibitory effects on COL1A1 mRNA
expression (Fig. 4). Furthermore,
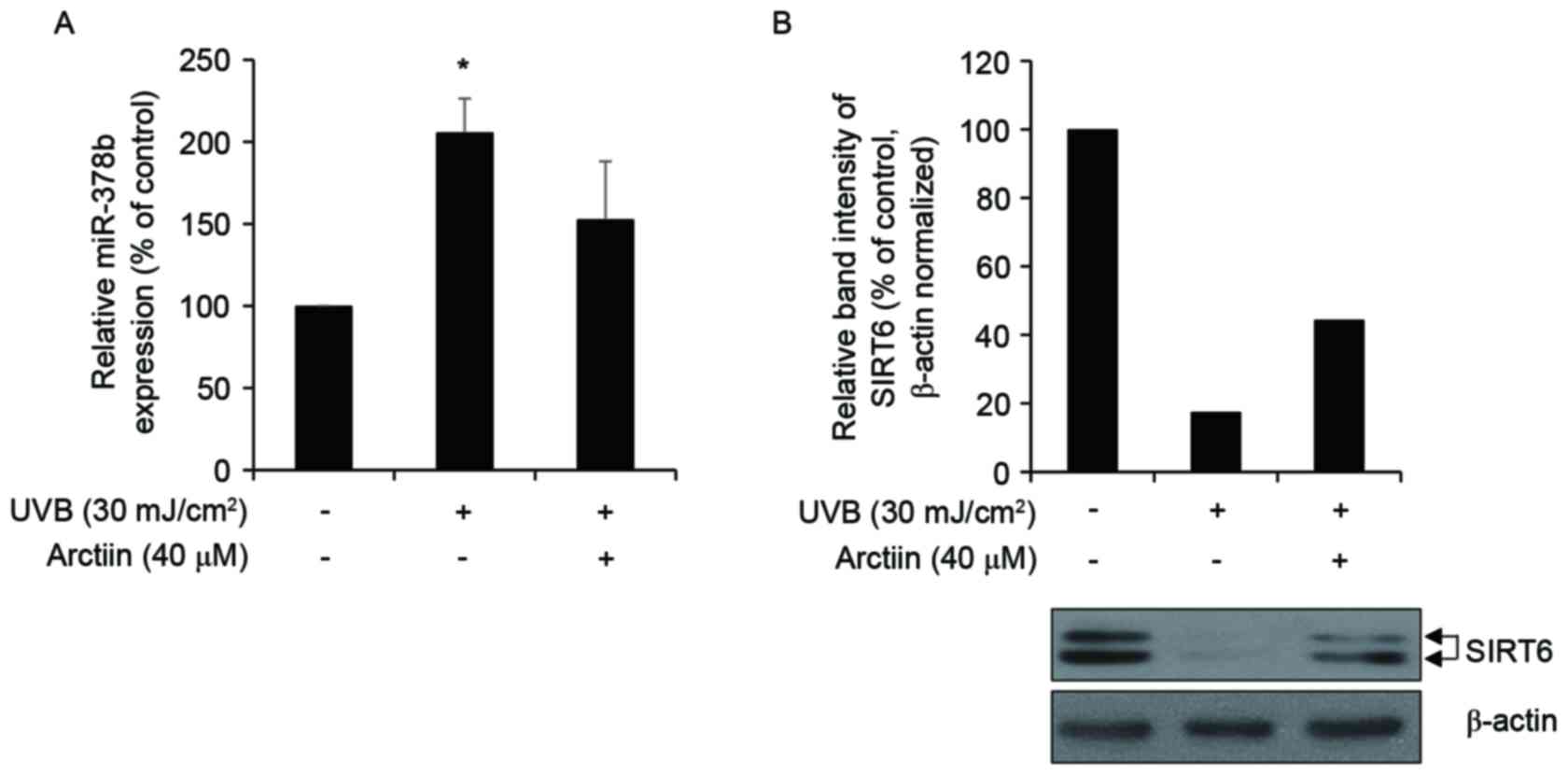
UVB treatment increased miR-378b expression and decreased SIRT6
protein expression in nHDFs (Fig. 5A
and B). Conversely, arctiin treatment decreased miR-378b
expression and increased SIRT6 in UVB-exposed nHDFs. These inverse
correlations indicated that arctiin elevates COL1A1 transcription
indirectly in UVB-exposed nHDFs through loss of miR-378b-mediated
inhibition of SIRT6. Alteration of Col1A1 expression, by enhanced
SIRT6, is induced by repression of NF-κB activation (35). In various aging mechanism,
activation NF-κB signaling is a key mediator of aging (36,37).
In UVB-induced photoaging, NF-κB activity is implicated, since
NF-κB is indirectly and directly regulated by collagens and matrix
metalloproteinases (38).
Therefore, the results suggest that miR-378b is useful in
preventing aging through blocking of SIRT6.
In conclusion, the study demonstrated that the
arctiin-induced upregulation of COL1A1 expression is regulated by
the miR-378/SIRT6 signaling pathway. Furthermore, it was revealed
that arctiin is able to prevent the UVB-induced reduction of COL1A1
expression, at least partially, through this same pathway.
Acknowledgements
The present study was supported by the Research
Professor Program of Konkuk University (to Professor Hwa Jun
Cha)and the Korean Health Technology R&D Project, Ministry of
Health & Welfare, Republic of Korea (grant no. HN13C0075).
References
|
1
|
Quan T and Fisher GJ: Role of
age-associated alterations of the dermal extracellular matrix
microenvironment in human skin aging: A mini-review. Gerontology.
61:427–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darlenski R, Kazandjieva J and Tsankov N:
Skin barrier function: Morphological basis and regulatory
mechanisms. J Clin Med. 4:36–45. 2011.
|
|
3
|
Eckert RL and Rorke EA: Molecular biology
of keratinocyte differentiation. Environ Health Perspect.
80:109–116. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Varani J, Dame MK, Rittie L, Fligiel SE,
Kang S, Fisher GJ and Voorhees JJ: Decreased collagen production in
chronologically aged skin: Roles of age-dependent alteration in
fibroblast function and defective mechanical stimulation. Am J
Pathol. 168:1861–1868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wulf HC, Sandby-Møller J, Kobayasi T and
Gniadecki R: Skin aging and natural photoprotection. Micron.
35:185–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Egbert M, Ruetze M, Sattler M, Wenck H,
Gallinat S, Lucius R and Weise JM: The matricellular protein
periostin contributes to proper collagen function and is
downregulated during skin aging. J Dermatol Sci. 73:40–48. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farage MA, Miller KW, Elsner P and Maibach
HI: Characteristics of the aging skin. Adv Wound Care (New
Rochelle). 2:5–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Debacq-Chainiaux F, Leduc C, Verbeke A and
Toussaint O: UV stress and aging. Dermatoendocrinol. 4:236–240.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katiyar SK and Mukhtar H: Green tea
polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin
prevents UVB-induced infiltration of leukocytes, depletion of
antigenpresenting cells, and oxidative stress. J Leukoc Biol.
69:719–726. 2001.PubMed/NCBI
|
|
10
|
Wlaschek M, Tantcheva-Poór I, Naderi L, Ma
W, Schneider LA, Razi-Wolf Z, Schüller J and Scharffetter-Kochanek
K: Solar UV irradiation and dermal photoaging. J Photochem
Photobiol B. 63:41–51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shindo Y, Witt E and Packer L: Antioxidant
defense mechanisms in murine epidermis and dermis and their
responses to ultraviolet light. J Invest Dermatol. 100:260–265.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee S, Shin S, Kim H, Han S and Kim K,
Kwon J, Kwak JH, Lee CK, Ha NJ, Yim D and Kim K: Anti-inflammatory
function of arctiin by inhibiting COX-2 expression via NF-κB
pathways. J Inflamm (Lond). 8:162011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu JG, Wu JZ, Sun LN, Han T, Du J, Ye Q,
Zhang H and Zhang YG: Ameliorative effects of arctiin from Arctium
lappa on experimental glomerulonephritis in rats. Phytomedicine.
16:1033–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Knott A, Reuschlein K, Mielke H, Wensorra
U, Mummert C, Koop U, Kausch M, Kolbe L, Peters N, Stäb F, et al:
Natural Arctium lappa fruit extract improves the clinical signs of
aging skin. J Cosmet Dermatol. 7:281–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha HJ, Lee GT, Lee KS, Lee KK, Hong JT,
Lee NK, Kim SY, Lee BM, An IS, Hahn HJ, et al: Photoprotective
effect of arctiin against ultraviolet B-induced damage in HaCaT
keratinocytes is mediated by microRNA expression changes. Mol Med
Rep. 10:1363–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Syed DN, Khan MI, Shabbir M and Mukhtar H:
MicroRNAs in skin response to UV radiation. Curr Drug Targets.
14:1128–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Honda N, Jinnin M, Kajihara I, Makino T,
Makino K, Masuguchi S, Fukushima S, Okamoto Y, Hasegawa M, Fujimoto
M and Ihn H: TGF-β-mediated down-regulation of microRNA-196a
contributes to the constitutive upregulated type I collagen
expression in scleroderma dermal fibroblasts. J Immunol.
188:3323–3331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamauchi M, Prisayanh P, Haque Z and
Woodley DT: Collagen cross-linking in sun-exposed and unexposed
sites of aged human skin. J Invest Dermatol. 97:938–941. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sohal RS and Weindruch R: Oxidative
stress, caloric restriction, and aging. Science. 273:59–63. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benedetto AV: The environment and skin
aging. Clinics Derm. 16:129–139. 1998. View Article : Google Scholar
|
|
22
|
Kwon DN, Chang BS and Kim JH: MicroRNA
dysregulation in liver and pancreas of CMP-Neu5Ac hydroxylase null
mice disrupts insulin/PI3K-AKT signaling. Biomed Res Int.
2014:2363852014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Gubory KH, Fowler PA and Garrel C: The
roles of cellular reactive oxygen species, oxidative stress and
antioxidants in pregnancy outcomes. Int J Biochem Cell Biol.
42:1634–1650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lucas K and Raikhel AS: Insect microRNAs:
Biogenesis, expression profiling and biological functions. Insect
Biochem Mol Biol. 43:24–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung HJ and Suh Y: MicroRNA in aging: From
discovery to biology. Curr Genomics. 13:548–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Banerjee J, Chan YC and Sen CK: MicroRNAs
in skin and wound healing. Physiol Genomics. 43:543–556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 9:597–610. 2010.
|
|
28
|
Greussing R, Hackl M, Charoentong P, Pauck
A, Monteforte R, Cavinato M, Hofer E, Scheideler M, Neuhaus M,
Micutkova L, et al: Identification of microRNA-mRNA functional
interactions in UVB-induced senescence of human diploid
fibroblasts. BMC Genomics. 14:2242013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi R and Fuchs E: MicroRNA-mediated
control in the skin. Cell Death Differ. 17:229–235. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hildebrand J, Rütze M, Walz N, Gallinat S,
Wenck H, Deppert W, Grundhoff A and Knott A: A comprehensive
analysis of microRNA expression during human keratinocyte
differentiation in vitro and in vivo. J Invest Dermatol. 13:20–29.
2011. View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsuzaki Y, Koyama M, Hitomi T, Yokota T,
Kawanaka M, Nishikawa A, Germain D and Sakai T: Arctiin induces
cell growth inhibition through the down-regulation of cyclin D1
expression. Oncol Rep. 19:721–727. 2008.PubMed/NCBI
|
|
33
|
Hayashi K, Narutaki K, Nagaoka Y, Hayashi
T and Uesato S: Therapeutic effect of arctiin and arctigenin in
immunocompetent and immunocompromised mice infected with influenza
A virus. Biol Pharm Bull. 33:1199–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Binic I, Lazarevic V, Ljubenovic M, Mojsa
J and Sokolovic D: Skin ageing: Natural weapons and strategies.
Evid Based Complement Alternat Med. 2013:8272482013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baohua Y and Li L: Effects of SIRT6
silencing on collagen metabolism in human dermal fibroblasts. Cell
Biol Int. 36:105–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tilstra JS, Clauson CL, Niedernhofer LJ
and Robbins PD: NF-κB in aging and disease. Aging Dis. 2:449–465.
2011.PubMed/NCBI
|
|
37
|
Osorio FG, López-Otín C and Freije JM:
NF-kB in premature aging. Aging (Albany NY). 4:726–727. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanaka K, Asamitsu K, Uranishi H,
Iddamalgoda A, Ito K, Kojima H and Okamoto T: Protecting skin
photoaging by NF-kappaB inhibitor. Curr Drug Metab. 11:431–435.
2010. View Article : Google Scholar : PubMed/NCBI
|